Abstract
Context:
Skeletal muscle from sedentary older adults exhibits reduced mitochondrial abundance and oxidative capacity.
Objective:
The primary objective was to determine whether 8 weeks of combined training (CT) has a more robust effect than endurance training (ET) or resistance training (RT) on mitochondrial physiology in healthy young (18–30 years) and older (≥65 years) adults.
Intervention:
Thirty-four young and 31 older adults were randomly assigned to 8 weeks of ET, RT, and control/CT. Control subjects completed 8 weeks of no exercise (control) followed by 8 weeks of CT. Body composition, skeletal muscle strength, and peak oxygen uptake were measured before and after the intervention. Vastus lateralis muscle biopsy samples were obtained before and 48 hours after the intervention. Mitochondrial physiology was evaluated by high-resolution respirometry and expression of mitochondrial proteins and transcription factors by quantitative PCR and immunoblotting.
Results:
ET and CT significantly increased oxidative capacity and expression of mitochondrial proteins and transcription factors. All training modalities improved body composition, cardiorespiratory fitness, and skeletal muscle strength. CT induced the most robust improvements in mitochondria-related outcomes and physical characteristics despite lower training volumes for the ET and RT components. Importantly, most of the adaptations to training occurred independent of age.
Conclusion:
Collectively, these results demonstrate that both ET and CT increase muscle mitochondrial abundance and capacity although CT induced the most robust improvements in the outcomes measured. In conclusion, CT provides a robust exercise regimen to improve muscle mitochondrial outcomes and physical characteristics independent of age.
Aging coupled with a sedentary lifestyle contributes to a progressive reduction in skeletal muscle health characterized by declines in skeletal muscle mass, strength, and quality (1, 2). Moreover, aged skeletal muscle from sedentary older adults also exhibits reduced mitochondrial abundance, oxidative enzyme activities, and oxidative capacity (OXPHOS) (3–8). Collectively, these age-related skeletal muscle deficits contribute to reductions in cardiorespiratory fitness (peak oxygen uptake [Vo2 peak]), exercise tolerance, and overall physical function (9, 10). Exercise represents an attractive therapeutic strategy to counteract age-related declines in these skeletal muscle deficits.
Both endurance training (ET) and resistance training (RT) are cornerstones of evidence-based exercise prescriptions designed to prevent, delay, or reverse the onset of age-related skeletal muscle deficits. It is widely accepted that mitochondrial abundance, oxidative enzyme activities, and OXPHOS increase in skeletal muscles from rodents to humans in response to exercise (11). However, the degree to which skeletal muscle mitochondria adapt to exercise probably depends on the mode of exercise (eg, ET vs RT). Moreover, the effect that aging has on exercise-induced mitochondrial adaptations remains incompletely defined. Chronic rigorous ET has been shown to nearly normalize mitochondrial protein expression as well as mitochondrial ATP production rates in healthy older adults to levels comparable to those seen in sedentary young adults (4). Short-term RT has also been reported to increase mitochondrial OXPHOS in previously sedentary older adults (12), although this could be considered counterintuitive and RT requires additional exploration alone and in combination with ET. Recent data demonstrate that ET, RT, and combined training (CT) improve mitochondrial abundance and OXPHOS in middle-aged adults with type 2 diabetes (13). To the best of our knowledge, the relative effects of ET, RT, and CT on mitochondrial adaptations have not been comprehensively examined in a single study targeting both young and older adults. Previous studies showed that RT in frail older people improves their ability to perform endurance activities (14). Here, we hypothesized that CT would enhance OXPHOS in skeletal muscle and directly compared the effects of CT, ET, and RT in young and older adults. We hypothesized that both ET and CT would increase mitochondrial OXPHOS, but to a greater extent for CT than for ET. We also hypothesized that the training-induced improvements in mitochondrial OXPHOS would be attenuated in the older subjects compared with those in the younger subjects.
Subjects and Methods
Subjects
Thirty-four young (18–30 years) and 31 older (≥65 years) sedentary (ie, exercising <2 days/wk) adults participated after providing written informed consent approved by the Mayo Clinic Institutional Review Board. Subjects completed an initial screening visit that included a medical history, physical examination, resting electrocardiogram, and biochemical tests of renal, hepatic, hematologic, and metabolic function. Exclusion criteria included evidence of diabetes, cardiovascular disease, and untreated thyroid dysfunction, history of alcohol abuse, substance abuse, smoking, or use of β-blockers or insulin sensitizers.
Study design
Subjects were randomly assigned to 1 of the 3 treatment arms: ET, RT, or control/CT. We previously reported detailed descriptions of the exercise training programs (15). In brief, the ET subjects performed cycling at ∼65% Vo2 peak for 1 hour, 5 days/wk for 8 weeks. The RT subjects performed 4 sets of 8 to 10 repetitions targeting multiple muscle groups 4 days/wk for 8 weeks. The control subjects performed no exercise training for 8 weeks (control), followed by crossover to a second condition, which was 8 weeks of combined ET and RT. The CT program consisted of cycling at 65% Vo2 peak for 30 minutes 5 days/wk and roughly two thirds the RT volume 4 days/wk. An exercise specialist supervised all exercise sessions. The training volume in all 3 training groups progressively increased over the first 3 weeks. The times spent exercising in the ET, RT, and CT groups were all 60 minutes or less (∼60 minutes × 5 days/wk [ET], ∼30 minutes × 5 days/wk [RT], and ∼50 minutes × 4 days/wk plus ∼60 minutes 1 day/wk [CT]. To document changes in Vo2 peak, strength, and skeletal muscle adaptations, subjects completed 1 pretraining outpatient visit and 1 pretraining inpatient visit, which were repeated in identical fashion after the 8-week training program. Participants who underwent the CT intervention after the control period were studied a third time. Each pretraining inpatient visit was scheduled more than 7 days after the baseline Vo2 peak assessment, whereas the posttraining inpatient visit was scheduled 48 hours after the last exercise bout. The Supplemental Methods provide a detailed description of the pertinent methodologies. In brief, the primary outcome measures were muscle mitochondrial OXPHOS (state 3 respiration) measured by high-resolution respirometry using a standard substrate inhibitor titration protocol in isolated mitochondria described previously (16). Secondary outcomes included abundance of mRNA encoding mitochondrial proteins and transcription factors measured by quantitative real-time PCR (qPCR) (17, 18) and protein abundance of mitochondrial proteins and transcription factors using standard immunoblotting (19). We also measured citrate synthase activity spectrophotometrically as described previously (18). We used dual-energy x-ray absorptiometry (20) and 1-cut computed tomography of the midthigh (21) to measure changes in body composition. Finally, we measured cardiorespiratory fitness (Vo2 peak) and skeletal muscle strength using an incremental exercise test with indirect calorimetry and a 1 − repetition maximum (1 − RM) test, respectively.
Statistical analysis
We conducted all statistical analyses using SAS Enterprise Software (version 5.1; SAS Institute). Data are expressed as means ± SEM. We used ANOVA to test for baseline differences between treatment and age groups. We used paired t tests to test for preintervention to postintervention percent changes (%Δ) within each treatment group. We used mixed-effects ANOVA models to test for mean differences between groups for the preintervention to postintervention %Δ scores. The model specification included parameters to estimate the exercise main effect (control, ET, RT, and CT), the age main effect (young vs old), and their interaction on the %Δ in the dependent variables. The models also included a random effect that represented the between- and within-subject error terms. We used post hoc 2-sample t tests to examine mean differences in the preintervention to postintervention %Δ between the treatment groups, with the exception of the comparison between the control and CT groups, for which we used paired t tests. The P values reported for the a priori pairwise comparisons between the exercise treatments and control (ie, ET vs control, RT vs control, and CT vs control) are unadjusted P values and the pairwise comparisons between exercise treatments (eg, ET vs RT, ET vs CT, and RT vs CT) are the Hochberg-adjusted P values. The overall type I error (α) = .05% using two-sided tests.
Results
Baseline demographics and physical characteristics (Table 1)
Table 1.
Baseline Demographics and Physical Characteristics Stratified by Age Group
| Control/CT |
ET |
RT |
ANOVA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Young (n = 12) | Older (n = 10) | Young (n = 11) | Older (n = 11) | Young (n = 11) | Older (n = 10) | Treatment | Age | Treatment × Age | |
| n | 12 (6 M/6 F) | 10 (5 M/5 F) | 11 (6 M/5 F) | 11 (5 M/6 F) | 11 (6 M/5 F) | 10 (5 M/5 F)) | |||
| Age, y | 26 (1) | 71 (2) | 25 (1) | 70 (1) | 25 (1) | 70 (1) | 0.59 | <0.0001 | 0.98 |
| Height, cm | 172 (2) | 167 (4) | 169 (2) | 169 (3) | 170 (2) | 169 (2) | 0.95 | 0.36 | 0.64 |
| Weight, kg | 81 (4) | 80 (5) | 69 (3) | 81 (4) | 72 (3) | 75 (5) | 0.34 | 0.08 | 0.32 |
| Body mass index, kg/m2 | 27 (1) | 28 (1) | 24 (1) | 28 (1) | 25 (1) | 27 (1) | 0.42 | 0.016 | 0.31 |
| Fat mass, % | 36 (2) | 40 (2) | 31 (2) | 39 (1) | 34 (3) | 36 (2) | 0.30 | 0.008 | 0.41 |
| Fat mass, kg | 28 (2) | 30 (2) | 20 (2) | 30 (2) | 23 (2) | 28 (3) | 0.23 | 0.009 | 0.36 |
| Lean mass, kg | 49 (3) | 45 (3) | 44 (2) | 46 (3) | 45 (2) | 47 (2) | 0.84 | 0.97 | 0.48 |
| Midthigh skeletal muscle, cm2a | 259 (26) | 230 (21)1 | 257 (16) | 251 (18) | 259 (15) | 242 (15) | 0.88 | 0.28 | 0.83 |
| Vo2 peak, mL/kg/min | 27 (2) | 16 (1) | 30 (1) | 18 (1) | 27 (2) | 19 (10) | 0.29 | <0.001 | 0.56 |
| Chest press 1 − RM, kg | 54 (8)2 | 30 (4) | 44 (5) | 34 (4) | 43 (6) | 36 (4) | 0.85 | 0.003 | 0.29 |
| Leg press 1 − RM, kg | 141 (17)3 | 88 (14) | 114 (11) | 81 (6) | 109 (14)2 | 88 (13) | 0.35 | 0.001 | 0.48 |
| Normalized leg press 1 − RM, kg/cm2 | 0.66 (0.19)3 | 0.33 (0.03)1 | 0.45 (0.04) | 0.33 (0.02) | 0.41 (0.05)2 | 0.35 (0.04) | 0.38 | 0.023 | 0.29 |
Abbreviations: F, female; M, male; Age groups: young, n = 18 females and 16 males; older, n = 16 females and 15 males. Data are presented as means (SEM).
Sum of right and left midthigh skeletal muscle cross sectional area; 1 n = 9; 2 n = 10; 3 n = 11.
At baseline there were no significant differences between the treatment groups for any of the demographic or physical characteristics (all P > .05). In contrast, the older subjects had a higher body mass index (P = .016), percent body fat (P = .008), and total fat mass (P = .009) than the younger subjects. The older subjects also exhibited lower Vo2 peak (P < .001), chest press 1 − RM (P = .003), leg press 1 − RM (P = .001), and leg press 1 − RM normalized to the total midthigh skeletal muscle cross-sectional area (P = .023). At baseline, there were also no significant differences in total physical activity assessed by accelerometry between the young and older subjects (273 ± 25 wear-time cpm [n = 23 young] vs 234 ± 14 wear-time cpm [n = 25 old], P > .05).
Training-induced changes in physical characteristics (Table 2)
Table 2.
Preintervention to Postintervention Percent Change (%Δ) in Body Composition, Cardiorespiratory Fitness, and Skeletal Muscle Strength
| Control |
ET |
RT |
CT |
ANOVA |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Young (n = 12) | Older (n = 10) | Young (n = 11) | Older (n = 11) | Young (n = 11) | Older (n = 10) | Young (n = 12) | Older (n = 9) | Treatment | Age | Treatment × Age | |
| Fat mass, % | 1.7 (1.2) | −0.1 (0.5) | −4.2 (1.9) | −1.3 (1.9) | −0.2 (1.5) | −.9 (.9) | −4.1 (.9)b | −1.3 (.8) | 0.006 | 0.35 | .11 |
| 0.9 (0.7) | −2.8 (1.0)a,e | −0.5 (0.9) | −2.9 (0.7)b,e | Control > ET and CT | |||||||
| Fat mass, kg | 2.8 (1.7) | 0.1 (0.9) | −3.2 (2.3) | −1.9 (0.7)a | 1.7 (2.2) | −.5 (1.3) | −4.9 (1.1)c | −2.2 (1.5) | 0.005 | 0.85 | .25 |
| 1.6 (1.0) | −2.5 (1.2)a,d | 0.7 (1.3) | −3.7 (0.9)c,e,g | Control > ET and CT, RT > CT | |||||||
| Lean mass, kg | 0.2 (0.4) | 0.1 (0.5) | 2.7 (0.8)b,h | 0.3 (0.5)a | 2.2 (0.5)b,h | .7 (.5) | 1.2 (.7) | 1.5 (.7) | 0.07 | 0.027 | .08 |
| 0.2 (0.3) | 1.5 (0.5)b,d | 1.5 (0.4)b,e | 1.3 (0.5)a | Control < ET and RT | |||||||
| Midthigh skeletal muscle, cm2 | 1.7 (2.9) | −2.1 (1.7)1 | 6.3 (2.2)a,h | 4.0 (1.5)a | 8.7 (3.0)a,h | 5.6 (3.4) | 5.2 (1.7)a,3 | 5.1 (2.8) | 0.026 | 0.18 | 0.90 |
| 0.0 (1.7) | 5.2 (1.3)c,d | 7.2 (2.2)b,d | 5.1 (1.5)b | Control < ET and RT | |||||||
| Vo2 peak, mL/kg/min | 2.3 (3.3) | 3.3 (3.6) | 9.5 (2.7)b | 14.9 (6.2)a,2 | −6.5 (4.5)h | 11.0 (6.2) | 12.1 (8.0) | 23.3 (4.8)b | 0.010 | 0.022 | 0.43 |
| 2.8 (2.4) | 12.1 (3.3)b,d | 1.8 (4.2) | 16.9 (5.0)b,d | Control < ET and CT | |||||||
| Chest press, kg | 4.4 (3.3)2 | 4.2 (2.8) | 0.5 (2.1)2 | 0.0 (4.1)2 | 18.5 (3.8)b,1 | 14.7 (4.4)b | 13.4 (2.6)c,2 | 12.1 (4.2)a | <0.0001 | 0.56 | 0.96 |
| 4.3 (2.3) | 0.3 (2.3) | 16.5 (2.9)c,e,f | 12.8 (2.4)c,d,f | Control < RT and CT; ET < RT and CT | |||||||
| Leg press, kg | 3.7 (6.0)3 | −1.1 (5.6) | 11.6 (5.2)a,2 | 11.4 (4.8)b | 42.0 (9.5)b,1 | 4.4 (1.1)b | 22.0 (6.1)b,3 | 47.8 (7.8)c | <0.0001 | 0.34 | 0.12 |
| 1.4 (4.1) | 11.5 (4.1)b | 41.1 (6.8)c,e,f | 33.6 (5.5)c,e,f | Control < RT and CT; ET < RT and CT | |||||||
| Normalized leg press, kg/cm2 | 2.5 (6.4)3 | 2.1 (7.2)1 | 5.6 (4.7)2 | 7.0 (3.9) | 28.4 (7.3)b,1 | 32.9 (8.7)b | 16.7 (6.0)a,2 | 41.0 (7.3)c | <0.0001 | 0.11 | 0.22 |
| 2.3 (4.7) | 6.3 (3.0)a | 30.8 (5.6)c,e,f | 28.2 (5.4)c,e,f | Control < RT and CT; ET < RT and CT | |||||||
Data are presented as means (SEM). Data in italics are the means (SEM) for the young and older pooled together.
P < .05 (pre vs post);
P < .01 (pre vs post);
P < 0.001 (pre vs post);
P < .05 (treatment vs control);
P < .01 (treatment vs control);
P < .05 (treatment vs ET);
P < .05 (treatment vs RT). 1 n = 9; 2 n = 10; 3 n = 11.
Both ET and CT reduced percent body fat (P = .015 and P = .001, respectively) and absolute fat mass (P = .044 and P = .001, respectively). The changes in these variables were significantly greater than those for the control (all P < .05) and were independent of age (all P > .05). All 3 exercise conditions increased lean body mass and midthigh skeletal muscle cross-sectional area (all P < .05). The increases in lean body mass in the ET and RT groups were also significantly greater than those in the control group (all P < .05). The ET- and RT-induced increases in lean body mass were significantly greater in the young than in the older subjects (P = .018 and P = .041, respectively). Both ET and CT increased Vo2 peak (P = .001 and P = .0003, respectively). Both RT and CT increased chest press 1 − RM (P < .0001), whereas all 3 exercise conditions increased leg press 1 − RM (P < .0001). The RT- and CT-induced increases in chest press and leg press 1 − RM were greater than those in the control and ET groups (all P < .05). Both RT and CT increased leg press 1 − RM normalized to total skeletal muscle cross-sectional area (P < .0001), which was also significantly greater than the changes in the control and ET groups (P < .05).
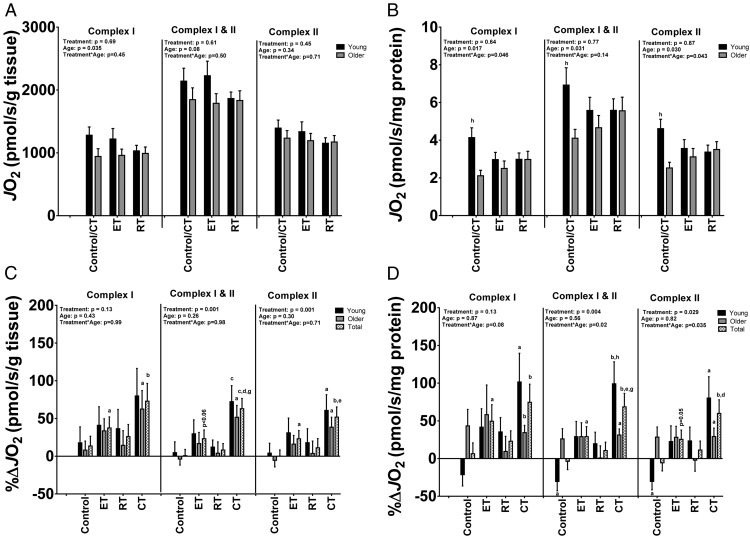
Baseline mitochondrial oxidative capacity (Figure 1)
Figure 1.
Skeletal muscle mitochondrial respiratory capacity was measured with high-resolution respirometry using substrates targeting complex I, complex I+II, and complex II. A and B, baseline state 3 oxygen flux rates (JO2) expressed per gram of tissue or per milligram mitochondrial protein, respectively. C and D, preintervention to postintervention percent change (%Δ) in state 3 flux JO2 in response to 8 weeks of control, ET, RT, or CT, expressed per gram of tissue or per milligram mitochondrial protein, respectively. Data are expressed as means ±SEM. At baseline, total n = 12 young/9 old (control/CT), n = 10 young/11 old (ET), and n = 11 young/10 old (RT) for complex I; n = 10 young/9 old (control/CT), n = 11 young/11 old (ET), and n = 11 young/10 old (RT) for complex I+II; and n = 11 young/8 old (control/CT), n = 10 young/11 old (ET), and n = 11 young/10 old (RT) for complex II-driven respiration. For the preintervention to postintervention %Δ, n = 12 young/9 old (control/CT), n = 10 young/9 old (ET), n = 11 young/10 old (RT), and n = 12 young/8 old (CT) for complex I; n = 10 young/9 old (control/CT), n = 11 young/11 old (ET), n = 11 young/10 old (RT), and n = 11 young/9 old (CT) for complex I+II; and n = 11 young/8 old (control/CT), n = 10 young/11 old (ET), n = 11 young/10 old (RT), and n = 12 young/8 old (CT) for complex II-driven respiration. Baseline data were analyzed by ANOVA. Paired t tests were used to test for preintervention to postintervention percent changes (%Δ) within each treatment group. ANOVA models were used to test for mean differences between groups for the preintervention to postintervention %Δ scores. a, P < .05 (pre vs post); b, P < .01 (pre vs post); c, P < .001 (pre vs post); d, P < .05 (treatment vs control); e, P < .01 (treatment vs control); f, P < .05 (treatment vs ET); g, P < .05 (treatment vs RT); h, P < .05 (young vs old).
Figure 1 shows mitochondrial OXPHOS (state 3 respiration) for complex I (glutamate + malate), complex I+II (glutamate + malate + succinate), and complex II (glutamate + malate + succinate + rotenone) in isolated mitochondria from the young and older subjects by treatment group at baseline as well as the %Δ from baseline. We expressed OXPHOS measurements per gram of muscle tissue wet weight (OXPHOSTissue) and per milligram of protein in the mitochondrial suspension (OXPHOSProtein). At baseline there were no significant differences among the treatment groups for any of the OXPHOSTissue or OXPHOSProtein measurements (all P > .05). The older subjects had a lower complex I OXHPHOSTissue and complex I OXPHOSProtein than the young (P = .035 and P = .017, respectively). Complex I+II and complex II OXPHOSProtein were also lower in the older subjects than in the young (P = .031 and P = .030, respectively). Finally, baseline citrate synthase activity was not statistically different between the young and the older subjects (8.3 ± 0.3 μmol/min/g tissue vs 8.1 + 0.3 μmol/min/g tissue).
Training-induced changes in mitochondrial oxidative capacity (Figure 1)
Overall, ET increased complex I, complex I+II, and complex II OXPHOSTisssue (P = .020, P = .059, and P = .048, respectively). CT increased complex I, complex I+II, and complex II OXPHOSTisssue (P = .006, P = .0002, and P = .001, respectively). The CT-induced increases in OXPHOSTissue were also greater than the increases in the control group for complex I+II and complex II (all P < .05) and greater than those in the RT groups for complex I+II (P < .05). ET increased complex I, complex I+II, and complex II OXPHOSProtein (P = .038, P = .041, and P = .050, respectively). CT increased complex I, complex I+II, and complex II OXPHOSProtein (P = .006, P = .001, and P = .004, respectively). The CT-induced increases in OXPHOSProtein were also greater than the increases in the control group for complex I+II and complex II (all P < .05). Moreover, the CT-induced increase in OXPHOSProtein for complex I+II was also greater than the increases for RT. Interestingly, the CT-induced increase in OXPHOSProtein was greater in the young than in the older subjects (P < .05) for complex I+II. In support of these findings from mitochondrial respirometry, CT increased citrate synthase activity (1.3 ± 0.6 μmol/min/g tissue, P = .05).
Baseline mRNA abundance
There were no significant differences in mRNA abundance between the old and young for pgc1α total (0.69 ± 0.06 arbitrary units [au] vs 0.64 ± 0.04 au [pgc1α/28s]), pgc1α1 (0.77 ± 0.09 au vs 0.98 ± 0.10 au [pgc1α1/28s]), or pgc1α4 (0.79 ± 0.09 au vs 0.81 ± 0.09 au [pgc1α4/28s]) (all P > .05). Because of the technical issues encountered in the analyses, we were unable to analyze baseline differences between the other gene transcripts.
Training-induced changes in mRNA abundance (Table 3)
Table 3.
Preintervention to Postintervention Percent Change (%Δ) in mRNA Abundance Measured by qPCR
| Control |
Endurance (ET) |
Resistance (RT) |
Combined (CT) |
ANOVA |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Young (n = 12) | Old (n = 10) | Young (n = 11) | Old (n = 11) | Young (n = 11) | Old (n = 9) | Young (n = 12) | Old (n = 9) | Treatment | Age | Treatment × Age | |
| pgc1α | −1 (6) | 13 (14) | 44 (17)a,2 | 15 (16) | 80 (62) | 36 (9)b | 25 (10)a | 58 (29) | 0.28 | 0.74 | 0.47 |
| 5 (7) | 29 (12)a | 60 (34) | 39 (14)a | ||||||||
| pgc1α1 | −17 (6)a | 8 (12) | 60 (17)b,2 | 15 (13) | 57 (45) | 13 (9) | 74 (31)a,3 | 74 (37) | 0.032 | 0.38 | 0.43 |
| −5 (7) | 36 (11)b,e | 38 (25) | 74 (23)b | Control < ET | |||||||
| pgc1α4 | −10 (15) | 13 (19) | 36 (19) | 12 (23) | 9 (17) | 17 (19) | 72 (23)b | 102 (61) | 0.007 | 0.61 | 0.73 |
| 0 (12) | 24 (15) | 13 (12) | 85 (28)b,d | Control < CT | |||||||
| tfam | −9 (5) | 10 (11) | 21 (11) | 6 (10) | 19 (18) | 1 (18) | 28 (13) | 28 (13) | 0.21 | 0.69 | 0.47 |
| 0 (6) | 13 (8) | 10 (13) | 28 (10)a | − | |||||||
| nrf1 | −6 (2)a | −1 (10) | −10 (6) | −7 (5) | 1 (13) | −17 (6)a | −4 (5) | 9 (8) | 0.46 | 0.87 | 0.24 |
| −3 (5) | −8 (4)a | −7 (16) | 1 (5) | − | |||||||
| nrf2 | 4 (9) | 16 (8) | 60 (14)b | 15 (14) | 30 (27) | 22 (15) | 12 (7) | 16 (11) | 0.23 | 0.39 | 0.22 |
| 10 (6) | 38 (11)b,d | 26 (16) | 14 (6)a | − | |||||||
| sirt1 | −1 (3) | −4 (5) | 0 (5) | −11 (6) | 3 (8) | −12 (6) | 2 (5) | 7 (4) | 0.25 | 0.15 | 0.28 |
| −2 (3) | −6 (4) | −4 (5) | 4 (3) | − | |||||||
| sirt3 | −5 (2) | −2 (6) | 24 (9)a | 11 (9) | 11 (10) | 11 (5)a | 24 (6)b | 37 (10)b | 0.004 | 0.89 | 0.36 |
| −4 (3) | 17 (6)a,e | 11 (6)d | 30 (6)c,e | Control < ET, RT, and CT | |||||||
| sdhb | 1 (7) | 0 (8) | 46 (11)b | 20 (11) | 26 (16) | 23 (18) | 38 (14)a | 61 (18)a | 0.004 | 0.89 | 0.34 |
| 1 (5) | 33 (8)b,e | 25 (12)a | 48 (11)c,e | Control < ET and CT | |||||||
| atpsf1 | 0 (6) | 7 (7) | 49 (7)c | 28 (10)a | 28 (19) | 25 (16) | 28 (8)b | 38 (14)a | 0.02 | 0.87 | 0.53 |
| 3 (5) | 39 (7)c,e | 26 (13)a | 32 (7)c,d | Control < ET and CT | |||||||
| cytobc | −3 (4) | 0 (5) | 24 (9)a | 16 (6)a | 12 (9) | 8 (5) | 28 (9)b | 23 (7)a | 0.002 | 0.46 | 0.89 |
| −2 (3) | 20 (5)b,e | 10 (5) | 26 (6)c,e | Control < ET and CT | |||||||
| cox3 | −14 (6) | 0 (10) | 47 (16)a | 38 (13)a | 50 (39) | 38 (18) | 65 (18)b | 84 (24)b | 0.002 | 0.83 | 0.84 |
| −8 (6) | 42 (70)c,e | 45 (22)d | 73 (14)c,e | Control < ET, RT, and CT | |||||||
| cox4 | −2 (6) | 2 (8) | 57 (13)b | 30 (13)a | 26 (20) | 29 (12)a | 57 (19)a | 66 (21)a | 0.0009 | 0.78 | 0.61 |
| 0 (5) | 43 (9)c,e | 27 (12)a,d | 61 (14)c, e | Control < ET, RT, and CT | |||||||
| nd4 | −10 (6) | 11 (16) | 41 (12)b | 25 (14) | 37 (26) | 24 (20) | 44 (11)b | 61 (20)a | 0.019 | 0.86 | 0.53 |
| 0 (8) | 33 (9)b,e | 31 (17) | 51 (10)c,e | Control < ET and CT | |||||||
| myolc | −7 (8) | −7 (11) | 15 (9) | 161 (139) | 14 (9) | 54 (53) | 51 (25) | 96 (54) | 0.34 | 0.16 | 0.61 |
| −7 (6) | 88 (70) | 32 (24) | 70 (27)a | ||||||||
| mhcI | −11 (11) | 6 (16) | 5 (10) | 26 (16) | 33 (21) | 4 (21) | 85 (57) | 60 (33) | 0.06 | 0.84 | 0.73 |
| −3 (9) | 15 (10) | 20 (15) | 74 (35) | ||||||||
| mhcIIa | 4 (13) | −15 (10) | 22 (14) | −6 (15) | 51 (33) | 3 (16) | 13 (11) | 40 (19) | 0.10 | 0.87 | 0.89 |
| −5 (8) | 8 (11) | 29 (20) | 25 (10)a | ||||||||
| mhcIIx | −9 (14) | 0 (15) | −31 (13)a | −34 (14)a | −38 (14)a | −52 (7)c | −30 (21) | −29 (17) | 0.06 | 0.87 | 0.89 |
| −5 (10) | −33 (9)b | −44 (8)c | −29 (14)a | ||||||||
Data are presented as mean (sem). Data in italics are the mean (sem) for the young and old pooled together.
P < .05 (pre vs post);
P < .01 (pre vs post);
P < 0.001 (pre vs post);
P < .05 (treatment vs control);
P < .01 (treatment vs control). 2 n = 10; 3 n = 11.
ET and CT increased the mRNA abundance >75% (11 of 14 and 12 of 14, respectively) of the gene transcripts encoding mitochondrial transcription factors and proteins (all P < .05). Compared with the control, ET and CT also increased approximately 60% (9 of 14 and 8 of 14, respectively) of the gene transcripts encoding mitochondrial transcription factors and proteins (all P < .05). RT increased the mRNA abundance of approximately 20% (3 of 14) of the gene transcripts encoding mitochondrial transcription factors and proteins (all P < .05). Compared with the control, RT also increased approximately 20% (3 of 14) of the gene transcripts encoding mitochondrial transcription factors and proteins (all P < .05). CT also increased mRNA abundance of genes encoding contractile (myolc, mhcI, and mhcIIa) proteins (P < .05). All 3 exercise training conditions reduced the mRNA abundance of the gene encoding mhcIIx (P < .05). There was no significant effect of age on the mRNA response to exercise (P > .05).
Baseline protein abundance
There were no significant differences in protein abundance for peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) (1.7 ± 0.1 au vs 1.6 ± 0.1 au), sirtuin 3 (SIRT3) (1.2 ± 0.1 au vs 1.2 ± 0.1 au), transcription factor A, mitochondrial (TFAM) (1.6 ± 0.3 au vs 1.2 ± 0.1 au), or vinculin (1.0 ± 0.1 au vs 1.0 ± 0.1 au) between the young and older subjects (all P > .05, n = 30 per group) at baseline. There were also no significant differences in protein abundance for the total OXPHOS electron transport chain (ETC) proteins (0.8 ± 0.05 au vs 0.8 ± 0.04 au) between young and older subjects (P > .05, n = 30 young and 29 older) at baseline. There were random differences in the protein expression of PGC1α and total OXPHOS ETC proteins between the treatment groups at baseline. Specifically, the control group had higher PGC1α protein abundance (2.1 ± 0.2 au) than either the ET (1.6 ± 0.1 au) or the RT (1.3 ± 0.2 au) group (P < .05) at baseline. The ET group had higher total OXPHOS protein abundance (0.9 ± 0.1 au) than either the control (0.7 ± 0.1 au) or the RT (0.8 ± 0.1 au) groups (P < .05) at baseline. There were no differences between treatment groups for protein abundance for SIRT3, TFAM, or vinculin at baseline (data not shown).
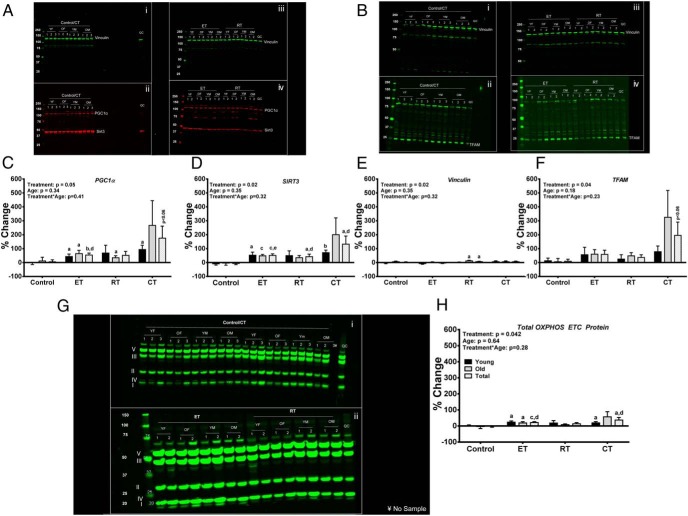
Training-induced changes in protein abundance (Figure 2)
Figure 2.
Panels A and B present representative blots for PGC1α, SIRT3, Vinculin, and TFAM. Blots Ai and Aii are the same blot incubated in antibodies for Vinculin (Ai), PGC1α (Aii), and SIRT3 (Aii) for the control/combined (CT) group. Blots Aiii and Aiv are the same blot incubated in antibodies for Vinculin (Aiii), PGC1α (Aiv), and SIRT3 (Aiv) for the endurance training (ET) and resistance training (RT) groups. Blots Bi and Bii are the same blot incubated in antibodies for Vinculin (Bi) and TFAM (Bii) for the control/CT group. Blots Biii and Biv are the same blot incubated in antibodies for Vinculin (Biii) and TFAM (Biv) for the ET and RT groups. Panels C–F present the densitometry for PGC1α, SIRT3, Vinculin, and TFAM, respectively. The densitometry for Vinculin was performed on the PGC1α/SIRT3 blots (Panel A). Panel G presents representative blots for the Mitosciences Total OXPHOS Electron Transport Chain (ETC) protein abundance for the control/CT (Gi) and ET (Gii) and RT (Gii) groups.Panel H presents the integrated intensity of bands detected by the Mitosciences Total OXPHOS ETC protein antibody cocktail ranging from ∼10 kDa through ∼60 kDa was quantified and used to represent the abundance for the OXPHOS ETC proteins. One older ET participant's sample was excluded from the total OXPHOS ETC analysis due to a smear in the bands. Data are expressed as mean percentage change ± SEM. Total n = 60; n = 10 young, 10 old in each intervention group, 50/50 male/female. Data were analyzed as described in Figure 1. A, P < .05 (pre vs post); b, P < .01 (pre vs post); c, P < 0.001 (pre vs post); d, P < 0.05 (treatment vs control). YF, Young Female; OF, Old Female; YM, Young Male; and YO, Old Male.
ET increased PGC1α and SIRT3 protein abundance (P = .001 and P = .0004, respectively), which was greater than the increase in the control group (P < .05). RT increased SIRT3 protein abundance (P = .033), which was also greater than the increase in the control group (P < .05). CT increased SIRT3 protein abundance (P = .036), which was greater than the increase in the control group (P < .05). CT showed a trend to increase PGC1α and TFAM protein abundance (P < .06). ET and CT both increased the total OXPHOS ETC protein abundance (P < .001, and P = .030, respectively). Both the ET- and CT-induced increases in the total OXPHOS ETC protein abundance were greater than the increase in the control group (P = .014 and P = .035, respectively).
Discussion
The present study demonstrates that both ET and CT significantly increased (1) OXPHOS measured by high-resolution respirometry and (2) mRNA and protein abundance of mitochondrial transcription factors and mitochondrial proteins measured by qPCR and immunoblotting. Importantly, these exercise-induced improvements were largely independent of age. Finally, CT resulted in the most robust improvements in most of the mitochondrial-related outcomes, supporting the current exercise recommendations to routinely incorporate both ET and RT to maximize the salutary effects of exercise on muscle health.
The results indicate that CT improved not only whole-muscle oxidative capacity but also the intrinsic function of mitochondria as OXPHOS expressed per tissue wet weight and protein content. We hypothesized that training-induced improvements would be attenuated in the older subjects compared with those in the younger subjects. However, ET increased OXPHOS independent of age, consistent with prior observations that ET increases skeletal muscle mitochondrial bioenergetic function across the lifespan (4, 12, 22, 23). Moreover, improvements in OXPHOS in response to CT were also independent of age. In contrast to prior studies in healthy young (24) and older adults (12, 25), RT did not significantly affect OXPHOS in the present investigation regardless of age. Differences in the OXPHOS response to RT between the present study and prior studies may be due to differences in training volume, intensity, duration, and types of exercises. The present results also demonstrated that in nondiabetic young and older adults, 8 weeks of CT provides the most robust improvements in OXPHOS and thus supports results in type 2 diabetic individuals undergoing 9 months of CT in comparison with ET and RT training alone (13).
Both mRNA and protein abundance of PGC1α generally increased in response to ET and CT, showing similar directions of change in both age groups but reaching statistical significance specifically in the young; this finding supports the notion that increased OXPHOS in response to ET and CT is mediated in part by PGC1α, which is a master regulator of mitochondrial biogenesis (26). It is similarly of interest that even RT, a modality not traditionally associated with mitochondrial adaptation, affected PGC1α expression, and this finding demonstrates that the molecular distinction between different exercise modalities can become blurred to some extent when real-world exercise interventions are studied. Moreover, the observation of the increase in the mRNA abundance for pgc1α1, which is an isoform of PGC1α that promotes an oxidative phenotype in skeletal muscle (15), further supports this PCG-1–related mechanism for muscular adaptation to exercise training. These results, particularly those in the young participants, build upon the original report from this cohort showing that both ET and CT increased pgc1α1 expression with the CT effect being greater than the ET effect (15). The CT-induced increase in pgc1α4 is also consistent with its ability to stimulate skeletal muscle hypertrophy (15). However, both ET and RT produced nonstatistically significant increases in pgc1α4 (∼24% and ∼13%, respectively) despite significantly increasing skeletal muscle cross-sectional area. We expected that pgc1α4 would have increased after RT based on its putative role in regulating muscle hypertrophy (15). There are numerous potential nuances in the manner in which pgc1α4 responds to training. For example, sex appears to affect exercise-induced expression of pgc1α4. Indeed, recent data demonstrate that acute bouts of both ET and RT increase the expression of pgc1α4 in men (26). Consistently, in the male participants in the current study, pgc1α4 increased in response to ET (45 ± 2%, P = .04), RT (39 ± 2%, P = .06), and CT (59 ± 3%, P = .03). This observation may partly explain the greater skeletal mass in men, and the interaction between pgc1α4 expression and sex hormones remains unknown. It is possible that pgc1α4 only plays a permissive role in skeletal muscle hypertrophy in response to exercise, which is widely recognized to be under the regulation of the mammalian target of rapamycin (27, 28). We also suspect that the lack of a statistically significant increase in pgc1α4 after RT as well as ET may also reflect in part a type II error. Consistent with the CT-induced increase in pgc1α4, we also observed a concomitant increase in the mRNA abundance of Metrnl in a subanalysis (Supplemental Figure 1). These training-induced elevations are also consistent with the acute increase in Metrnl expression after a single bout of combined endurance and resistance exercise (29). In mice, elevated expression and secretion of Metrnl has been associated with increased thermogenesis and glucose homeostasis (29).
We also assessed the impact that the 3 exercise training conditions had on key mitochondrial transcription factors such as TFAM. All exercise conditions induced increases in mRNA and protein abundance of some of the mitochondria-related transcription factors. Of interest, CT resulted in the most consistent and statistically significant increases in the mRNA and protein abundance of these transcription factors. Overall, CT showed a trend to increase the mRNA and protein abundance of TFAM. However, there was no statistically significant treatment, age, or treatment × age interaction for the preintervention to postintervention change in the mRNA abundance of tfam. In contrast, there was a significant treatment effect on the preintervention to postintervention change in TFAM protein abundance. We have previously reported that chronic rigorous ET (4) as well as 8 weeks of ET in this cohort increases SIRT3 expression (30); to our knowledge this is the first report that SIRT3 expression is increased in response to RT or CT. SIRT3 recently has been reported to play an important role in regulating metabolic flexibility of muscle (31) as well as regulating muscle mass and OXPHOS (32). Thus, SIRT3 may be an important mediator of muscle quality in response to exercise. We recognize that other molecular targets including AMP-activated protein kinase (AMPK), p38 MAPK, calcium/calmodulin-dependent protein kinase II (CAMKII), myocyte enhancer factor 2 (MEF2), and activating transcription factor (ATF) also play important roles in regulating exercise-induced mitochondrial biogenesis and skeletal muscle adaptations, promoting a more oxidative phenotype (33). However, these important regulatory enzymes/transcription factors are rapidly turned on and off by phosphorylation and dephosphorylation. As a result, the signal from these molecules is expected to be low 48 hours after the last exercise session.
Another important finding of the current study is that both ET and CT consistently increased mitochondrial abundance, evident from elevations in the mRNA abundance of gene transcripts encoding mitochondrial proteins measured by qPCR and protein abundance of total OXPHOS ETC proteins measured by immunoblotting. In agreement with the OXPHOS data, CT also resulted in the most consistent statistically significant increases in markers of mitochondrial abundance. These findings support results in type 2 diabetic individuals receiving 9 months of CT in comparison with ET and RT training alone (13). Previous studies have also reported that ET increases mitochondrial abundance using electron microscopy-, cardiolipin-, and proteomic-based analyses (4, 22, 34, 35).
Of interest, all 3 training modalities significantly increased fat free mass and midthigh cross-sectional area. These results are consistent with those of previous publications, demonstrating the beneficial effects of ET, RT, and CT on body composition, skeletal muscle strength, and cardiorespiratory fitness (36–38). It may seem counterintuitive for ET to increase lean body mass and midthigh cross-sectional area; however, in deconditioned subjects, cycling may provide sufficient stimulus to enhance skeletal muscle hypotrophy. A longer duration of training (eg, 6 months) may have allowed for greater divergence in the exercise training–induced elevations in lean body mass and skeletal muscle cross-sectional area. Another interesting finding was that RT and CT increased leg press 1 − RM, both absolute strength and that normalized to leg lean mass, supporting the notion that these training modalities improve overall muscle quality. CT appears to be especially effective for increasing the overall muscle quality in the older subjects, supporting our view that CT is a desirable approach for treating sarcopenia/dynapenia in older people. The exercise training–induced changes in body composition, skeletal muscle strength, and cardiorespiratory fitness were largely independent of age.
The current study also demonstrated lower OXPHOS through complex I in the older than in the younger subjects at baseline when expressed per tissue wet weight. These findings are consistent with recent data indicating that maximal mitochondrial ATP production rates in response to a supply of complex I substrates are lower in older than in younger adults (35, 39). Moreover, intrinsic mitochondrial capacity (expressed per mitochondrial protein content) was also lower in the older than in the younger subjects in response to a supply of complex I, complex I+II, and complex II substrates. These findings indicate that the age-related reductions in skeletal muscle oxidative capacity are due in part to reductions in organelle abundance and also to a decline in the inherent function of mitochondria.
Among the strengths of the present study was the comprehensive assessment of the exercise training–induced effects on the skeletal muscle mitochondria in a relatively large number of individuals. A potential limitation is the fact that the exercise training interventions were not matched for energy expenditure, which could have affected some of the molecular adaptations to exercise. Because of the complex nature of whole-body resistance training, we were unable to quantify precisely the absolute energy cost of each exercise condition. However, it is likely that the absolute energy cost of exercise was greatest in the ET group and least in the RT group. Likewise, the younger participants also probably had higher absolute energy costs of exercise than the older participants, although the relative exertion levels (percentage of maximal exercise capacity) were similar between young and older individuals in our study.
Collectively, the present results demonstrate that 8 weeks of ET and CT increase skeletal muscle mitochondrial OXPHOS, mRNA, and protein abundance of mitochondrial transcription factors and proteins in healthy young and older adults. Importantly, most of the exercise training–induced improvements in these outcomes occurred independent of age. Moreover, CT also resulted in the most robust improvements in most of the mitochondria-related outcomes and improvements in muscle strength and overall muscle quality in the older subjects, supporting the observation that CT is the preferred exercise regime for older people to treat sarcopenia. CT provides a robust exercise regimen for improving skeletal muscle OXPHOS, muscle strength, overall muscle quality, and physical characteristics independent of age.
Acknowledgments
We thank Jill Schimke, Dawn Morse, Katherine Klaus, Daniel Jakaitis, Beth Will, Audrey Weymiller, Roberta Soderberg, Deborah Sheldon, Lynne Johnson, and Melissa Aakre for the skillful assistance.
This work was supported by the National Institutes of Health (grants R01AG09531, R01DK41973 [to K.S.N.], KL2RR024151 [to B.A.I.], and UL1 TR000135), the Mayo Foundation, the Murdock-Dole Professorship (to K.S.N.), and the National Center for Advancing Translational Sciences, a component of the National Institutes of Health (Clinical and Translational Science Awards Grant UL1-TR000135). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
The study has been registered at Clinicaltrials.gov under registration number NCT01489930.
Present address for G.C.H.: Newomics Inc., Emeryville, CA 94608.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- au
- arbitrary units
- CT
- combined training
- ET
- endurance training
- ETC
- electron transport chain
- OXPHOS
- oxidative capacity
- PCG1α
- peroxisome proliferator-activated receptor gamma coactivator 1α
- qPCR
- quantitative real-time PCR
- RM
- repetition maximum
- RT
- resistance training
- SIRT3
- sirtuin 3
- TFAM
- transcription factor A, mitochondrial
- Vo2 peak
- peak oxygen uptake.
References
- 1. Brotto M. Lessons from the FNIH-NIA-FDA sarcopenia consensus summit. IBMS BoneKEy. 2012;9:210(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102:5618–5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1:637–639. [DOI] [PubMed] [Google Scholar]
- 8. McCully KK, Fielding RA, Evans WJ, Leigh JS, Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol. 1993;75:813–819. [DOI] [PubMed] [Google Scholar]
- 9. Karakelides H, Irving BA, Short KR, O'Brien P, Nair KS. Age, obesity, and sex effects on insulin sensitivity and skeletal muscle mitochondrial function. Diabetes. 2010;59:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 12. Jubrias SA, Esselman PC, Price LB, Cress ME, Conley KE. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol. 2001;90:1663–1670. [DOI] [PubMed] [Google Scholar]
- 13. Sparks LM, Johannsen NM, Church TS, et al. Nine months of combined training improves ex vivo skeletal muscle metabolism in individuals with type 2 diabetes. J Clin Endocrinol Metab. 2013;98:1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ades PA, Ballor DL, Ashikaga T, Utton JL, Nair KS. Weight training improves walking endurance in healthy elderly persons. Ann Intern Med. 1996;124:568–572. [DOI] [PubMed] [Google Scholar]
- 15. Ruas JL, White JP, Rao RR, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanza IR, Nair KS. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 2009;457:349–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Asmann YW, Stump CS, Short KR, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–3319. [DOI] [PubMed] [Google Scholar]
- 18. Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proc Natl Acad Sci USA. 2003;100:7996–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zabielski P, Blachnio-Zabielska A, Lanza IR, et al. Impact of insulin deprivation and treatment on sphingolipid distribution in different muscle subcellular compartments of streptozotocin-diabetic C57BL/6 mice. Am J Physiol Endocrinol Metab. 2014;306:E529–E542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68:867–873. [DOI] [PubMed] [Google Scholar]
- 21. Irving BA, Weltman JY, Brock DW, Davis CK, Gaesser GA, Weltman A. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity (Silver Spring). 2007;15:370–376. [DOI] [PubMed] [Google Scholar]
- 22. Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. [DOI] [PubMed] [Google Scholar]
- 24. Pesta D, Hoppel F, Macek C, et al. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1078–R1087. [DOI] [PubMed] [Google Scholar]
- 25. Parise G, Brose AN, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol. 2005;40:173–180. [DOI] [PubMed] [Google Scholar]
- 26. Ydfors M, Fischer H, Mascher H, Blomstrand E, Norrbom J, Gustafsson T. The truncated splice variants, NT-PGC-1α and PGC-1α4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol Rep. 2013;1:e00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf). 2010;199:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rao RR, Long JZ, White JP, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnson ML, Irving BA, Lanza IR, et al. Differential effect of endurance training on mitochondrial protein damage, degradation, and acetylation in the context of aging. J Gerontol. [published online ahead of print December 10, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jing E, O'Neill BT, Rardin MJ, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes. 2013;62:3404–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin L, Chen K, Khalek WA, et al. Regulation of skeletal muscle oxidative capacity and muscle mass by SIRT3. PLoS One. 2014;9:e85636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lanza IR, Sreekumaran Nair K. Regulation of skeletal muscle mitochondrial function: genes to proteins. Acta Physiol (Oxf). 2010;199:529–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1271–R1278. [DOI] [PubMed] [Google Scholar]
- 35. Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCartney N, Hicks AL, Martin J, Webber CE. Long-term resistance training in the elderly: effects on dynamic strength, exercise capacity, muscle, and bone. J Gerontol A Biol Sci Med Sci. 1995;50:B97–B104. [DOI] [PubMed] [Google Scholar]
- 37. Ferrara CM, Goldberg AP, Ortmeyer HK, Ryan AS. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J Gerontol A Biol Sci Med Sci. 2006;61:480–487. [DOI] [PubMed] [Google Scholar]
- 38. Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tatpati LL, Irving BA, Tom A, et al. The effect of branched chain amino acids on skeletal muscle mitochondrial function in young and elderly adults. J Clin Endocrinol Metab. 2010;95:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]