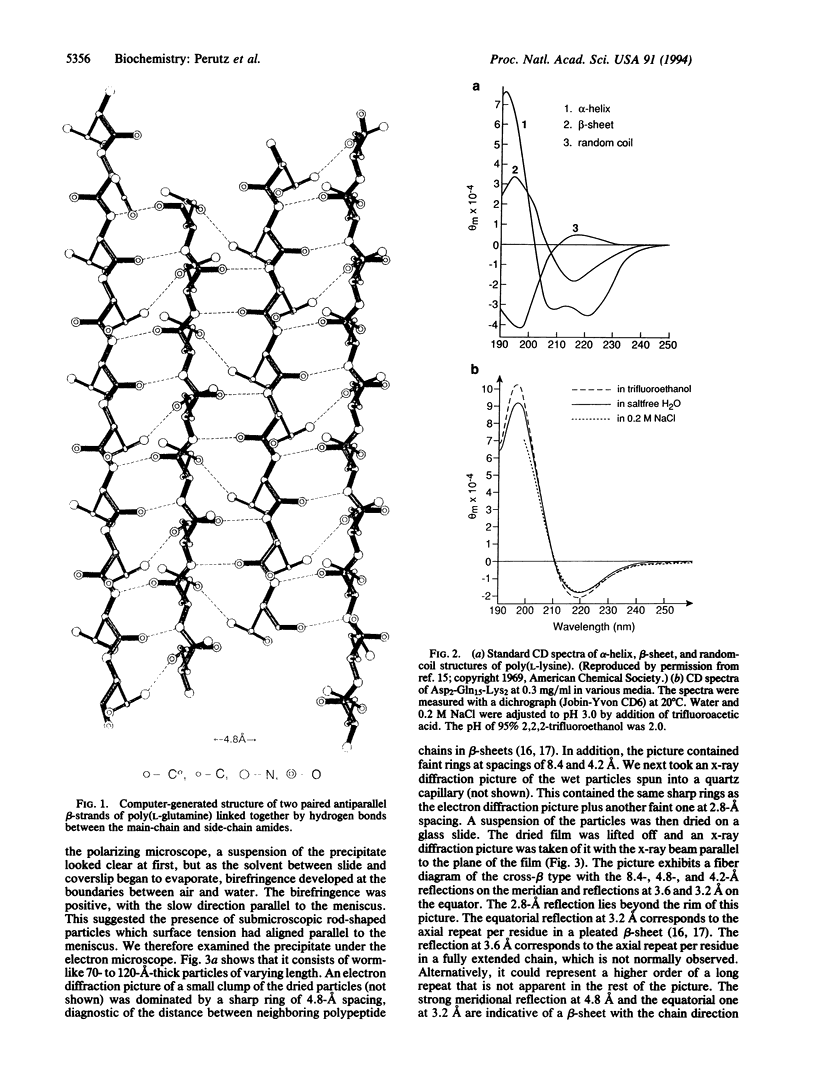
Abstract
Four inherited neurodegenerative diseases are linked to abnormally expanded repeats of glutamine residues in the affected proteins. Molecular modeling followed by optical, electron, and x-ray diffraction studies of a synthetic poly(L-glutamine) shows that it forms beta-sheets strongly held together by hydrogen bonds. Glutamine repeats may function as polar zippers, for example, by joining specific transcription factors bound to separate DNA segments. Their extension may cause disease either by increased, nonspecific affinity between such factors or by gradual precipitation of the affected proteins in neurons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali N., Bienz M. Functional dissection of Drosophila abdominal-B protein. Mech Dev. 1991 Aug;35(1):55–64. doi: 10.1016/0925-4773(91)90041-4. [DOI] [PubMed] [Google Scholar]
- Arnott S., Dover S. D., Elliott A. Structure of beta-poly-L-alanine: refined atomic co-ordinates for an anti-parallel beta-pleated sheet. J Mol Biol. 1967 Nov 28;30(1):201–208. doi: 10.1016/0022-2836(67)90252-5. [DOI] [PubMed] [Google Scholar]
- Ashida T., Tanaka I., Yamane T. Beta-pleated sheets in oligopeptide crystals. Int J Pept Protein Res. 1981 Mar;17(3):322–329. doi: 10.1111/j.1399-3011.1981.tb01998.x. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989 Dec 1;59(5):827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- Haynes S. R., Mozer B. A., Bhatia-Dey N., Dawid I. B. The Drosophila fsh locus, a maternal effect homeotic gene, encodes apparent membrane proteins. Dev Biol. 1989 Jul;134(1):246–257. doi: 10.1016/0012-1606(89)90094-8. [DOI] [PubMed] [Google Scholar]
- Hoogeveen A. T., Willemsen R., Meyer N., de Rooij K. E., Roos R. A., van Ommen G. J., Galjaard H. Characterization and localization of the Huntington disease gene product. Hum Mol Genet. 1993 Dec;2(12):2069–2073. doi: 10.1093/hmg/2.12.2069. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Kao C. C., Lieberman P. M., Schmidt M. C., Zhou Q., Pei R., Berk A. J. Cloning of a transcriptionally active human TATA binding factor. Science. 1990 Jun 29;248(4963):1646–1650. doi: 10.1126/science.2194289. [DOI] [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Roling D. B., Harding A. E., Warner C. L., Spiegel R., Hausmanowa-Petrusewicz I., Yee W. C., Fischbeck K. H. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992 Dec;2(4):301–304. doi: 10.1038/ng1292-301. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Martin J. B. Molecular genetics of neurological diseases. Science. 1993 Oct 29;262(5134):674–676. doi: 10.1126/science.8235586. [DOI] [PubMed] [Google Scholar]
- Matilla T., Volpini V., Genís D., Rosell J., Corral J., Dávalos A., Molins A., Estivill X. Presymptomatic analysis of spinocerebellar ataxia type 1 (SCA1) via the expansion of the SCA1 CAG-repeat in a large pedigree displaying anticipation and parental male bias. Hum Mol Genet. 1993 Dec;2(12):2123–2128. doi: 10.1093/hmg/2.12.2123. [DOI] [PubMed] [Google Scholar]
- Mhatre A. N., Trifiro M. A., Kaufman M., Kazemi-Esfarjani P., Figlewicz D., Rouleau G., Pinsky L. Reduced transcriptional regulatory competence of the androgen receptor in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1993 Oct;5(2):184–188. doi: 10.1038/ng1093-184. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Staden R., Moens L., De Baere I. Polar zippers. Curr Biol. 1993 May 1;3(5):249–253. doi: 10.1016/0960-9822(93)90174-m. [DOI] [PubMed] [Google Scholar]
- Ross C. A., McInnis M. G., Margolis R. L., Li S. H. Genes with triplet repeats: candidate mediators of neuropsychiatric disorders. Trends Neurosci. 1993 Jul;16(7):254–260. doi: 10.1016/0166-2236(93)90175-l. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Boyer T. G., Berk A. J. Factors (TAFs) required for activated transcription interact with TATA box-binding protein conserved core domain. Genes Dev. 1993 Feb;7(2):180–187. doi: 10.1101/gad.7.2.180. [DOI] [PubMed] [Google Scholar]