Abstract
There is increasing evidence that local thyroid hormone (TH) availability changes profoundly in inflammatory conditions due to altered expression of deiodinases that metabolize TH. It is largely unknown, however, how inflammation affects TH availability via the expression of TH transporters. In this study we examined the effect of bacterial lipopolysaccharide (LPS) administration on two TH transporters that are critically important for brain TH homeostasis, organic anion-transporting polypeptide 1c1 (OATP1c1), and monocarboxylate transporter 8 (MCT8). MRNA levels were studied by in situ hybridization and qPCR as well as protein levels by immunofluorescence in both the rat and mouse forebrain. The mRNA of both transporters decreased robustly in the first 9 hours after LPS injection, specifically in brain blood vessels; OATP1c1 mRNA in astrocytes and MCT8 mRNA in neurons remained unchanged. At 24 and/or 48 hours after LPS administration, OATP1c1 and MCT8 mRNAs increased markedly above control levels in brain vessels. OATP1c1 protein decreased markedly in vessels by 24 hours whereas MCT8 protein levels did not decrease significantly. These changes were highly similar in mice and rats. The data demonstrate that OATP1c1 and MCT8 expression are regulated in a parallel manner during inflammation at the blood-brain barrier of rodents. Given the indispensable role of both transporters in allowing TH access to the brain, the results suggest reduced brain TH uptake during systemic inflammation.
It is well known that thyroid hormone (TH) homeostasis is altered in association with acute and chronic illnesses, a disorder commonly referred to as the nonthyroidal illness syndrome and manifested by reduced serum TH levels and an inappropriately low TSH (1–3). However, there is limited understanding about how TH availability changes at the level of specific target cells and tissues under these conditions. Local TH availability is largely determined by the presence of TH transporters that enable the passage of TH across cellular membranes, and deiodinases that metabolize TH (4, 5). Deiodinases have been studied extensively in animal models of inflammatory conditions (1), particularly type 2 deiodinase (D2) that activates T4 to the biologically more potent T3 (4). In these models, D2 expression increases in several organs (6–15), including novel expression in cells and tissues where it is not normally present (6, 8, 15), suggesting a local anti-inflammatory role for TH (6, 8, 16–18). In contrast, it is largely unknown how inflammation affects TH availability via TH transporters. qPCR studies reported decreased monocarboxylate transporter 8 (MCT8; coded by Slc16a2) expression in several organs of septic pigs (19) and in the adipose tissue of septic patients (20), whereas organic anion-transporting polypeptide 1c1 (OATP1c1; coded by Slco1c1) and monocarboxylate transporter 10 mRNAs increased in the hypothalamus of rabbits in prolonged critical illness (21). Details of these responses, such as cell-type specificity, temporal characteristics, and whether mRNA changes translate into protein levels, have not yet been studied. Shedding light on these aspects of TH handling would be important to better understand TH availability during disease at the cellular level.
Previously, we described how inflammation affects D2 expression in the brain of different rodent species, and demonstrated inflammation-induced D2 expression in the leptomeninges, choroid plexus and a subset of brain blood vessels (15). The present study was conducted to obtain a detailed, cell type–specific insight into the inflammatory regulation of TH transporters. Of the several proteins capable of TH transport and present in the rodent brain (22, 23), we focused on OATP1c1 and MCT8 because they are critically important for brain TH homeostasis (24). Both transporters facilitate TH traffic in multiple cell types (25–30) and are indispensable for TH entry into the brain via the blood-brain and/or blood-cerebrospinal fluid barrier (24, 31–34). In fact, almost the entire T3 uptake into the brain is facilitated by MCT8 (32), whereas both MCT8 and OATP1c1 contribute to T4 uptake (24). Importantly, a recent study by Mayerl et al (24) demonstrated that the lack of both transporters results in a severely hypothyroid brain, with T3 and T4 contents being of only 10% of wild-type levels.
In this study, we examined the effect of bacterial lipopolysaccharide (LPS) administration, an acute systemic inflammatory challenge, on OATP1c1 and MCT8 mRNA and protein expression in the brain using in situ hybridization, qPCR, and immunofluorescence. Given that we previously found major differences in the LPS-induced D2 expression between rats and mice (15), we performed the experiments in both species, with special attention to the rat meninges.
Materials and Methods
Animals
The experiments were carried out on adult, male, Sprague-Dawley (Taconic Biosciences) and Wistar rats (TOXI-COOP KKT) weighing 220–260 g, and adult, male C57Bl/6 mice (Taconic Biosciences and Charles River Laboratories), weighing 19–21 g. Animals were housed under standard conditions (lights on between 0600 and 1800 h, temperature, 22 ± 1°C, rodent chow and water ad libitum). All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at Tufts Medical Center and the Animal Welfare Committee at the Institute of Experimental Medicine of the Hungarian Academy of Sciences.
Injections and tissue preparation
LPS injections
Rats and mice were injected ip with LPS from Escherichia coli (serotype O55:B5; Sigma-Aldrich), dissolved in saline, at a dose of 2.5 mg/kg body weight to all animals according to the Guide of the American Thyroid Association (35). Control animals received the same volume of saline.
Experiment for qPCR analysis
Groups of Wistar rats (n = 6 per group) and C57Bl/6 mice (n = 8 per group) were injected with LPS or saline, and were decapitated 9 or 48 hours later. The brains were dissected and samples from the cerebral cortex collected and stored at −80°C until subjected to reverse transcription real-time PCR. In another experiment, leptomeninges of the basal forebrain were collected from six control and eight LPS-treated Sprague-Dawley rats 9 hours after the injections.
Time course experiment for in situ hybridization and immunofluorescence studies
Groups of Sprague-Dawley rats and C57Bl/6 mice (n = 4 or 5 in each group) were injected with LPS and 2, 4, 9, 24, or 48 hours later were anesthetized with ketamine-xylazine, then decapitated. Control mice (n = 5) were euthanized at 9 hours, and control rats were euthanized at 9 hours (n = 3) or 24 hours (n = 2) because in situ hybridization and immunofluorescent signals for OATP1c1 and MCT8 were of the same intensity in 9- and 24-hour control rat brains. The brains were removed, snap-frozen on powdered dry ice, and 16-μm thick coronal sections were cut using a Leica CM3050 S cryostat (Leica Microsystems). Sections were thaw-mounted on Superfrost Plus slides (Thermo Fisher Scientific), air-dried and stored at −80°C until processed for in situ hybridization.
RNA isolation and real-time PCR analyses
RNA was isolated using the RNeasy Lipid Tissue Mini Kit (QIAGEN) from cortex samples and with RNeasy Tissue Mini Kit (QIAGEN) from leptomeninges according to the manufacturer's instructions. The purity and concentration of the RNA were analyzed using Spectrophotometer (Bio-Rad, SmartSpec Plus). Reverse transcription was performed with 1 μg of RNA to convert the total RNA to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems by Life Technologies). Concentration of the generated cDNA was determined using the Qubit 2.0 Fluorometer with the Qubit ssDNA Assay Kit (Life Technologies). Expression of OATP1c1, MCT8, and D2 was measured by real-time quantitative TaqMan RT-PCR reaction with a ViiA 7 Real-Time PCR System (Life Technologies), using commercially available TaqMan probes (code numbers are summarized in Supplemental Table 1) on 10-ng cDNA template in duplicates. Glyceraldehyde-3-phosphate dehydrogenase was used as housekeeping gene. Expression of the housekeeping gene did not vary between the experimental groups.
Generation of hybridization probes
Template cDNA fragments were generated with RT-PCR using standard procedures. Amplification was performed on cDNA synthesized from rat brain for OATP1c1 and mouse or rat liver for MCT8. Fragments were cloned into pGemT vector (Promega) and confirmed by sequencing. Probe sequences were as follows: rat OATP1c1 probe corresponds to nt 375-2525 of NM_053441.1 and has 93% homology with the corresponding mouse sequence, nt 368-2522 of NM_021471.2; mouse MCT8 probe nt 2569-3428 of NM_009197.2; rat MCT8 probe nt 41-648 of NM_147216.1, which has 98% homology with the mouse sequence NM_009197.2 between nt 227-834; rat D2 probe was reported previously (15). Antisense riboprobes were synthesized using SP6 or T7 RNA polymerase (Promega) in the presence of [35S]-uridine 5′-(α-thio) triphosphate (PerkinElmer), and purified with Mini Quick Spin RNA columns (Roche Applied Sciences). For fluorescent in situ hybridization, the OATP1c1 probe was labeled with digoxigenin-11-UTP (Roche).
Isotopic in situ hybridization
Isotopic in situ hybridization was performed as previously described (15, 36) using 50 000 cpm/μl radiolabeled probe concentrations. The same probe was used to detect OATP1c1 mRNA in both species; the rat MCT8 probe was used for rat sections, whereas both the rat and mouse MCT8 probes that are nonoverlapping were used on mouse sections to increase hybridization sensitivity. Following stringency washes, sections were dehydrated in ascending ethanol series, air dried, and placed on Amersham Hyperfilm autoradiography film (GE Healthcare Biosciences) for 6 days (rat OATP1c1) or 8 days (mouse OATP1c1 and MCT8). Slides were then dipped in Kodak NTB autoradiography emulsion (Carestream Health Inc), and stored at 4°C until developed. Exposure times were as follows: 9 days for mouse OATP1c1; 24 days for mouse MCT8; two exposure times, 5 days and 9 days, were used for two different hybridizations for rat OATP1c1; 35 days for rat MCT8; and 14 days for rat D2. Autoradiograms were developed with Kodak D19 developer (Eastman Kodak Co). Sections were immersed in 0.0005% cresyl violet acetate (Sigma-Aldrich) for 2 minutes to obtain fluorescent labeling of cell nuclei, dehydrated in ascending ethanol series and xylenes, and coverslipped with DPX (Sigma-Aldrich). Darkfield images of the emulsion autoradiographs and fluorescent images of the cresyl violet signal were captured using a Zeiss Axioplan 2 microscope equipped with a SPOT Slider digital camera (Spot Imaging Solutions).
Isotopic OATP1c1 in situ hybridization combined with glial fibrillary acidic protein immunofluorescence
Sections from the control and 9-hour LPS groups from both species were hybridized for OATP1c1 as above, then processed further for immunofluorescence. The sections were treated with the mixture of 0.5% Triton X-100 and 0.5% H2O2 for 15 minutes, rinsed in PBS (3 × 10 minutes), immersed in maleate buffer (pH 7.5) for 10 minutes, and then in 1% blocking reagent for nucleic acid hybridization (Roche). The sections were incubated overnight in a mouse monoclonal antibody against the astrocyte marker, glial fibrillary acidic protein (GFAP) (Cat No. MAB360; Millipore; diluted 1:1,000), and subsequently in Alexa Fluor 488-conjugated donkey antimouse IgG (1:400; Life Technologies) for 2 hours. Sections were dehydrated and dipped in Kodak NTB autoradiography emulsion. The autoradiograms were developed after 9 days. The fluorescent signal of Alexa Fluor 488 was pseudocolored to red for better visibility of dual-labeled cells.
Fluorescent OATP1c1 in situ hybridization combined with GFAP immunofluorescence
Hybridizations were performed on both fresh-frozen control rat sections, and sections from four normal paraformaldehyde-perfused rats. Paraformaldehyde perfusion was performed as previously described (36). Briefly, rats were anesthetized with an overdose of pentobarbital (50 mg/kg) and perfused transcardially with PBS followed by 4% paraformaldehyde. The brains were postfixed by immersion in 4% paraformaldehyde, cryoprotected in 20% sucrose in PBS overnight, then snap frozen on dry ice. Serial 20-μm coronal sections were cut on a cryostat, collected in cryoprotectant solution and stored at −20°C until used. Fluorescent in situ hybridization for fresh-frozen and paraforaldehyde-perfused sections was performed as previously described (15, 36). The digoxigenin-labeled probe was detected with peroxidase-conjugated Fab fragments of sheep antidigoxigenin antibody (1:100, Roche). The hybridization signal was amplified with biotinylated tyramide for 30 minutes using the TSA amplification kit (PerkinElmer), and visualized by Alexa 488-conjugated Streptavidin (1:500; Life Technologies). Sections were reacted with murine GFAP antibody as above, and detected with Cy3-conjugated donkey antimouse IgG (Jackson ImmunoResearch). Sections were then coverslipped with Vectashield containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories).
Immunofluorescence for OATP1c1 and MCT8
For MCT8 immunofluorescence, mounted fresh-frozen sections were fixed with 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) for 15 minutes. For OATP1c1 immunofluorescence, sections were fixed with methanol at −20°C for 5 minutes. Sections were permeabilized with 0.25% Triton-X-100 for 30 minutes, blocked with 2% normal horse serum in PBS, and incubated overnight in a rabbit antiserum against human MCT8 (raised against amino acids 527–539; No.1306, gift from Dr Theo J. Visser) or a rabbit antiserum against human OATP1c1 (raised against amino acids 697–712; No. 3516, gift from Dr Theo J. Visser), both diluted at 1:1000. The primary antisera were detected with Alexa Fluor 488-conjugated donkey antirabbit IgG diluted at 1:400 (Jackson ImmunoResearch). Immunostaining with both antisera resulted in the same patterns reported earlier (26, 37).
Image analysis
In situ hybridization signals were compared between sections from a single hybridization experiment where all conditions and exposure times were identical. Darkfield images of OATP1c1 hybridization were analyzed with ImageJ software (public domain at http://rsb.info.nih.gov/ij). To maximize the analysis of OATP1c1 signal in blood vessels, hybridizations were analyzed in the hypothalamus where no significant signal was detected in astrocytes after 9 days exposure in mice or 5 days exposure in rats. Images were taken with a 5× objective at the level of the paraventricular nucleus (mice) or the dorsomedial nucleus (rats), and integrated density values were calculated. From the two rat OATP1c1 hybridizations, only the 5-day exposure experiment was used for quantitative image analysis. Images from both the 9- and 5-day exposure experiments were used to illustrate the magnitude of changes in OATP1c1 hybridization signal. To quantify vascular MCT8 hybridization in mice, blood vessel segments with clear hybridization signal were counted in the thalamus and hypothalamus in three sections per mouse at the rostro-caudal level of the median eminence. In these regions, the low neuronal hybridization signal allowed easier identification of labeled blood vessels than in cortical regions. OATP1c1 immunofluorescence in blood vessels was quantified in rats using ImageJ. Images were taken with a 20× objective of an area with high density of blood vessels in the midline thalamus. The area covered by immunofluorescently labeled vessels (pixel number) was measured by subtracting nonlabeled areas (ie, dark pixels) from the image using the threshold tool and the same threshold value for all images. Hematoxylin and eosin staining was used to facilitate the identification of arachnoid veins in rats.
Statistical analysis
Data are presented as mean ± SEM. qPCR data were expressed as relative quantification values (RQ; mean ± SEM). qPCR data from cortex and image analysis data were compared between groups by one-way ANOVA and Newman-Keuls multiple comparison post-hoc test. qPCR data from the leptomeninges were compared by Student t test.
Results
Effect of LPS on OATP1c1 expression in the brain
OATP1c1 mRNA
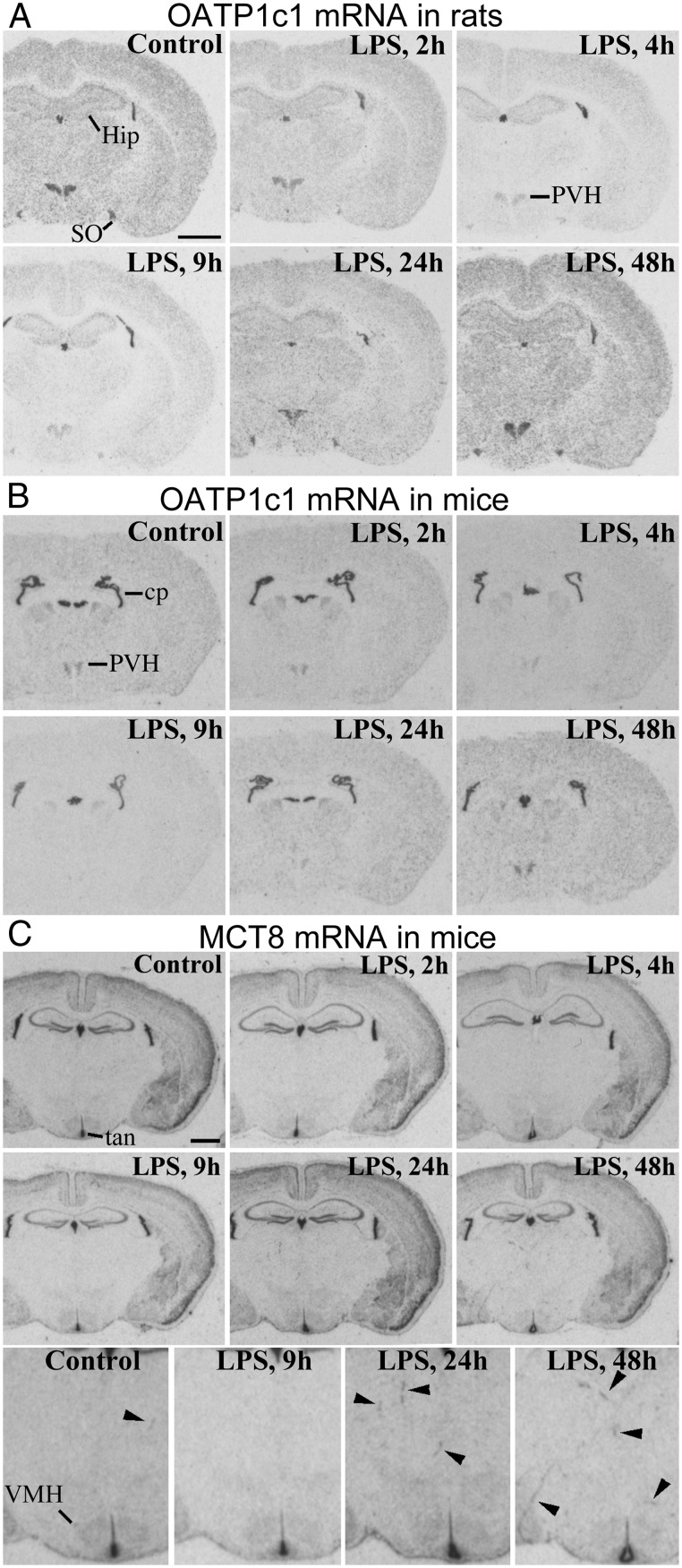
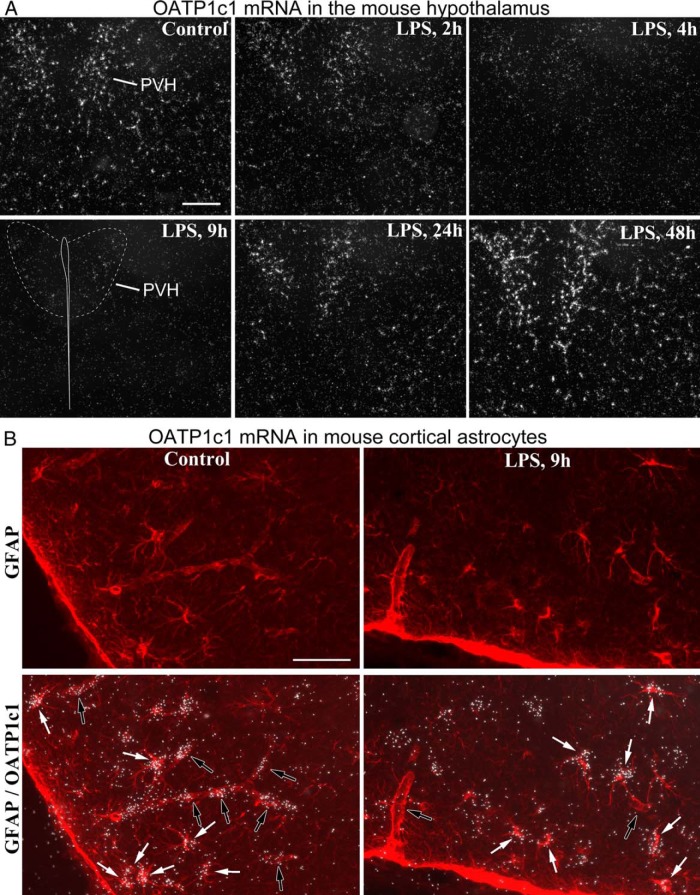
LPS administration similarly affected OATP1c1 expression in mice and rats. OATP1c1 mRNA levels decreased profoundly within 9 hours after LPS injection, as demonstrated by a striking reduction in hybridization signal across the forebrain (Figure 1, A and B). Emulsion autoradiography revealed that this reduction was due to a loss of hybridization signal specifically in blood vessels, but not astrocytes. Vascular OATP1c1 expression decreased uniformly in the forebrain, but the course of this response could be followed most clearly in the hypothalamus (Figure 2A) where hybridization signal in astrocytes was the lowest among forebrain regions. Hybridization signal in vessels decreased significantly as soon as 2 hours after LPS, and markedly by 4 hours (Figure 2A). Vascular hybridization signal was virtually absent in mice 9 hours after LPS injection, except light labeling of the highly vascularized hypothalamic paraventricular nucleus (Figure 2A). A similar reduction was observed in rats, where only scattered blood vessels with light hybridization signal were detected at 9 hours after LPS (in the hybridization experiment with the longer, 9-d autoradiography exposure). Moderate labeling remained only in some vessels of the paraventricular and supraoptic nuclei (Figure 3A; also in Figure 1A); in fact, vessels in these two nuclei were more intensely labeled than elsewhere, even in control rats. The loss of OATP1c1 hybridization signal in blood vessels is also demonstrated in images from the cerebral cortex in Figures 2B and 3A.
Figure 1. X-ray film autoradiograms of isotopic in situ hybridizations for rat and mouse OATP1c1, and mouse MCT8.
A, OATP1c1 mRNA expression in the rat forebrain in the function of time following LPS injection. Hybridization signal decreases after LPS injection; at 4 and 9 h, the remaining visible signal in the hypothalamic paraventricular and supraoptic nuclei is vascular, whereas in other regions, such as the hippocampus and cortex, is primarily astrocytic. B, LPS has the same effect on OATP1c1 mRNA in mice as in rats. In mice, OATP1c1 mRNA decreases even in the choroid plexus at 9 h. C, MCT8 mRNA in the mouse brain following LPS administration. Neuronal labeling remains unchanged. The bottom panel shows magnified views of the diencephalic area, where several blood vessels (arrowheads) are labeled at 24 and 48 h, but none at 9 h after LPS. cp, choroid plexus; Hip, hippocampus; PVH, hypothalamic paraventricular nucleus; SO, supraoptic nucleus; tan, tanycytes; VMH, hypothalamic ventromedial nucleus. Scale bars = 2 mm.
Figure 2. A, Darkfield emulsion autoradiography images demonstrate the time course of OATP1c1 mRNA expression (silver grain accumulation, white) in the mouse hypothalamus after LPS injection. Hybridization signal labeled only blood vessels but not astrocytes in the hypothalamus. The signal virtually disappears by 9 h, when only light signal remains visible in the paraventricular nucleus. At 48 h, OATP1c1 mRNA increases significantly. Scale bar = 200 μm. B, Combination of in situ hybridization for OATP1c1 (silver grains) and GFAP immunofluorescence (red) demonstrates that LPS did not affect OATP1c1 mRNA in astrocytes. Images were taken from the piriform cortex (control) and adjacent cortical amygdala (LPS). White arrows in the overlay images indicate astrocytes with OATP1c1 hybridization signal, open arrows point to blood vessel segments. Note that hybridization signal labels vessel segments in the control, but not in LPS-injected brain. Scale bar = 50 μm.
Figure 3. A, Darkfield emulsion autoradiography images illustrate the effect of LPS on OATP1c1 mRNA in the rat brain. The decrease in OATP1c1 mRNA levels at 9 h is demonstrated in hybridizations with longer exposure time, whereas shorter exposure was necessary to visualize the increase at 48h. The top panel shows the dramatic decrease of OATP1c1 mRNA in vessels of the hypothalamic paraventricular nucleus. Middle panel demonstrates the effect in the cortex, where hybridization in vessels is almost completely absent 9 h after LPS, and the remaining signal labels predominantly astrocytes. Arrows indicate longer vessel segments in the control cortex. The bottom panel demonstrates the robust increase in OATP1c1 mRNA levels in blood vessels at 48 h after LPS; images were taken from the hypothalamic dorsomedial nucleus. PVH, hypothalamic paraventricular nucleus. Scale bar = 100 μm. B, Fluorescent in situ hybridization demonstrates OATP1c1 mRNA in different astrocyte populations in the rat. Hybridization on paraformaldehyde-perfused sections shows high levels of OATP1c1 mRNA (green) in astrocytes of the hippocampus, cortex, and striatum. Note the numerous intense green puncta denoting OATP1c1 mRNA in the cytoplasm and proximal processes of astrocytes, which were identified by immunofluorescence for GFAP (red). In contrast, more sensitive hybridization using fresh-frozen sections was necessary to detect OATP1c1 mRNA in hypothalamic astrocytes. In these cells, hybridization signal concentrated in only a few, 5–8, smaller-sized puncta. Cell nuclei are labeled by the blue fluorescence of DAPI. Scale bar = 20 μm.
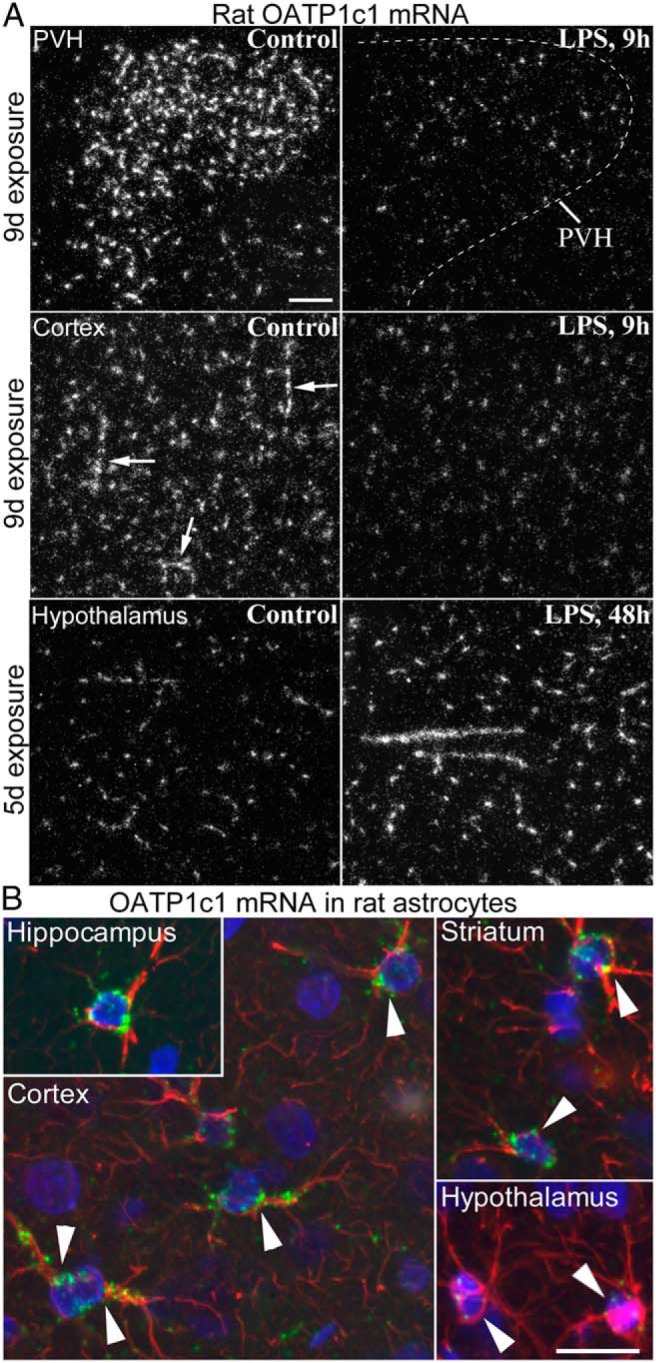
Hybridization signal in astrocytes did not change noticeably in either species, and remained comparable to control levels 9 hours after LPS, as demonstrated in images from the mouse and rat cortex (Figures 2B and 3A). Notably, OATP1c1 mRNA in astrocytes varied greatly between brain regions, confirming recent studies (23, 27). In mice, OATP1c1 mRNA was easily detected in hippocampal and cortical astrocytes, whereas it was not detected in hypothalamic astrocytes with the exposure time used in this experiment. In rats, differences in astrocytic OATP1c1 expression were confirmed by both fluorescent (Figure 3B) and radioactive in situ hybridization. Hybridization signal was moderate to intense in astrocytes of the cortex, hippocampus, striatum, and ventrolateral thalamus, whereas it was much lighter in astrocytes of the hypothalamus, such as in the ventromedial nucleus (Figure 3B).
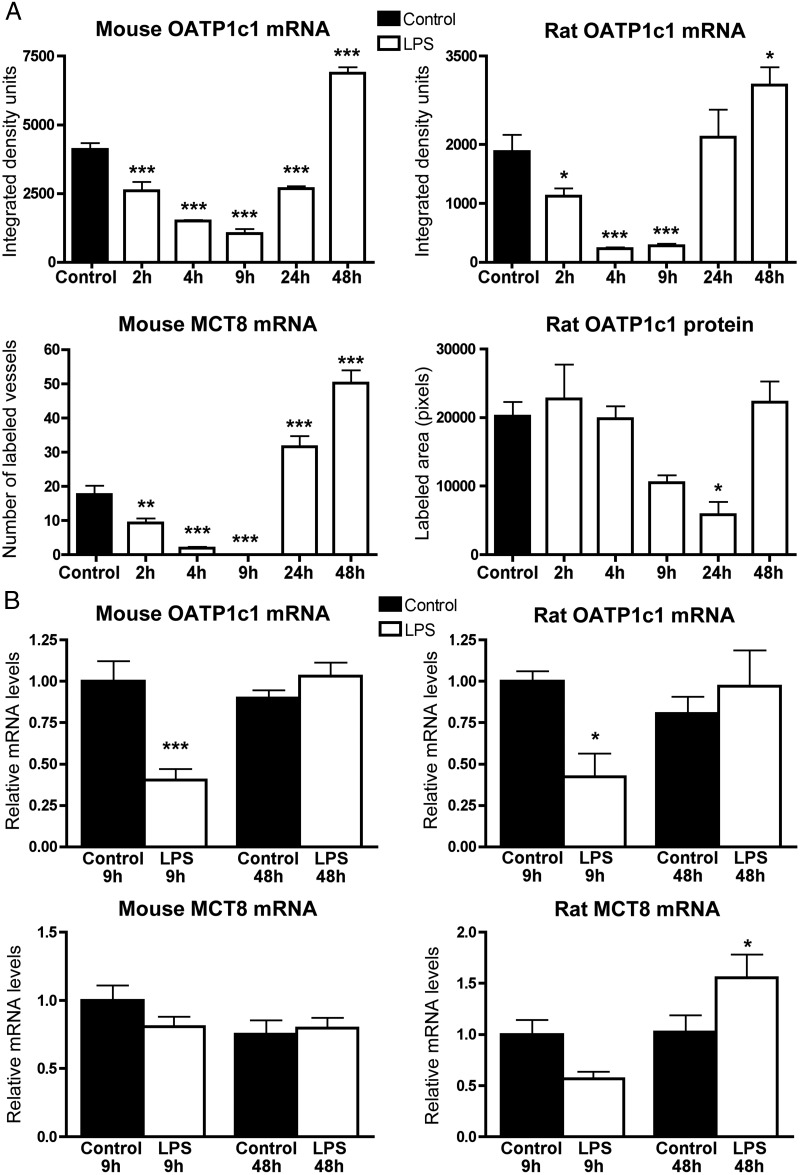
In both species, vascular OATP1c1 hybridization signal increased above control levels during the recovery phase from endotoxemia. In mice, OATP1c1 mRNA was still below control levels at 24 hours, but increased markedly at 48 hours after LPS (Figure 2A). In rats, up-regulation of OATP1c1 mRNA in blood vessels could be observed at both 24 and 48 hours after LPS (Figure 3A). Conspicuously increased signal in vessels was observed in one of four rats in the 24-hour LPS group, and three of four rats in the 48-hour LPS group. Markedly increased OATP1c1 mRNA at 24 hours after LPS was confirmed in another experiment (two of two rats; data not shown). This suggests that the timing of postendotoxemic increase in vascular OATP1c1 mRNA can vary among individual rats. Image analysis results from mice and rats representing vascular OATP1c1 mRNA expression at each time point following LPS injection are presented in Figure 4A. qPCR data from cortical samples confirmed the in situ hybridization results, as OATP1c1 mRNA was significantly decreased 9 hours after LPS injection in both species (Figure 4B). At 48 hours after LPS, OATP1c1 mRNA levels were not different from control levels by qPCR (Figure 4B).
Figure 4. A, Results of semiquantitative image analysis of OATP1c1 and MCT8 in situ hybridization, and OATP1c1 immunofluorescence. OATP1c1 hybridization signal was quantified in the hypothalamus and represents mRNA expression specifically in blood vessels, as astrocytes were not labeled with the used exposure times. In the mouse MCT8 in situ hybridization experiment, the number of labeled blood vessels were counted in the diencephalic area in three sections per mouse, and the average number of vessels per section was calculated and presented in the graph. OATP1c1 immunofluorescence in rats was quantified in images taken from the thalamus by measuring the area (pixel number) covered by immunolabeled vessels. Sample sizes: four or five rats or mice/group. *, P < .05; **, P < .01; ***, P < .001 vs control. B, qPCR results from the mouse and rat cortex 9 and 48 h after LPS injection. OATP1c1 mRNA is significantly reduced 9 h after LPS injection in both species: In rats, MCT8 mRNA tended to decrease at 9 h but did not reach significance (P = .07). Sample sizes: n = 6 rats/group and n = 8 mice/group. *, P < .05, ***, P < .001 vs the corresponding controls.
In the choroid plexus, OATP1c1 hybridization signal was consistently reduced 9 hours after LPS in mice (Figure 1B) but not rats. In tanycytes, OATP1c1 mRNA expression was extremely intense in rats (Supplemental Figure 1), but conspicuously absent in mice (Supplemental Figure 2). LPS did not affect OATP1c1 expression in rat tanycytes.
OATP1c1 protein
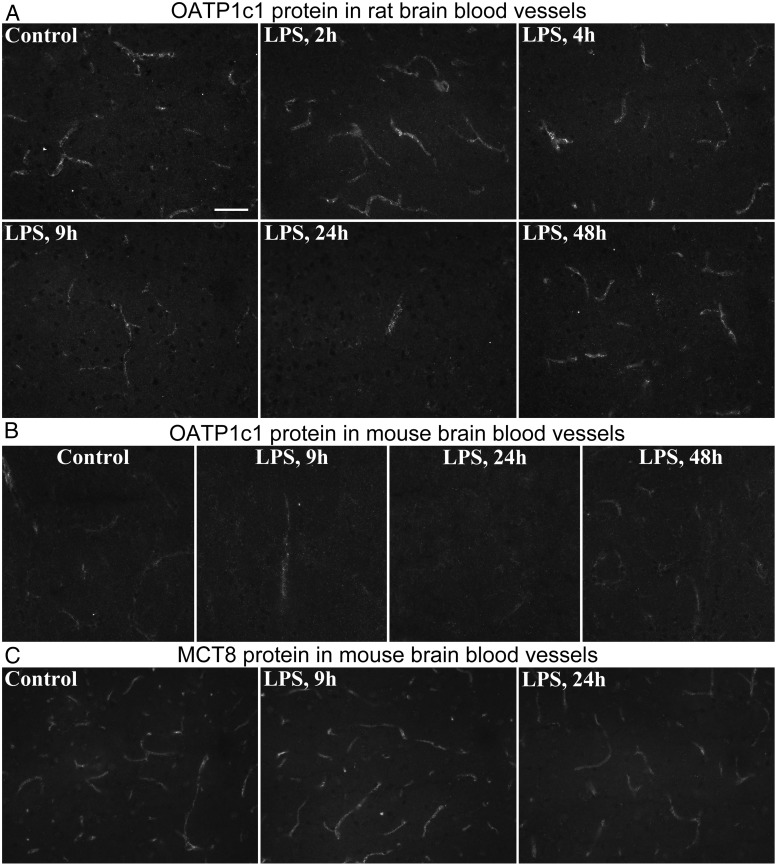
Immunofluorescence for OATP1c1 gave clear labeling of blood vessels in the rat brain with low background, whereas in mice the labeling was less sensitive and less clear due to higher background levels. The antiserum did not label astrocytes. Immunostaining of blood vessels did not differ between control, 2-, and 4-hour LPS groups, but visibly decreased at 9- and 24 hours (Figure 5, A and B). At 24 hours, when the decrease was significant by image analysis (Figure 4A), only scattered major vessel segments were labeled in both species (Figure 5, A and B). OATP1c1 immunostaining in vessels at 48 hours after LPS was similar to controls (Figure 5, A and B). OATP1c1 immunostaining was intense in rat tanycytes (Supplemental Figure 1), whereas in mice it was observed only in lateral beta (β-1) tanycytes in the floor of the third ventricle (Supplemental Figure 2). The presence of OATP1c1 immunoreactivity in mouse tanycytes is in agreement with a previous report by Roberts et al (26) that used highly specific antibodies.
Figure 5. A, OATP1c1 immunofluorescence shows the time course of OATP1c1 protein in blood vessels of rats following LPS injection. Images were taken from the thalamus; note the decrease in labeling at 9 h, and that only scattered vessel segments are labeled at 24 h. B, The same phenomenon is shown in mice in images taken from the rostral perifornical area. C, MCT8 immunfluorescence in blood vessels of the mouse thalamus; labeling intensity is only mildly reduced at 24 h after LPS. Scale bar = 50 μm.
Effect of LPS on MCT8 expression in the brain
MCT8 mRNA
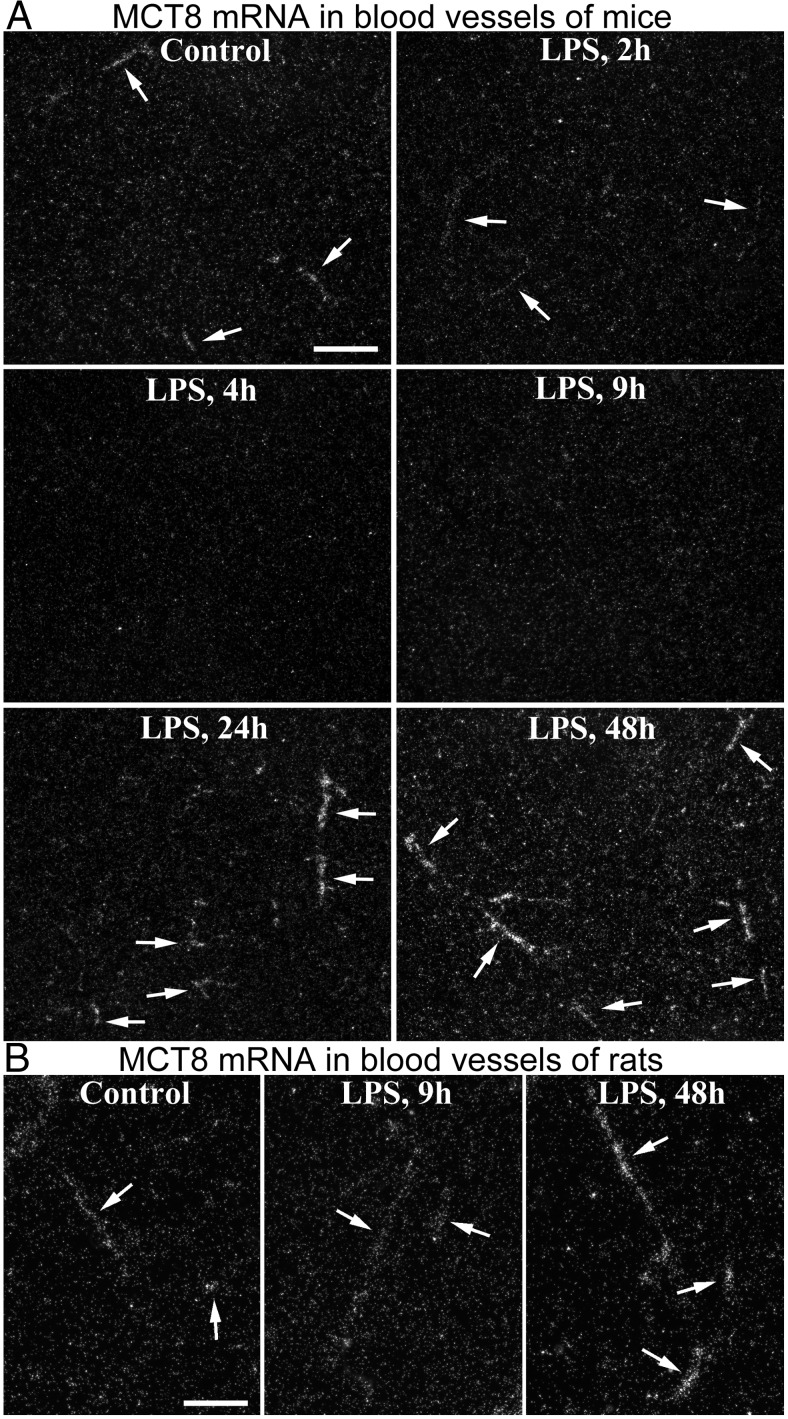
LPS similarly affected MCT8 expression in mice and rats. The hybridization patterns in control brains were identical to what was previously described (25). Several neuronal populations were labeled with variable, light-to-intense signal, and scattered larger blood vessels were labeled with moderate or light signal (Figure 1C). Rat tanycytes were intensely labeled, and extremely intense signal labeled mouse tanycytes and the choroid plexus in both species (Figure 1C). Neuronal MCT8 expression did not noticeably change at any time after LPS injection in both species (Figure 1C). In contrast, MCT8 mRNA expression in blood vessels changed markedly in response to LPS. In mice, the intensity of vascular labeling decreased gradually from 2 until 9 hours, when hybridization signal was no longer detected in vessels (Figure 6A). A similar trend was observed in rats, with noticeably less numerous and less intensely labeled blood vessels at 4h, and only occasional, lightly labeled vessels visible at 9 hours (Figure 6B).
Figure 6. A, Darkfield emulsion autoradiography images demonstrate the effect of LPS on MCT8 mRNA in brain blood vessels. Images were taken from the thalamus where labeled vessels (arrows) stand out due to virtually undetectable hybridization signal in neurons. The vascular signal vanishes completely by 9 h after LPS. At 24 h, and especially at 48 h, more intense hybridization signal labels a higher number of vessels than in control mice. Scale bar = 200 μm. B, A similar phenomenon is illustrated in images taken from the rat hippocampus. At 9 h after LPS only occasional vessels with faint hybridization signal were detected, whereas several vessels with intense signal were observed at 48 h. Scale bar = 100 μm.
In mice, both the number of detected vessel segments and their signal intensity increased at 24 hours after LPS compared with control mice, and even more strikingly at 48 hours (Figures 1C and 6A). The quantification of labeled vessels in mice is presented in Figure 4A. In rats, labeling of blood vessels was comparable to control levels at 24 hours, but increased markedly by 48 hours after LPS (Figure 6B). qPCR from the mouse cortex did not detect significant changes in MCT8 mRNA after LPS (Figure 4B). In the rat cortex, MCT8 mRNA tended to decrease at 9 hours, albeit not significantly, but significantly increased 48 hours after LPS (Figure 4B).
MCT8 protein
MCT8 immunolabeling in blood vessels was not different from control levels at 2, 4, 9, or 48 hours after LPS in both species. Only a slight decrease in labeling was observed at 24 hours in mice (Figure 5C). Intense MCT8 immunofluorescence was observed in axons in both species, in accordance with our previous description of specific axonal MCT8 labeling (37). MCT8-containing axons were present in most brain regions, but unaffected by LPS.
Effect of LPS on OATP1c1, MCT8, and D2 mRNAs in the rat leptomeninges
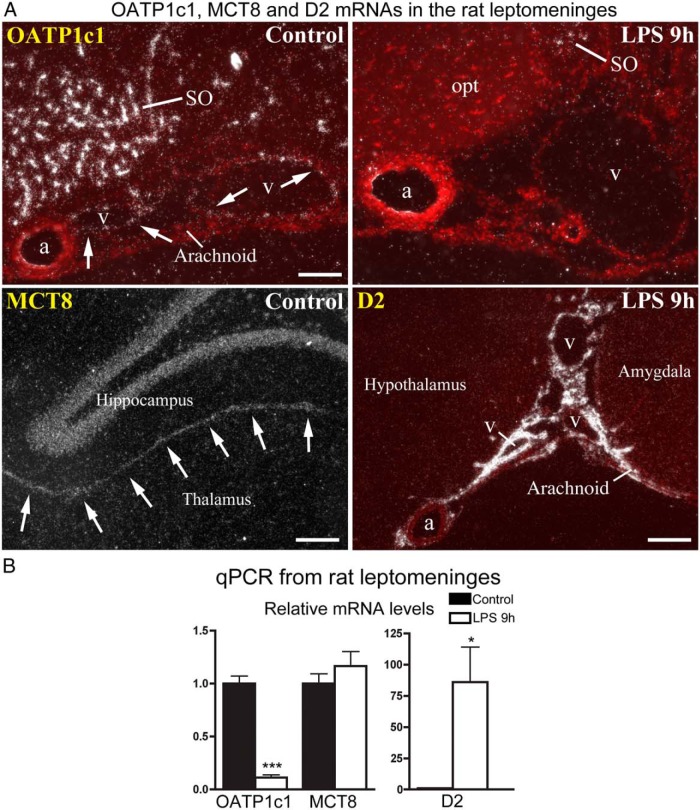
Because we previously observed that LPS administration induces robust D2 expression in the leptomeninges of rats (15), we also studied the expression of TH transporters in this tissue. OATP1c1 mRNA-expressing cells were observed in arachnoid veins, but not arteries or in the arachnoid tissue itself (Figure 7A). Following LPS, OATP1c1 hybridization signal could no longer be detected in the wall of the veins at 2, 4, or 9 hours later (Figure 7A), but returned to control levels by 24 hours, and remained normal at 48 hours. Conversely, D2 mRNA was induced in the wall of the arachnoid veins but not arteries following LPS (Figure 7A), which we did not previously recognize (15). Moderate-to-intense MCT8 hybridization signal was observed in the leptomeningeal layers between the hippocampus and thalamus (Figure 7A), and the dorsal third ventricle adjacent to the choroid plexus. This signal did not change significantly following LPS administration. MCT8 hybridization signal in the arachnoid covering the outer brain surface was uncertain, as control hybridizations with the sense transcript also yielded labeling in this location, although less intensely than the antisense probe. By qPCR from leptomeninges removed from the basal forebrain, OATP1c1 mRNA was reduced to approximately 10% of control level 9 hours after LPS injection, MCT8 mRNA did not change, whereas D2 mRNA increased 86-fold on average (Figure 7B).
Figure 7. A, In situ hybridization for OATP1c1, MCT8, and D2 in the rat leptomeninges. Red fluorescent cresyl violet counterstaining is overlaid on darkfield emulsion autoradiography images to help identify tissue locations (OATP1c1 and D2 images). Top: OATP1c1 mRNA is expressed specifically in the wall of veins (v) that run in the arachnoid, but not in arteries (a), or the arachnoid tissue itself. Labeling of veins is absent 9 h after LPS injection. Bottom left: MCT8 mRNA is expressed in the leptomeningeal layers between the hippocampus and thalamus (arrows). Bottom right: D2 mRNA is expressed in both the arachnoid tissue and wall of arachnoid veins (v), but not arteries (a) 9 h after LPS injection. a, artery; opt, optic tract; SO, supraoptic nucleus; v, vein. Scale bar = 100 μm in top, 200 μm in bottom panels. B, qPCR results from rat leptomeningeal samples obtained from six control and eight LPS-treated rats. OATP1c1 mRNA decreases to 10% of control value, whereas D2 increases ∼86-fold on average. *, P < .05; ***, P < .0001 vs control.
Discussion
Although data have been accumulating on cell type–specific regulation of TH action in the brain, regulation of TH transport is poorly understood. In the present study, regulation of TH transporters was studied in the forebrain using a model of nonthyroidal illness syndrome, a condition known to evoke marked changes in tissue TH availability (1, 2). In particular, we report changes in OATP1c1 and MCT8 expression in the rodent brain in response to LPS administration, with the following main findings: 1) OATP1c1 and MCT8 mRNAs decrease rapidly and robustly in blood vessels; 2) this effect is cell-type specific as OATP1c1 and MCT8 mRNA levels remain unaltered in astrocytes and neurons; 3) a robust decrease in OATP1c1 but not MCT8 protein levels in vessels follows hours later; 4) during recovery from endotoxemia, OATP1c1 and MCT8 mRNA levels increase markedly in blood vessels above control levels; 5) these changes occur in both mice and rats. In addition, we describe OATP1c1 expression in arachnoid veins but not arteries that respond to LPS in a similar way as parenchymal blood vessels, and MCT8 mRNA in part of the rat leptomeninges.
MCT8 and OATP1c1 have complementary functions at the blood-brain barrier, and work in tandem to allow TH access to the brain (24). The parallel changes in OATP1c1 and MCT8 mRNAs in brain vessels during endotoxemia further corroborates the functional link between these transporters, and suggest a common mechanism regulating the expression of these genes during inflammation. The mechanisms by which rapid down-regulation of both transporter mRNAs occurs is unknown, but could be a direct effect of cytokines or other inflammatory signals that are associated with endotoxin administration. However, this response was specific to cells comprising the brain vasculature, such as endothelial cells known to express these transporters (26, 34, 38) and possibly also pericytes, whereas OATP1c1 and MCT8 mRNA levels remained unchanged in astrocytes and neurons. One possibility to explain the latter phenomenon is that the extracellular signal that down-regulates MCT8 and OATP1c1 expression in vessels has limited access to astrocytes and neurons inside the blood-brain barrier. Alternatively, the signal may be present in the brain parenchyma, but due to intrinsic properties of astrocytes and neurons, has no effect on OATP1c1 or MCT8 mRNA. The latter might be explained by the lack of receptors for the signal molecule, or that the signaling pathway activated is not coupled to the mechanism that changes the transcription of the transporter genes.
The marked increase in OATP1c1 and MCT8 mRNAs during the recovery period from endotoxemia may be secondary to low intracellular TH levels in microvascular cells, supported by data that OATP1c1 in rat brain microvessels is up-regulated by hypothyroidism (28). However, regulation of OATP1c1 and MCT8 mRNAs by TH was not observed in congenitally hypothyroid mice (25), raising the possibility that increased OATP1c1 and MCT8 mRNA expression may be a rebound effect independent of TH concentration.
At the protein level, OATP1c1 decreased in brain vessels in a similarly robust manner as OATP1c1 mRNA, but only several hours later. This delay likely reflects the turnover rate of OATP1c1 protein in cells of the blood-brain barrier. In contrast, only a modest decrease in vascular MCT8 immunostaining was observed in mice. A possible explanation is that the half-life of MCT8 may be substantially longer than that of OATP1c1, and in our model, the inflammatory state may have been too transitory to see a significant decrease in the MCT8 protein. Thus, an inflammation model of longer duration may be necessary to more definitively address whether MCT8 decreases similar to OATP1c1. Given that our analysis did not provide information on the subcellular location of the transporters, it is also conceivable that internalization of OATP1c1 and/or MCT8 from the cell membrane could result in a more rapid decrease in TH transport before a decrease in protein levels is realized.
Access of TH to most of the brain parenchyma primarily occurs via the blood-brain barrier, and much less via the blood–cerebrospinal fluid barrier (39). Given that deficiency in either OATP1c1 or MCT8 is sufficient to reduce brain TH uptake (24, 32), down-regulation of these transporters in brain vessels during inflammation suggests diminished TH uptake into the brain parenchyma and may contribute to common symptoms associated with illness such as fatigue, depressed mood, and impaired neurocognitive function. In support, a recent clinical study reported that certain OATP1c1 polymorphisms are associated with fatigue and depression in a population of patients with adequately treated hypothyroidism (40).
Inflammatory regulation of OATP1c1 and MCT8 was strikingly different from that observed for D2 that is highly inducible by the inflammatory transcription factor, nuclear factor κB (11, 41). LPS induces D2 via nuclear factor κB in tanycytes (7, 15), and probably in meningeal fibroblasts (15). In contrast, OATP1c1 and MCT8 expression did not increase following LPS in any cell type in the brain, and markedly decreased in cells of brain blood vessels. The opposite regulation of D2 and OATP1c1/MCT8 by inflammation raises the possibility that during inflammation, TH availability may not be uniform throughout the brain. The down-regulation of OATP1c1 and MCT8 at the blood-brain barrier suggests decreased TH availability within the brain parenchyma, whereas induction of D2 suggests increased TH availability in the leptomeninges and choroid plexus (15). Therefore, inflammation may result in both hypothyroid and hyperthyroid compartments in the rat brain. As we previously hypothesized (15), increased TH levels in the leptomeninges and choroid plexus, where the proinflammatory reaction is the most intense following LPS administration (42–47), may serve to control inflammatory processes and improve macrophage function (6, 8, 16–18). It can be also speculated, however, that at the same time, decreased TH levels might be adaptive for cells in the brain parenchyma. Although additional research will be necessary to understand the pathophysiological role of these changes, the present findings underscore that TH availability may differ significantly for different cell types of a single organ during the nonthyroidal illness syndrome.
In conclusion, OATP1c1 and MCT8 expression is down-regulated at the blood-brain barrier during inflammation, suggesting decreased TH uptake into the rodent brain. TH uptake studies in inflammation models will be essential in the future to test the importance of TH transporter down-regulation on TH delivery to the brain.
Acknowledgments
We thank Dr Theo Visser for generously providing the MCT8 and OATP1c1 antisera.
This work was supported by National Institutes of Health Grant DK-37021, a grant from the Dr Gerald J. and Dorothy R. Friedman New York Foundation for Medical Research, EU FP7 contract Switchbox (Grant No. 259772), the Hungarian National Brain Research and Lendület Programs and OTKA109415.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- D2
- type 2 deiodinase
- GFAP
- glial fibrillary acidic protein
- LPS
- lipopolysaccharide
- MCT8
- monocarboxylate transporter 8
- OATP1c1
- organic anion-transporting polypeptide 1c1
- TH
- thyroid hormone.
References
- 1. Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: Local thyroid hormone metabolism during inflammation and infection. Endocr Rev. 2011;32:670–693. [DOI] [PubMed] [Google Scholar]
- 2. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35:159–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van den Berghe G. Non-thyroidal illness in the ICU: A Syndrome with different faces. Thyroid. 2014;24:1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gereben B, Zavacki AM, Ribich S, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Visser TJ. Thyroid hormone transporters and resistance. Endocr Dev. 2013;24:1–10. [DOI] [PubMed] [Google Scholar]
- 6. Kwakkel J, Surovtseva OV, de Vries EM, Stap J, Fliers E, Boelen A. A novel role for the thyroid hormone-activating enzyme type 2 deiodinase in the inflammatory response of macrophages. Endocrinology. 2014;155:2725–2734. [DOI] [PubMed] [Google Scholar]
- 7. de Vries EM, Kwakkel J, Eggels L, Kalsbeek A, Barrett P, Fliers E, Boelen A. NFκB signaling is essential for the lipopolysaccharide-induced increase of type 2 deiodinase in tanycytes. Endocrinology. 2014;155:2000–2008. [DOI] [PubMed] [Google Scholar]
- 8. Barca-Mayo O, Liao XH, DiCosmo C, et al. Role of type 2 deiodinase in response to acute lung injury (ALI) in mice. Proc Natl Acad Sci U S A. 2011;108:E1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boelen A, Kwakkel J, Chassande O, Fliers E. Thyroid hormone receptor β mediates acute illness-induced alterations in central thyroid hormone metabolism. J Neuroendocrinol. 2009;21:465–472. [DOI] [PubMed] [Google Scholar]
- 10. Boelen A, Kwakkel J, Thijssen-Timmer DC, Alkemade A, Fliers E, Wiersinga WM. Simultaneous changes in central and peripheral components of the hypothalamus-pituitary-thyroid axis in lipopolysaccharide-induced acute illness in mice. J Endocrinol. 2004;182:315–323. [DOI] [PubMed] [Google Scholar]
- 11. Fekete C, Gereben B, Doleschall M, et al. Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: Implications for the nonthyroidal illness syndrome. Endocrinology. 2004;145:1649–1655. [DOI] [PubMed] [Google Scholar]
- 12. Kwakkel J, Chassande O, van Beeren HC, Wiersinga WM, Boelen A. Lacking thyroid hormone receptor beta gene does not influence alterations in peripheral thyroid hormone metabolism during acute illness. J Endocrinol. 2008;197:151–158. [DOI] [PubMed] [Google Scholar]
- 13. Kwakkel J, van Beeren HC, Ackermans MT, et al. Skeletal muscle deiodinase type 2 regulation during illness in mice. J Endocrinol. 2009;203:263–270. [DOI] [PubMed] [Google Scholar]
- 14. Ma SF, Xie L, Pino-Yanes M, et al. Type 2 deiodinase and host responses of sepsis and acute lung injury. Am J Respir Cell Mol Biol. 2011;45:1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wittmann G, Harney JW, Singru PS, et al. Inflammation-inducible type 2 deiodinase expression in the leptomeninges, choroid plexus, and at brain blood vessels in male rodents. Endocrinology. 2014;155:2009–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y, Sjolinder M, Wang X, et al. Thyroid hormone enhances nitric oxide-mediated bacterial clearance and promotes survival after meningococcal infection. PLoS One. 2012;7:e41445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cheng AW, Bolognesi M, Kraus VB. DIO2 modifies inflammatory responses in chondrocytes. Osteoarthritis Cartilage. 2012;20:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perrotta C, Buldorini M, Assi E, et al. The thyroid hormone triiodothyronine controls macrophage maturation and functions: Protective role during inflammation. Am J Pathol. 2014;184:230–247. [DOI] [PubMed] [Google Scholar]
- 19. Castro I, Quisenberry L, Calvo RM, Obregon MJ, Lado-Abeal J. Septic shock non-thyroidal illness syndrome causes hypothyroidism and conditions for reduced sensitivity to thyroid hormone. J Mol Endocrinol. 2013;50:255–266. [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez-Perez A, Palos-Paz F, Kaptein E, et al. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clin Endocrinol (Oxf). 2008;68:821–827. [DOI] [PubMed] [Google Scholar]
- 21. Mebis L, Debaveye Y, Ellger B, et al. Changes in the central component of the hypothalamus-pituitary-thyroid axis in a rabbit model of prolonged critical illness. Crit Care. 2009;13:R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kinne A, Schulein R, Krause G. Primary and secondary thyroid hormone transporters. Thyroid Res. 2011;4 Suppl 1:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muller J, Heuer H. Expression pattern of thyroid hormone transporters in the postnatal mouse brain. Front Endocrinol (Lausanne). 2014;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayerl S, Müller J, Bauer R, et al. Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest. 2014;124:1987–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heuer H, Maier MK, Iden S, et al. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology. 2005;146:1701–1706. [DOI] [PubMed] [Google Scholar]
- 26. Roberts LM, Woodford K, Zhou M, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149:6251–6261. [DOI] [PubMed] [Google Scholar]
- 27. Schnell C, Shahmoradi A, Wichert SP, et al. The multispecific thyroid hormone transporter OATP1C1 mediates cell-specific sulforhodamine 101-labeling of hippocampal astrocytes. Brain Struct Funct. 2015;220:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sugiyama D, Kusuhara H, Taniguchi H, et al. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: High affinity transporter for thyroxine. J Biol Chem. 2003;278:43489–43495. [DOI] [PubMed] [Google Scholar]
- 29. Tohyama K, Kusuhara H, Sugiyama Y. Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood-brain barrier. Endocrinology. 2004;145:4384–4391. [DOI] [PubMed] [Google Scholar]
- 30. Wirth EK, Roth S, Blechschmidt C, et al. Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J Neurosci. 2009;29:9439–9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology. 2006;147:4036–4043. [DOI] [PubMed] [Google Scholar]
- 32. Trajkovic M, Visser TJ, Mittag J, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117:627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ceballos A, Belinchon MM, Sanchez-Mendoza E, et al. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3′-triiodo-L-thyronine. Endocrinology. 2009;150:2491–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mayerl S, Visser TJ, Darras VM, Horn S, Heuer H. Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology. 2012;153:1528–1537. [DOI] [PubMed] [Google Scholar]
- 35. Bianco AC, Anderson G, Forrest D, et al. American thyroid association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24:88–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521:3287–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kallo I, Mohacsik P, Vida B, et al. A novel pathway regulates thyroid hormone availability in rat and human hypothalamic neurosecretory neurons. PLoS One. 2012;7:e37860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grijota-Martínez C, Díez D, Morreale de Escobar G, Bernal J, Morte B. Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology. 2011;152:1713–1721. [DOI] [PubMed] [Google Scholar]
- 39. Dratman MB, Crutchfield FL, Schoenhoff MB. Transport of iodothyronines from bloodstream to brain: Contributions by blood:brain and choroid plexus:cerebrospinal fluid barriers. Brain Res. 1991;554:229–236. [DOI] [PubMed] [Google Scholar]
- 40. van der Deure WM, Appelhof BC, Peeters RP, et al. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin Endocrinol (Oxf). 2008;69:804–811. [DOI] [PubMed] [Google Scholar]
- 41. Zeöld A, Doleschall M, Haffner MC, et al. Characterization of the nuclear factor-kappa B responsiveness of the human dio2 gene. Endocrinology. 2006;147:4419–4429. [DOI] [PubMed] [Google Scholar]
- 42. Elmquist JK, Breder CD, Sherin JE, et al. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381:119–129. [DOI] [PubMed] [Google Scholar]
- 43. Lacroix S, Feinstein D, Rivest S. The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol. 1998;8:625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J Neurochem. 1998;70:452–466. [DOI] [PubMed] [Google Scholar]
- 45. Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–293. [DOI] [PubMed] [Google Scholar]
- 46. Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: A view from the blood-brain barrier. Neuroscience. 1999;93:1449–1464. [DOI] [PubMed] [Google Scholar]
- 47. Nadeau S, Rivest S. Regulation of the gene encoding tumor necrosis factor alpha (TNF-alpha) in the rat brain and pituitary in response in different models of systemic immune challenge. J Neuropathol Exp Neurol. 1999;58:61–77. [DOI] [PubMed] [Google Scholar]