Abstract
Context:
The changing hormonal milieu around menopause is implicated in the development of sleep disturbances. No studies have assessed the association between concurrent physiological measures of sleep and serum hormone concentrations in perimenopausal women.
Objective:
This study aimed to assess the interaction between physiological sleep and reproductive hormone measures in perimenopausal women.
Design and Participants:
This was a cross-sectional laboratory study of 33 perimenopausal women age 43–52 years (17 with no sleep complaints and 16 with a clinical diagnosis of insomnia). Eleven premenopausal women without sleep complaints (18–27 y), were included to determine whether hormone-sleep relationships differed depending on reproductive stage.
Main Outcome Measures:
Concurrent polysomnographic sleep indices and serum hormone levels (estradiol and follicle stimulating hormone [FSH]) were measured.
Results:
FSH was positively associated with polysomnographic-defined wakefulness after sleep onset, and number of awakenings and arousals in perimenopausal women (P < .05) without sleep complaints independent of age, body mass index, and hot flashes. Similarly, FSH correlated with wakefulness after sleep onset and light N1 sleep in premenopausal women (P < .05). In contrast, in perimenopausal insomniacs amount of sleep correlated with anxiety and depression (P < .05) but not with FSH. Estradiol did not correlate with sleep in perimenopausal groups but correlated negatively with arousals in premenopausal women (P < .01).
Conclusion:
Our results suggest an interaction between the hypothalamic-pituitary-ovarian (HPO) axis and sleep-wake regulatory systems in pre- and peri-menopausal women without sleep complaints. There was no relationship between hormones and sleep in perimenopausal insomniacs, whose sleep may be influenced by other factors intrinsic to insomnia, such as hyperactivity, poor mood, and night-to-night variability.
The approach to menopause is associated with significant changes in the hormonal milieu with an increase in FSH due principally to the decline in negative feedback from inhibin as follicular reserves in the ovaries decline (1). Estradiol levels are erratic during the early menopausal transition before declining to low levels after menopause (1). In association with this changing hormonal environment, there is increased prevalence of symptoms including depressed mood, hot flashes, and sleep problems (2). Sleep problems are one of the most prevalent and bothersome symptoms during perimenopause, being reported by ∼40% of women (3), with frequent night-time awakenings being the most common and severe symptom (3, 4). Increased prevalence of sleep problems during perimenopause is attributed to several factors, including hot flashes, poor physical or psychological health, presence of a sleep disorder, stress exposure, and the hormonal changes themselves (2, 4).
Population studies have investigated the association between reproductive hormone levels and sleep in pre- and perimenopausal women; however, these have mostly relied on self-reported sleep, and findings are mixed (4–11). Low or decreasing estradiol (4, 9, 10), high or increasing FSH (4, 9, 11), or low or decreasing inhibin B (6, 8) have all been associated with poor subjective sleep quality or sleep disruption in late-stage reproductive or perimenopausal women.
Few studies have related reproductive hormone levels with measures of sleep derived from polysomnographic (PSG) recordings. FSH levels were associated with more wakefulness after sleep onset (WASO) in mostly pre and early perimenopausal women in the Study of Women Across the Nation (SWAN) sleep study but this relationship did not survive correction for covariates including age and body mass index (BMI) (12). Also, a faster rate of change in FSH over a 5–7-year period was associated with a higher percentage of delta (N3) sleep and longer total sleep time (TST), implying that a faster transition through menopause is associated with better sleep. Estradiol was unrelated to PSG measures (12). However, a caveat of this study is that blood was sampled 3–6 months before the PSG. A small study (13) showed a positive association between concurrent FSH levels and WASO and a negative correlation between FSH and N3 sleep in a combined sample of depressed pre- and postmenopausal women, but correlations were not adjusted for age. Others have reported a negative association between concurrent estradiol levels and movement arousals during N3 sleep but not with other PSG measures in postmenopausal women; no relationships were found between FSH and sleep measures (5). Sleep architecture also is modulated by the menstrual cycle (14). We previously found that estradiol and progesterone levels correlated negatively with rapid eye movement (REM) sleep and positively with WASO in the luteal phase (15). These data correspond, in part, with data from animal studies showing that estradiol and progesterone administration to ovariectomized rats reduces REM and non-REM sleep and increases WASO (16).
Other physiological changes associated with menopause could mediate a relationship between reproductive hormones and disturbed sleep. Hot flashes, a key symptom of menopause, disrupt sleep (7, 17, 18). Hot flashes increase in prevalence and severity in association with an increase in FSH and decline in estradiol in the approach to menopause (7, 19, 20) suggesting that hot flashes may mediate the association between hormones and sleep. Other key elements may have a role in the hormone-sleep relationship. Insomnia, clinically diagnosed based on persistent self-reported poor sleep, is associated with significant distress, increases in prevalence in peri- and postmenopausal women (21), and is associated with a specific psychophysiological profile characterized by high neuroticism and obsessive compulsive personality (22), more anxiety and depression symptoms (21, 23), and greater prevalence and distress from hot flashes (18, 22). These data suggest that menopausal insomniacs may have a heightened sensitivity to reproductive hormone changes.
Our primary aim was to investigate the interaction between female reproductive hormones, particularly FSH and estradiol, and physiological sleep measures in perimenopausal women with and without a clinical diagnosis of insomnia. We considered potential confounding factors such as the presence of objective hot flashes, BMI, and age. We hypothesized that higher levels of FSH and lower levels of estradiol would be associated with poor sleep outcomes (eg, more wakefulness) in perimenopausal women and that relationships would be stronger in those with insomnia. Finally, we investigated whether relationships between hormones and sleep varied according to reproductive stage by analyzing hormone-sleep associations in a group of premenopausal women without sleep complaints.
Materials and Methods
Participants
Women were recruited from the San Francisco Bay Area community. The study was reviewed and approved by SRI International's Institutional Review Board. All participants provided written informed consent, and received compensation for study participation. Perimenopausal women were screened as described in (22). Premenopausal women were screened as described in (15). Inclusion criteria for perimenopausal groups included being in the menopausal transition according to Stages of Reproductive Aging Workshop criteria (24) ie, menstrual cycle lengths that differed by more than 7 days from normal (early menopause transition) or amenorrhea interval of more than 60 days (late menopausal transition), BMI no more than 32 kg/m2, intact uterus, and at least one ovary. Inclusion criteria for premenopausal women included regular menstrual cycles, between 24 and 35 days and BMI no more than 30 kg/m2. Exclusions for all groups were use of hormone therapy or hormonal contraception for the previous 3 months, hypertension or arrhythmias, long-term medication use, or current sleep medication, and/or antidepressant use. Thirty-three perimenopausal and 11 premenopausal women participated (Table 1).
Table 1.
Characteristics and Serum Reproductive Hormone Concentrations (Mean (sd)) for Each Participant Group
| Premenopausal Women No Sleep Complaints (n = 11) | Perimenopausal Women |
||
|---|---|---|---|
| No Sleep Complaints (n = 17) | Insomniacs (n = 16) | ||
| Age, y | 29.1 (7.3)b | 48.9 (2.3) | 49.1 (2.7) |
| BMI, kg/m2 | 22.4 (2.4) | 25.6 (3.8) | 24.0 (3.9) |
| Ethnicity, No. Caucasian | 5 | 14 | 11 |
| Menopause status, No. in early MT | – | 16 | 15 |
| Pittsburgh Sleep Quality Index | 3.3 (1.4) | 3.9 (1.7) | 8.9 (3.5)b |
| STAI, Form Y-2 | 32.9 (7.5) | 29.6 (6.1) | 37.9 (11.1)c |
| Beck Depression Inventory | 2.9 (3.8) | 3.0 (3.6) | 11.0 (8.1)b |
| No. self-report nocturnal hot flashes | – | 3 | 6 |
| No. Objective nocturnal hot flashes | – | 2 | 2d |
| FSH, IUa | 5.6 (2.5)b | 22.3 (17.5) | 23.7 (17.3) |
| FSH range, IU | 2.3–10.0 | 2.5–62.0 | 3.4–55.8 |
| Estradiol, pg/mla | 68.1 (38.5) | 72.2 (65.1) | 50.3 (33.3) |
| Estradiol range, pg/ml | 25.7–143.2 | 5.3–227.6 | 8.9–123.1 |
| Progesterone, ng/mla | 0.6 (0.3) | 0.7 (0.6) | 0.8 (0.5) |
| Progesterone range, ng/ml | 0.2–1.2 | 0.2–2.0 | 0.2–2.1 |
Abbreviation: MT, menopause transition.
Analyses are based on log-transformed data.
Significantly different from the other two groups.
Significantly different from the perimenopausal women without sleep complaints.
Skin conductance data available from 13 women.
Screening and study protocol
All women completed a phone screen and potentially eligible participants completed the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) Axis I Disorders (25). Perimenopausal women also completed a sleep history interview that queried DSM-IV criteria for insomnia. Based on the clinical interview, 16 women met criteria for diagnosis of insomnia. Specifically, they presented with a prominent self-described disturbance in sleep characterized by difficulty falling asleep, maintaining sleep, early morning awakening, and/or a feeling of nonrestorative sleep, evident at least three times per week for at least a month and that caused clinically significant distress or impairment; sleep disturbance was coincident with the onset of the menopause transition, and was not accounted for by another mental disorder, a breathing-related sleep disorder, periodic limb movement disorder, or narcolepsy (based on self-reported absence of symptoms and confirmed with a clinical in-lab PSG assessment). The remaining 17 women in the perimenopausal group reported absent or minimal sleep difficulties. Premenopausal women did not have sleep complaints. All participants must have had no lifetime history of DSM-IV insomnia before perimenopause or current axis I disorders (other than nicotine dependence or insomnia). Women completed questionnaires to assess sleep quality in the past month (Pittsburgh Sleep Quality Index) (26), trait anxiety (State-Trait Anxiety Inventory [STAI], Form Y-2) (27), and depression symptoms (Beck Depression Inventory; BDI-II) (28). Participants had an adaptation/screening night in the laboratory with a clinical PSG to adapt to the laboratory and to confirm the absence of a clinically-significant sleep disorder (apnea-hypopnea index > 5 and periodic leg movement index > 10).
Participants then were scheduled for a PSG recording in the follicular phase of their menstrual cycle (5–11 d after menstrual flow onset). On study days, the women were requested not to drink caffeinated beverages after 1500 h, not to drink alcoholic beverages, and not to nap. Participants registered 0 on the breathalyzer at each recording. They slept in sound-attenuated bedrooms in the sleep laboratory at SRI International. Ambient temperature was maintained between 20°C and 22°C. Lights-out and lights-on times were self selected by participants.
Hormone analysis
A blood sample was collected from participants at their visit, either in the evening (2000–2200 h) of the overnight PSG recording or the following morning (0700–0900 h). Serum samples were frozen at −70°C until analysis. Samples were analyzed for FSH, progesterone, and estradiol using standard immunoassay kits. Intra-assay and interassay coefficients of variations were 6.7% and 7.6%, respectively, for the estradiol assay (sensitivity, 4 pg/ml) (Beckman Coulter Inc., Fullerton, CA), and 2.6% and 5.5%, respectively, for the FSH assay (sensitivity, 0.1 mIU/mL) (Siemens Healthcare Diagnostics, Los Angeles, CA). Intra-assay and interassay coefficients of variations were 8.8% and 9.7%, respectively, for the progesterone assay (sensitivity, 0.1 ng/ml) (Siemens Healthcare Diagnostics).
Sleep assessment
Electroencephalographic (EEG), electrooculographic, and electromyographic recordings were made using Compumedics amplifiers and Profusion software (Compumedics, Abbotsford, Victoria, Australia). EEG electrodes were placed according to the international 10–20 system and cross referenced to contralateral mastoids. Sleep was scored according to American Academy of Sleep Medicine (AASM) criteria (29). EEG signals were digitized at 256 Hz and filtered (0.03–35 Hz). Thirty-second epochs were scored by two scorers (inter-rater reliability set at 0.90; discrepancies solved by a third scorer). TST (min) is time spent asleep minus in-bed wakefulness during time in bed (min). Sleep efficiency (SE; %) is the percentage of TST during time in bed. Sleep-onset latency (min) is the time from lights out to the first epoch of any stage of sleep. WASO was calculated as the total wake time minus the wake before sleep onset (min). The time between sleep onset and the first epoch of REM sleep is REM onset latency (min). Time spent in each sleep stage (N1, N2, N3, and REM; min) and number of awakenings was calculated. Brief arousals, defined as an increase in fast EEG activity relative to background activity, lasting at least 3 seconds and less than 15 seconds, were scored according to AASM rules (29). The arousal index (ArI) was calculated as number of arousals multiplied by 60, divided by TST.
Hot flash assessment
Nocturnal hot flashes were assessed during laboratory visits in perimenopausal women based on self report and measures of sternal skin conductance according to standard criteria (30) with a BioDerm Skin Conductance Meter (model 2701; UFI, Morro Bay, CA). Hot flashes were manually evaluated by two scorers, blinded to PSG scoring, for fluctuations (increase in conductance of ≥ 2 micro-Siemens within 30 s) (31). Due to technical failure, skin conductance measures were missing in three perimenopausal insomniacs (two of them did not subjectively report having hot flashes; one woman reported having a hot flash).
Data analyses
Statistical analyses were performed using Predictive Analytics SoftWare Statistics 18 (SPSS Inc., Chicago, IL). P < .05 was considered statistically significant.
Demographics and serum reproductive hormone concentrations, anxiety, depression, subjective sleep quality, and objective PSG sleep measures were compared with one-way ANOVAs with group as a categorical predictor. If ANOVA was significant, Student-Newman-Keuls post-hoc tests for multiple comparisons were used to identify the origin of any difference. Hormone levels were log transformed before analysis to assure normal distribution.
Associations between FSH and estradiol levels and PSG variables were investigated in all three groups combined as well as separately within each participant group using Pearson correlations. The observed power of correlations was calculated using G*Power version 3.1.9 (Universität Kiel, Germany). When correlations were significant, we ran stepwise multiple regression models with age and BMI as covariates.
Results
Demographics, reproductive hormones, mood, and subjective sleep quality
All but two women in the perimenopausal group were in the early menopause transition (24). Perimenopausal women were older (P < .001) and had higher FSH levels (P < .001) than premenopausal women (Table 1). As expected for the follicular phase, progesterone levels were low in all groups and correlations between progesterone and sleep variables were therefore not investigated. Perimenopausal insomniacs reported poorer sleep quality (P < .001) and had higher depression scores compared with perimenopausal (P < .001) and premenopausal (P < .01) women without sleep complaints (Table 1). They also had higher anxiety scores compared with perimenopausal women without sleep complaints (P < .05).
Physiological sleep
Perimenopausal insomniacs had a shorter TST than perimenopausal women without sleep complaints (P < .05) and premenopausal women (P < .01) (Table 2). They also had a lower SE than premenopausal women (P < .05). Groups did not differ on other PSG outcomes.
Table 2.
Variables Derived from the Polysomnogram (Mean (sd)) for Each Participant Group
| Premenopausal Women No Sleep Complaints (n = 11) | Perimenopausal Women |
||
|---|---|---|---|
| No sleep Complaints (n = 17) | Insomniacs (n = 16) | ||
| Time in bed, min | 459.8 (56.8) | 455.1 (33.8) | 429.0 (34.1) |
| Total sleep time, min | 426.6 (54.5) | 407.6 (36.2) | 376.7 (30.4)a |
| Sleep onset latency, min | 7.5 (7.1) | 10.9 (8.2) | 11.6 (8.5) |
| Latency to REM, min | 86.1 (33.4) | 88.2 (27.0) | 90.7 (34.8) |
| Sleep efficiency, % | 92.8 (3.2) | 89.6 (5.3) | 88.0 (5.3)b |
| WASO, min | 25.7 (14.7) | 36.6 (19.6) | 40.7 (23.7) |
| Time in N1, min | 31.3 (13.3) | 32.5 (12.1) | 30.8 (14.7) |
| Time in N2, min | 226.3 (43.9) | 223.6 (32.1) | 209.7 (24.7) |
| Time in N3, min | 66.3 (21.7) | 62.7 (14.3) | 49.5 (28.4) |
| Time in REM, min | 97.1 (26.5) | 88.7 (19.4) | 86.6 (19.8) |
| Awakenings, No. | 21.6 (10.9) | 20.8 (7.1) | 19.5 (6.9) |
| Arousals, No. | 53.3 (20.6) | 53.6 (17.4) | 52.0 (24.9) |
| ArI, No./h | 7.5 (2.7) | 8.0 (2.9) | 8.3 (4.0) |
Significantly different from the other two groups.
Significantly different from the premenopausal women without sleep complaints.
Interactions between sleep and reproductive hormones
Combined group
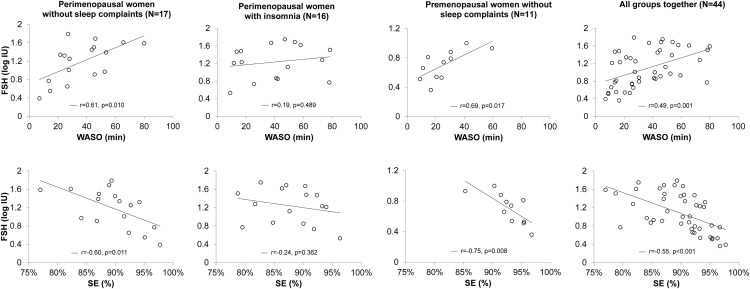
FSH correlated with SE (r = −0.55; P < .001), sleep onset latency (r = 0.34; P < .023), WASO (r = 0.49; P = .001), time spent in REM (r = −0.38; P < .011) and N1 (r = 0.33; P = .031), number of arousals (r = 0.30; P = .048), and ArI (r = 0.40; P = .007). Multiple regression models with age and BMI as covariates confirmed that FSH was significant in all models (P < .05). Estradiol correlated with SE (r = 0.30; P = .048), WASO (r = −0.30; P = .042), time in N2 (r = 0.35; P = .019), number of arousals (r = −0.32; P = .033), and ArI (r = −0.35, P = .018). The correlation between estradiol and SE was no longer significant after controlling for age and BMI. All other relationships remained significant.
Perimenopausal women without sleep complaints
FSH correlated with SE (r = −0.60; P = .011), WASO (r = 0.61; P = .010), number of awakenings (r = 0.55; P = .021), number of arousals (r = 0.58; P = .015), and ArI (r = 0.60; P = .010). Multiple regression models with age and BMI as covariates were all significant (P < .05) and confirmed FSH as the only significant predictor in all models (P < .05). There were no significant correlations between FSH and time spent in any sleep stage. Estradiol was not significantly related to PSG variables. Only two women had objectively recorded hot flashes during the PSG recording, with both women having a single hot flash. All relationships remained significant after excluding these women.
Perimenopausal insomniacs
There were no significant relationships between FSH and PSG variables. Estradiol correlated with time spent in N2 (r = 0.50; P = .048) in univariate analysis but this relationship was no longer significant after controlling for effects of age and BMI. Two of 13 women in whom hot flashes were monitored had a single objectively recorded hot flash during the PSG. Results were unchanged when these women were excluded.
Premenopausal women
FSH correlated with SE (r = −0.75; P = .008), WASO (r = 0.69; P = .017), and time in N1 (r = 0.73; P = .012). Estradiol correlated negatively with number of arousals (r = −0.80; P = .003) and ArI (r = −0.76; P = .006). All relationships were maintained in multiple regression models with age and BMI as covariates: FSH was the only significant predictor for SE, WASO, and time in N1 (P < .05), and estradiol was the only significant predictor for number of arousals and ArI (P < .05).
Relationships between FSH and WASO, and FSH and SE are represented in Figure 1, in the combined group as well as separately for perimenopausal women with and without insomnia and premenopausal women without sleep complaints.
Figure 1.
Relationship between FSH levels and polysomnographic-measured WASO and sleep efficiency (SE) in 17 perimenopausal women without sleep complaints, 16 perimenopausal insomniacs, 11 premenopausal women without sleep complaints, and in the combined group. Coefficients of correlation (r) and P-values are shown.
Association between sleep, anxiety, and depression in perimenopausal insomniacs
Given the lack of relationships between hormones and sleep in perimenopausal insomniacs, we explored associations between PSG measures and critical features of insomnia like anxiety and depression. Pearson's correlations suggested that high anxiety and depression scores were associated with reduced time in bed (anxiety: r = −0.53; P = .036; depression: r = −0.65; P = .007) and TST (anxiety: r = −0.52; P = .038; depression: r = −0.59; P = .015).
Discussion
Higher levels of FSH are strongly associated with PSG-derived measures of wakefulness in pre and perimenopausal women without sleep complaints. However, none of these relationships was evident in perimenopausal women with a diagnosis of insomnia. Together, these data suggest the existence of a normal interaction between the HPO axis and the sleep-wake regulatory system in pre- and perimenopausal women but that the relationship is absent in the presence of insomnia associated with the transition to menopause.
To our knowledge, this study is the first to examine associations between concurrent measures of physiological sleep and serum hormone levels in groups of pre and perimenopausal women.
Increasing FSH levels herald the approach to menopause and reflect endocrine changes occurring across the HPO axis. Our finding of an association between high FSH levels and several indicators of physiological arousal supports findings from the SWAN study of an association between higher FSH levels and self-reported trouble sleeping (11) and in-bed awakenings (9) in late-reproductive stage or perimenopausal women.
Our study is unique in that we distinguished perimenopausal women with and without sleep complaints and found discordance in the relationship between FSH and wakefulness depending on a diagnosis of insomnia. In contrast with our hypothesis, PSG outcomes were not associated with hormone levels in perimenopausal insomniacs. Rather, the amount of sleep was negatively associated with high anxiety and depression. As shown by others (32), insomniacs had higher levels of anxiety and depression than women without sleep complaints; although none of them was diagnosed with a depressive or anxiety disorder. Also, insomniacs had a shorter TST, mainly due to a shorter time in bed, compared with women without insomnia. However, the two groups did not differ in other physiological sleep measures, which is not surprising given the high night-to-night variability that characterizes sleep in insomniacs (33). A DSM-IV diagnosis of insomnia is based on self-reported sleep disturbance and distress associated with that complaint, and not on an objective criterion of poor sleep. Of note, the perimenopausal insomniacs in our study were not chronic insomniacs; they had developed insomnia recently in association with the menopause transition. A poor mood profile as well as high night-to-night variability may influence an insomniac's sleep pattern and sleep quality. Also, poor perceived sleep quality in insomniacs is attributed to a general state of psychophysiological hyperactivation that prevents them from obtaining a refreshing sleep (34).
The prevalence of hot flashes increases across the menopausal transition, correlating strongly with increasing FSH levels (7, 20). Hot flashes significantly affect sleep (17, 35), suggesting that the relationship between FSH and wakefulness could be mediated by hot flashes. However, in our sample of perimenopausal women early in the menopause transition we measured only four objective hot flashes; thus, the effect of hot flashes on hormone-sleep relationships was negligible.
In the combined group of women, higher estradiol levels were associated with a better sleep profile (less WASO and a lower ArI). When investigated in separate groups, the negative association between estradiol and ArI was maintained in premenopausal women, but not in perimenopausal groups. These findings contrast those in premenopausal women in the late-luteal phase (15) and animal studies (16), where higher estradiol levels were associated with more WASO. However, others found a relationship between high estradiol levels and fewer movement arousals during slow-wave sleep in postmenopausal women (5), or associations between low or decreasing levels of estradiol and poor self-reported sleep in late-stage reproductive or perimenopausal women (9, 10). However, no association was found between estradiol or rate of change in estradiol over time and PSG measures of sleep in SWAN sleep study participants (12) and estradiol was also unrelated to trouble sleeping across the menstrual cycle in SWAN participants (11).
Mechanisms of how HPO hormones may interact with sleep-wake regulatory systems in women are poorly understood. There is a regulated coordination between hypothalamic release of GnRH, anterior pituitary release of FSH and LH, and release of hormones including estradiol, progesterone, and inhibin from the ovaries. The HPO axis is a closed-loop negative feedback system in which ovarian steroids and inhibins can act at the hypothalamus and the anterior pituitary to provide negative feedback on secretion of gonadotropins (36). As such, an association between FSH and sleep measures may be mediated by relationships downstream or upstream of FSH. Estradiol may play a critical role; estrogen receptors are found in several brain areas, including those involved in sleep regulation, and estradiol influences arousal in rodent models (16). Inhibin may also be important; serum levels of inhibin B decrease as women enter the menopause transition with a consequent increase in FSH (36) and low inhibin B levels have previously been associated with poor subjective sleep quality in late-menopausal transition and early postmenopausal women (6). There also are links between the HPO axis and the orexin system, which is involved in the regulation of arousal and may be a potential pathway to influence wakefulness. Orexin receptors in the anterior pituitary are under GnRH influence and orexin receptor 1 expression in the hypothalamus and anterior pituitary is influenced by estradiol (37). Finally, reproductive hormones could influence sleep regulation via effects on other neurotransmitter systems involved in sleep regulation that remain to be determined.
Our study has limitations. Analysis of the relationship between FSH and sleep is correlational; therefore, we cannot determine directionality of the relationship or establish causation. We investigated associations between hormone levels measured in a single blood sample and PSG-measures on a single night. A single measure of either hormones or sleep cannot capture variability over time and may not accurately reflect the stable state of a woman's physiology. Multiple measurements, although challenging, may be needed to obtain a more reliable picture of hormone-sleep relationships. Also, we did not sample blood throughout the night and cannot establish the dynamics between pulsatile gonadotropin release and sleep stages or sleep-wake transitions; pulsatile gonadotropin secretion slows during sleep and pulses typically follow brief awakenings in young women (38). Our study was cross-sectional. Further longitudinal studies are needed to better understand the association between variability in FSH and sleep across the menopause transition. In fact, as suggested (8, 39), variability in hormones could be an important factor to consider in addition to absolute hormone levels. Another limitation is the relatively small sample size. However, observed power for all correlations between FSH and the main sleep-wake measures (SE and WASO) ranged from 80–99%.
Our study also has several strengths. Sleep recordings and blood samples were temporally proximate and occurred in the follicular phase of the menstrual cycle when hormone levels show good intra-individual reproducibility (40). We included a group of premenopausal women and could show that sleep-hormone associations found in perimenopause are also evident during the reproductive stage. We confirmed that none of the participants had clinically significant sleep apnea or periodic limb movement disorder, which increase in prevalence as women transition into menopause and are known causes of disrupted sleep (2). Finally, we evaluated sleep with objective PSG measures and accounted for several covariates including hot flashes. Our findings should stimulate further research into mechanisms behind interactions between the HPO axis and sleep in women.
Acknowledgments
We would like to thank Justin Greco, Rebecca Carr, David Sugarbaker, David Dresser, Stephanie Claudatos, and Sarah Inkelis for their effort in the data collection process.
This work was supported by the National Institutes of Health Grants HL103688 and HL088088 (to F.C.B.). Blood sample analysis was supported by National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction Research) Grant U54-HD28934, “University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.”
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AASM
- American Academy of Sleep Medicine
- ArI
- arousal index
- BDI
- Beck Depression Inventory
- BMI
- body mass index
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- EEG
- electroencephalography
- FSH
- follicle stimulating hormone
- HPO
- hypothalamic-pituitary-ovarian
- PSG
- polysomnographic
- REM
- rapid eye movement
- SE
- sleep efficiency
- STAI
- State-Trait Anxiety Inventory
- SWAN
- Study of Women Across the Nation
- TST
- total sleep time
- WASO
- wakefulness after sleep onset.
References
- 1. Santoro N. The menopausal transition. Am J Med. 2005;118:(Suppl. 12B):18–13. [DOI] [PubMed] [Google Scholar]
- 2. Joffe H, Petrillo LF, Koukopoulos A, et al. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011;96:E1044–E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kravitz HM, Joffe H. Sleep during the perimenopause: A SWAN story. Obstet Gynecol Clin North Am. 2011;38:567–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women's Health Study. Sleep. 2010;33:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polo-Kantola P, Erkkola R, Irjala K, Helenius H, Pullinen S, Polo O. Climacteric symptoms and sleep quality. Obstet Gynecol. 1999;94:219–224. [DOI] [PubMed] [Google Scholar]
- 6. Pien GW, Sammel MD, Freeman EW, Lin H, DeBlasis T. Predictors of sleep quality in women in the menopausal transition. Sleep. 2008;31:991–999. [PMC free article] [PubMed] [Google Scholar]
- 7. Woods N, Smith-Dijulio K, Percival D, Tao E, Taylor H, Mitchell E. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: Observations from the Seattle Midlife Women's Health Study. J Womens Health (Larchmt). 2007;16:667–677. [DOI] [PubMed] [Google Scholar]
- 8. Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007;110:230–240. [DOI] [PubMed] [Google Scholar]
- 9. Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 10. Hollander L, Freeman E, Sammel M, Berlin J, Grisso J, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98:391397. [DOI] [PubMed] [Google Scholar]
- 11. Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165:2370–2376. [DOI] [PubMed] [Google Scholar]
- 12. Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 13. Antonijevic I, Murck H, Frieboes R, Uhr M, Steiger A On the role of menopause for sleep-endocrine alterations associated with major depression. Psychoneuroendocrinology. 2003;28:401418. [DOI] [PubMed] [Google Scholar]
- 14. Driver HS, Werth E, Dijk D-J, Borbély AA. The menstrual cycle effects on sleep. Sleep Med Clin. 2008;3:1–11. [Google Scholar]
- 15. Baker FC, Sassoon SA, Kahan T, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mong J, Baker F, Mahoney M, et al. Sleep, rhythms, and the endocrine brain: Influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Zambotti M, Colrain I, Javitz H, Baker F. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102:1708–1715.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohayon M. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–1268. [DOI] [PubMed] [Google Scholar]
- 19. Casper R, Yen S, Wilkes M. Menopausal flushes: A neuroendocrine link with pulsatile luteninizing hormone secreation. Science (New York, NY). 1979;205:823–825. [DOI] [PubMed] [Google Scholar]
- 20. Randolph JF, Jr, Sowers M, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–6112. [DOI] [PubMed] [Google Scholar]
- 21. Terauchi M, Hiramitsu S, Akiyoshi M, et al. Associations between anxiety, depression and insomnia in peri- and post-menopausal women. Maturitas. 2012;72:61–65. [DOI] [PubMed] [Google Scholar]
- 22. Sassoon SA, de Zambotti M, Colrain IM, Baker FC. Association between personality traits and DSM-IV diagnosis of insomnia in peri- and postmenopausal. Menopause. 2014;21:602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zervas IM, Lambrinoudaki I, Spyropoulou AC, et al. Additive effect of depressed mood and vasomotor symptoms on postmenopausal insomnia. Menopause. 2009;16:837–842. [DOI] [PubMed] [Google Scholar]
- 24. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Climacteric. 2001;4:267–272. [PubMed] [Google Scholar]
- 25. First M, Spitzer R, Gibbon M, Williams J, Benjamin L. Structured clinical interview for DSM-IV axis I disorders (SCID) version 2.0. New York, NY: Biometrics Research Department, NY State Psychiatric Institute; 1998. [Google Scholar]
- 26. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 27. Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Anxiety Inventory: STAI (Form Y). Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 28. Beck A, Steer R, Brown G. Beck Depression Inventory-II (BDI-II). San Antonio, TX: Psychological Corporation; 1996 [Google Scholar]
- 29. Iber C, Ancoli-Israel S, Chesson A, Quan SF, for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, Ill.: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 30. Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6:209–215. [DOI] [PubMed] [Google Scholar]
- 31. Freedman R. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–579. [DOI] [PubMed] [Google Scholar]
- 32. Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–1464. [DOI] [PubMed] [Google Scholar]
- 33. Vallières A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14:447–453. [DOI] [PubMed] [Google Scholar]
- 34. Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. [DOI] [PubMed] [Google Scholar]
- 35. Joffe H, White DP, Crawford SL, et al. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: Results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause. 2013;20:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burger H, Hale G, Dennerstein L, Robertson D. Cycle and hormone changes during perimenopause: The key role of ovarian function. Menopause (New York, NY). 2008;15:603–612. [DOI] [PubMed] [Google Scholar]
- 37. Silveyra P, Cataldi NI, Lux-Lantos VA, Libertun C. Role of orexins in the hypothalamic-pituitary-ovarian relationships. Acta Physiol Oxf. 2010;198:355–360. [DOI] [PubMed] [Google Scholar]
- 38. Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab. 2005;90:2050–2055. [DOI] [PubMed] [Google Scholar]
- 39. Ryan J, Burger HG, Szoeke C, et al. A prospective study of the association between endogenous hormones and depressive symptoms in postmenopausal women. Menopause. 2009;16:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muti P, Trevisan M, Micheli A, et al. Reliability of serum hormones in premenopausal and postmenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev. 1996;5:917–922. [PubMed] [Google Scholar]