Abstract
Patients with cancer are at increased risk of venous thromboembolism (vte). Anticoagulation therapy has been shown to prevent vte; however, unique clinical circumstances in patients with cancer can often complicate the decisions surrounding the administration of prophylactic anticoagulation. No national Canadian guidelines on the prevention of cancer-associated thrombosis have been published. We therefore aimed to develop a consensus-based, evidence-informed guideline on the topic.
PubMed was searched for clinical trials and meta-analyses published between 2002 and 2013. Reference lists of key articles were hand-searched for additional publications. Content experts from across Canada were assembled to review the evidence and make recommendations.
Low molecular weight heparin can be used prophylactically in cancer patients at high risk of developing vte. Direct oral anticoagulants are not recommended for vte prophylaxis at this time. Specific clinical scenarios, including renal insufficiency, thrombocytopenia, liver disease, and obesity can warrant modifications in the administration of prophylactic anticoagulant therapy. There is no evidence to support the monitoring of anti–factor Xa levels in clinically stable cancer patients receiving prophylactic anticoagulation; however, factor Xa levels could be checked at baseline and periodically in patients with renal insufficiency. The use of anticoagulation therapy to prolong survival in cancer patients without the presence of risk factors for vte is not recommended.
Keywords: Low molecular weight heparin, prophylaxis, anticoagulation, venous thromboembolism, pulmonary embolism, deep vein thrombosis, clots, practice guideline
1. INTRODUCTION
Patients with cancer are at increased risk of developing venous thromboembolism (vte), including deep vein thrombosis (dvt) and pulmonary embolism1,2. Hypercoagulability in this population can occur as a result of cancer treatment (particularly chemotherapy) and of the cancer itself3,4. Compared with patients having local disease, those with metastatic disease have a significantly higher risk of developing vte5.
The approximate annual incidence of vte in cancer patients treated with chemotherapy is estimated at 1 in 200; in the general population, the estimate is 117 in 100,0006. Importantly, data show that the incidence of vte in cancer patients is on the rise, possibly because of longer survival and the older age of cancer patients7. Table i summarizes several patient-, cancer-, and treatment-related factors that adversely affect the risk of vte8,9. The risk of dying after an acute thrombotic event is higher by a factor of 4 to 8 in cancer patients than in patients without cancer, and vte is the second-leading cause of non-cancer death in cancer patients8–13.
TABLE I.
Factors associated with venous thromboembolism in patients with cancer
| Category | Factors |
|---|---|
| Patient-related |
|
| Cancer-related |
|
| Treatment-related |
|
| Biochemical |
|
Although several pharmacologic agents are available to prevent vte, administration of anticoagulants is not always straightforward in the oncology setting. Patients with cancer undergo complex treatment protocols and could have other comorbidities such as renal or hepatic insufficiency and thrombocytopenia that can affect the efficacy and safety of anticoagulation. Several groups have published guidelines on the management of vte in oncology patients—most recently, the American Society of Clinical Oncology14 and CancerControl Alberta (part of Alberta Health Services)15. Other published guidelines include those from the U.S. National Comprehensive Cancer Network16, the European Society for Medical Oncology17, and the American College of Chest Physicians18.
Despite the wealth of guidelines, several key issues surrounding the use of prophylactic anticoagulation and its monitoring in specific subpopulations have not been addressed. Moreover, most of the existing guidelines are largely general oncology guidelines with a subsection on vte. No national guidelines are specifically dedicated to the prophylaxis of cancer-associated vte.
Our overall aim was to develop national recommendations that are evidence-based (or consensus-based where evidence is lacking) on the prevention of vte in patients with cancer. The recommendations are meant to provide guidance to physicians, nurses, and other frontline medical professionals involved in the management of patients with cancer. We address vte prophylaxis in ambulatory, hospitalized, and surgical patients, and the use of anticoagulation in specific clinical scenarios such as renal and hepatic insufficiency, brain metastases, and thrombocytopenia. We also discuss the use of anticoagulation to prolong survival and the monitoring of anticoagulation therapy.
2. METHODS
2.1. Literature Search Strategy
The U.S. National Library of Medicine’s PubMed database was searched for relevant articles published between 2002 and March 2013. Search terms included “neoplasm” or “cancer” and “thrombosis prophylaxis” or “vte prophylaxis,” and results were limited to randomized controlled trials (rcts) and meta-analyses published between 2008 and March 2013. Trials that did not report outcomes related to the prophylaxis of vte were excluded. In addition, the U.S. National Guidelines Clearinghouse was searched for guidelines published between 2007 and March 2013. Updated results of relevant clinical trials published after March 2013 were also included. Because of a lack of translation services, non-English-language articles were excluded from the review of the evidence.
2.2. Development of Recommendations
The development and review process for the recommendations was modelled after these sources: the U.K. National Institute for Health and Clinical Excellence19, the Archives of Pediatrics and Adolescent Medicine20, and the agree collaboration21. Clinical questions and initial recommendations were developed by two medical oncologists (JCE and PK) and a cancer research methodologist (MAS) based on clinical experience and the literature review. The University of Oxford Centre for Evidence-Based Medicine grading system was used to grade the recommendations22. Briefly, the levels of evidence were these:
Level 1: a systematic review of homogenous rcts or a single rct with a narrow confidence interval
Level 2: a systematic review of homogenous cohort studies, or an individual cohort study or a low-quality rct
Level 3: a systematic review of case–control studies or an individual case–control study
Level 4: case series and poor-quality cohort and case–control studies
Level 5: expert opinion without explicit critical appraisal
These grades were defined:
Grade A: consistent level 1 studies
Grade B: consistent level 2 or 3 studies or extrapolations from level 1 studies
Grade C: level 4 studies or extrapolations from level 2 or 3 studies
Grade D: inconsistent or inconclusive studies of any level
The recommendations were reviewed by an expert panel of medical oncologists, hematologic oncologists, hematologists, and an internist, representing the provinces of Nova Scotia, Quebec, Ontario, Manitoba, Saskatchewan, Alberta, and British Columbia. A total of 11 specialists contributed directly to the development of all recommendations. Recommendations pertaining to renal insufficiency were further reviewed by a nephrologist and a pharmacist with expertise in renal insufficiency.
Recommendations were initially reviewed using a Web-based survey to capture the level of agreement with each statement on a 5-point scale ranging from “strongly agree” to “strongly disagree” and including an option of “unsure.” An evidence summary accompanied each statement, and panelists were instructed to consider the level of evidence when rating each statement. In addition to the rating scales, panelists were given the opportunity to comment on each statement. Based on panelist responses, recommendations were categorized as “consensus” (that is, statements with which most panelists agreed, with no more than 3 “neutral” or “unsure” responses allowed) or “non-consensus” (that is, statements with which at least 1 panelist disagreed or those that had 4 or more “neutral” or “unsure” responses). Non-consensus statements were reviewed once with the entire panel via webinar (Cisco WebEx, San Jose, CA, U.S.A.) to better understand the rationale for any disagreement or uncertainty and to determine where additional discussion was needed to reach consensus. The non-consensus statements were divided into two categories: “monitoring and dosing” and “special populations.” Panel members were assigned to working groups to address statements in one of the two categories. Working groups met a final time via webinar to discuss and revise the statements; consensus methods were used.
3. RESULTS
The literature review identified sixty-five publications, including three clinical practice guidelines. Meta-analyses and rcts were considered strong (higher-level) evidence in developing the recommendations. Several relevant retrospective case series were also included in the discussion, but were considered to be weak (lower-level) evidence. Based on the Web survey responses, consensus was reached immediately on 15 of the 22 final recommendation statements (68%). The remaining 7 recommendations were further discussed by the assigned panel members to reach consensus. Consensus was eventually reached for all 22 recommendation statements (Table ii).
TABLE II.
Guideline questions and recommendations related to the prophylaxis of venous thromboembolism (vte)
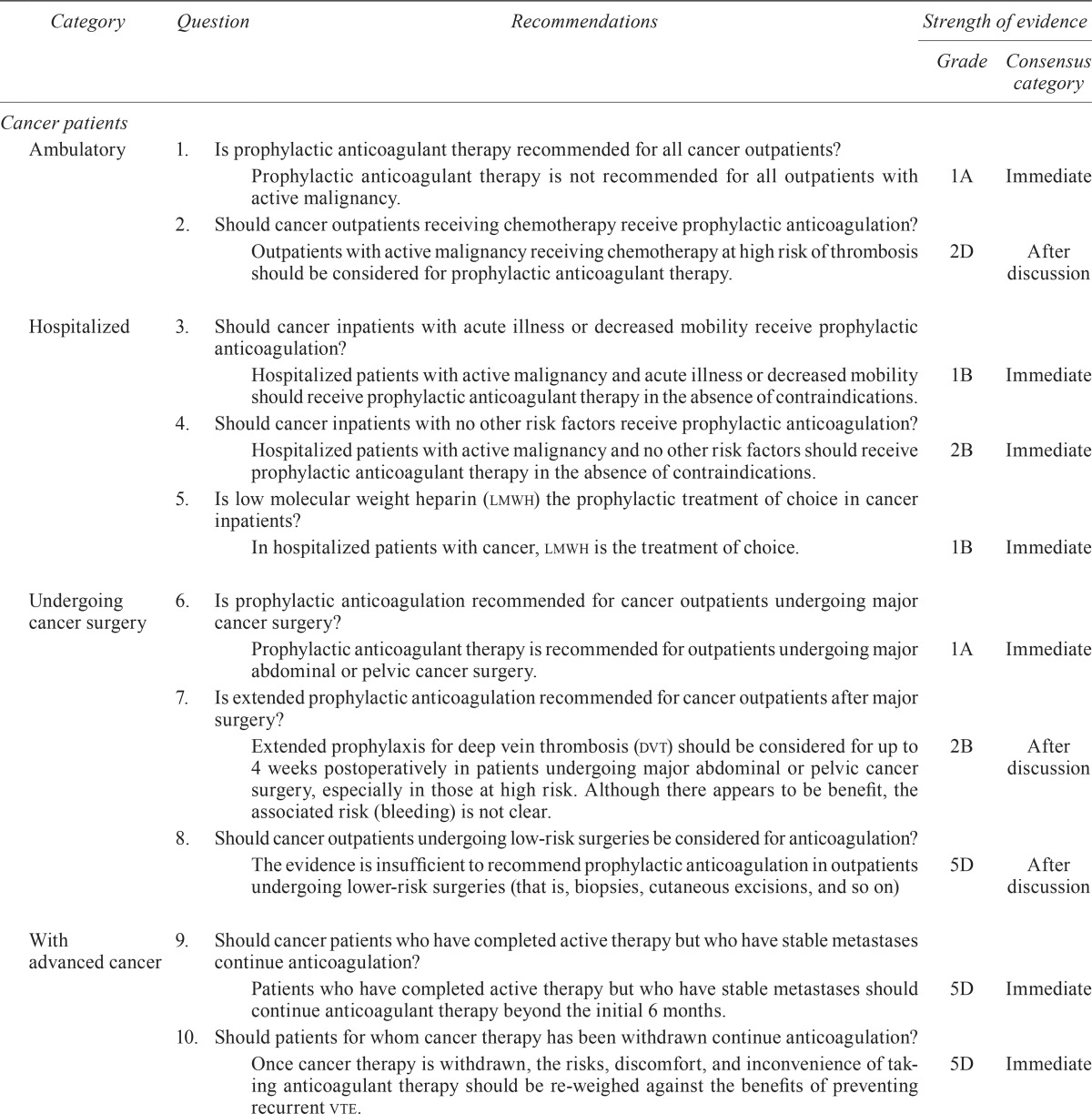
| Category | Question | Recommendations | Strength of evidence | |
|---|---|---|---|---|
|
| ||||
| Grade | Consensus category | |||
| Cancer patients | ||||
| Ambulatory | 1. | Is prophylactic anticoagulant therapy recommended for all cancer outpatients? | ||
| Prophylactic anticoagulant therapy is not recommended for all outpatients with active malignancy. | 1A | Immediate | ||
| 2. | Should cancer outpatients receiving chemotherapy receive prophylactic anticoagulation? | |||
| Outpatients with active malignancy receiving chemotherapy at high risk of thrombosis should be considered for prophylactic anticoagulant therapy. | 2D | After discussion | ||
| Hospitalized | 3. | Should cancer inpatients with acute illness or decreased mobility receive prophylactic anticoagulation? | ||
| Hospitalized patients with active malignancy and acute illness or decreased mobility should receive prophylactic anticoagulant therapy in the absence of contraindications. | 1B | Immediate | ||
| 4. | Should cancer inpatients with no other risk factors receive prophylactic anticoagulation? | |||
| Hospitalized patients with active malignancy and no other risk factors should receive prophylactic anticoagulant therapy in the absence of contraindications. | 2B | Immediate | ||
| 5. | Is low molecular weight heparin (lmwh) the prophylactic treatment of choice in cancer inpatients? | |||
| In hospitalized patients with cancer, lmwh is the treatment of choice. | 1B | Immediate | ||
| Undergoing cancer surgery | 6. | Is prophylactic anticoagulation recommended for cancer outpatients undergoing major cancer surgery? | ||
| Prophylactic anticoagulant therapy is recommended for outpatients undergoing major abdominal or pelvic cancer surgery. | 1A | Immediate | ||
| 7. | Is extended prophylactic anticoagulation recommended for cancer outpatients after major surgery? | |||
| Extended prophylaxis for deep vein thrombosis (dvt) should be considered for up to 4 weeks postoperatively in patients undergoing major abdominal or pelvic cancer surgery, especially in those at high risk. Although there appears to be benefit, the associated risk (bleeding) is not clear. | 2B | After discussion | ||
| 8. | Should cancer outpatients undergoing low-risk surgeries be considered for anticoagulation? | |||
| The evidence is insufficient to recommend prophylactic anticoagulation in outpatients undergoing lower-risk surgeries (that is, biopsies, cutaneous excisions, and so on) | 5D | After discussion | ||
| With advanced cancer | 9. | Should cancer patients who have completed active therapy but who have stable metastases continue anticoagulation? | ||
| Patients who have completed active therapy but who have stable metastases should continue anticoagulant therapy beyond the initial 6 months. | 5D | Immediate | ||
| 10. | Should patients for whom cancer therapy has been withdrawn continue anticoagulation? | |||
| Once cancer therapy is withdrawn, the risks, discomfort, and inconvenience of taking anticoagulant therapy should be re-weighed against the benefits of preventing recurrent vte. | 5D | Immediate | ||
| With special clinical scenarios | 11. | Should cancer outpatients with a central venous catheter (cvc) and no other risk factors receive prophylactic anticoagulation? | ||
| A cvc alone is not an indication for prophylactic anticoagulation therapy in outpatients with active malignancy. | 1B | Immediate | ||
| 12. | Are there special considerations for elderly patients receiving prophylactic anticoagulant therapy? | |||
| Elderly patients more than 70 years of age with reduced creatinine clearance could be at greater risk of lmwh-induced complications such as bleeding. | 4D | Immediate | ||
| 13. | Is there a preferred prophylactic anticoagulant therapy for elderly patients with active malignancy? | |||
| There is no high-level evidence to recommend one lmwh or unfractionated heparin (ufh) over another in elderly patients with active malignancy. | 2B | After discussion | ||
| 14. | Is there a preferred prophylactic anticoagulant therapy for patients with impaired renal function? | |||
| There is no high level evidence to recommend one lmwh or ufh over another in patients with impaired renal function. Enoxaparin might have a less favourable biologic profile than tinzaparin and dalteparin in patients with impaired renal function. | 2B | Immediate | ||
| 15. | Should patients with persistent or severe thrombocytopenia receive prophylactic anticoagulation? | |||
| Patients with persistent or severe thrombocytopenia should be referred to a hematologist or thrombosis expert where possible. | 5D | After discussion | ||
| In patients with significant thrombocytopenia, lmwh or ufh is preferred over vitamin K agonists if anticoagulation is necessary. | 5D | After discussion | ||
| 16. | Can prophylactic anticoagulant therapy be used in patients with central nervous system malignancy with vte? | |||
| Anticoagulant therapy can be used in patients with central nervous system malignancy. | 4D | Immediate | ||
| 17. | Are there special considerations for the administration of prophylactic anticoagulation in obese cancer patients? | |||
| Administration of lmwh should be based on actual body weight rather than ideal body weight. | 2C | Immediate | ||
| Prophylactic therapy | ||||
| Preferred type | 18. | What is the preferred lmwh for vte prophylaxis in the cancer outpatient setting? | ||
| There is no preferred lmwh for vte prophylaxis in cancer outpatients; the choice of anticoagulant is at the discretion of the treating physician. | 5D | After discussion | ||
| 19. | Can direct oral anticoagulant agents (that is, apixaban, dabigatran, rivaroxaban) be used for the prophylaxis of cancer-associated thrombosis? | |||
| Direct oral anticoagulant agents (that is, apixaban, dabigatran, rivaroxaban) have not yet been proved to be efficacious or safe in oncology patients and are currently not recommended for the prophylaxis of cancer-associated thrombosis. | 2C | Immediate | ||
| To improve overall survival | 20. | Should anticoagulant therapy be used to prolong survival in cancer patients without the presence of risk factors for vte? | ||
| The use of adjuvant anticoagulant therapy in patients without established vte or unselected (low-risk) patients to prolong survival is not recommended. | 2B | Immediate | ||
| Monitoring | 21. | Should levels of anti–factor Xa be monitored in patients receiving prophylactic anticoagulation? | ||
| Monitoring of anti–factor Xa is generally not recommended in most patients receiving prophylactic anticoagulation. | 1A | Immediate | ||
| 22. | Should levels of anti–factor Xa be monitored in patients with renal insufficiency receiving prophylactic anticoagulation? | |||
| Anti–factor Xa could be checked at baseline and periodically in patients with renal insufficiency at the discretion of the treating physician, with clinical correlation. | 5C | After discussion | ||
4. DISCUSSION
Patients with active malignancy are at increased risk of vte and increased risk of vte-related mortality10,12,13. Successful prevention of vte is a high priority, and dvt prophylaxis in the general population has been well established in multiple medical and surgical populations18.
4.1. Prophylaxis in Ambulatory Cancer Patients
None of the current guidelines recommend routine thromboprophylaxis in ambulatory cancer patients, but all suggest that it be considered for very select high-risk patients14,16,17. The outpatient prophylaxis score developed by Khorana et al.4 (Table iii) and validated in randomized trials identifies cancer patients at risk for vte. Anticoagulants tested in cancer patients include dalteparin, enoxaparin, tinzaparin, semuloparin, certoparin, bemiparin, nadroparin, and warfarin23–42, of which enoxaparin, dalteparin, and tinzaparin are readily available in Canada. A Cochrane systematic review43 of 21 rcts that included 9861 ambulatory patients with cancer receiving chemotherapy showed that, compared with inactive control and warfarin, low molecular weight heparin (lmwh) was associated with a 45% reduction in the overall vte incidence [risk ratio: 0.55; 95% confidence interval (ci): 0.34 to 0.88; p < 0.05] and a nonsignificant increase in bleeding. Despite those very interesting findings, which accord with other major guidelines, our consensus was that prophylactic anticoagulation is not advised for all outpatients, but can be used in select cases where indicated.
TABLE III.
Predictive model for chemotherapy-associated venous thromboembolism4
| Patient characteristic | Risk scorea |
|---|---|
| Site of cancer | |
| Very high risk (cancers of the stomach, pancreas, and brain) | 2 |
| High risk (cancers of the lung, bladder, and testes; lymphoma; gynecologic malignancies) | 1 |
| Pre-chemotherapy platelet count ≥ 350,000/μL | 1 |
| Hemoglobin < 10 g/dL or use red blood cell growth factors | 1 |
| Pre-chemotherapy leucocyte count > 11,000/μL | 1 |
| Body mass index ≥ 35 kg/m2 | 1 |
A score of 3 or greater is indicative of a 7% risk (for example, high risk); a score of 1–2 is indicative of a 2% risk; a score of 0 is indicative of a 0.5% risk.
4.2. Prophylaxis in Hospitalized Cancer Patients
Most hospitalized patients with cancer require thromboprophylaxis throughout hospitalization14–16,18. A systematic review comparing lmwh, unfractionated heparin (ufh), and placebo in medically ill patients (6.7% with current or previous cancer) demonstrated lower rates of dvt with lmwh than with placebo [odds ratio (or): 0.60; 95% ci: 0.47 to 0.75], but no difference when lmwh was compared with ufh (or: 0.92; 95% ci: 0.56 to 1.52). The groups showed no differences in rates of death, vte, or bleeding44. The exclaim trial, which compared lmwh (enoxaparin) with placebo in 5963 acutely ill inpatients (1.6% cancer patients), showed a nonsignificant reduction (2.5% vs. 4.0%) in the rate of vte events with lmwh. The rate of major bleeding was slightly higher with lmwh, but not statistically different from the rate with placebo37. However, the medenox trial, which compared lmwh (enoxaparin) with placebo in 1102 hospitalized patients, showed a significant reduction in the incidence of vte with lmwh (5.5% vs. 14.9%, p < 0.001)45.
The lmwhs have not been compared head-to-head exclusively in cancer patients, but in other populations, no advantages in vte incidence or bleeding rates were observed for any one agent46–51. Unfortunately, a need for cancer-specific dvt prophylaxis trials remains. Recommendations for dvt prophylaxis in cancer inpatients are typically extrapolated from non-cancer-specific dvt prophylaxis trials; only subgroup analyses of non-cancer specific trials are currently available. A meta-analysis by Carrier et al.52 of all available data on cancer inpatients showed no significant reduction in the occurrence of vte with either lmwh or fondaparinux prophylaxis [relative risk (rr): 0.91; 95% ci: 0.21 to 4.0]. Although dvt prophylaxis is likely effective in cancer populations because of a higher rate of vte, cancer-specific trials are necessary to help select appropriate patients and effective regimens of dvt prophylaxis.
4.3. Prophylaxis in Patients Undergoing Cancer Surgery
Consensus recommendations support vte prophylaxis in patients undergoing major cancer surgery. That consensus is based on the increased risk of vte in patients undergoing surgery that is at least 1 hour in length (Table i). Including a 2011 Cochrane meta-analysis of 16 rcts with 11,847 patients53, there are data, all relating to preoperative prophylactic anticoagulation, that show neither a beneficial nor a harmful effect of lmwh compared with ufh in terms of mortality, symptomatic dvt, pulmonary embolism, and minor or major bleeding. However, the analyses included data from patients enrolled in non-cancer-specific trials and were based largely on older data using positive screening radiologic imaging events as an endpoint. The canbesure trial, published in 2010, compared bemiparin with placebo in 625 cancer surgery patients and found that the rate of vte was significantly less in patients treated with bemiparin (0.8% vs. 4.6%, p = 0.01)29. The use of anticoagulation in patients undergoing major cancer surgery has been endorsed elsewhere14.
Current guidelines recommend extending postoperative prophylaxis for up to 4 weeks in patients undergoing abdominal or pelvic cancer surgery14–18. A meta-analysis comparing the extended use of lmwh (3–4 weeks after surgery) with conventional in-hospital prophylaxis (for the period of time in hospital) evaluated data from patients undergoing major abdominal surgery. The analyzed trials showed that extended prophylaxis significantly reduced the incidence of vte (rr: 0.44; 95% ci: 0.28 to 0.70; p < 0.05), dvt (rr: 0.46; 95% ci: 0.29 to 0.74; p < 0.05), and proximal dvt (rr: 0.24; 95% ci: 0.09 to 0.67; p < 0.05), with no significant differences in major or minor bleeding54. Using data from four trials, a Cochrane meta-analysis comparing prolonged lmwh thromboprophylaxis with control treatment or placebo showed that prolonged thromboprophylaxis with lmwh was associated with a 78% lower risk of developing symptomatic vte (or: 0.22; 95% ci: 0.06 to 0.80; p = 0.02)55. In both meta-analyses, the trials were non-cancer-specific, and the primary endpoint was positive radiologic screening, with only a few symptomatic events noted.
Currently, only limited data are available from investigations of the role of anticoagulant therapy in patients undergoing low-risk cancer surgery. Risk factors for vte in patients undergoing outpatient surgery include an operative time greater than 120 minutes (or: 1.69; p = 0.027), arthroscopic surgery (or: 5.16; p < 0.001), saphenofemoral junction surgery (or: 13.20; p < 0.001), and venous surgery not involving the great saphenous vein (or: 15.61; p < 0.001)56. However, data on the use of anticoagulation in those patients are lacking.
4.4. Preferred Prophylactic Therapy
The preferred anticoagulation therapy in the prophylactic setting is lmwh. There are no clinical data comparing tinzaparin, enoxaparin, and dalteparin in the prophylactic setting in patients with active malignancy, patients undergoing non-orthopedic surgery, or acutely ill patients. Based on data from hospitalized non-cancer patients indicating similar efficacy and bleeding rates47–50, it is reasonable to suggest that no particular lmwh is superior to another in that setting. Dalteparin is typically dosed subcutaneously at 5000 U daily25,27. Enoxaparin is typically dosed subcutaneously at 40 mg daily or, in patients with severe renal impairment, at 30 mg daily35,37,38,57. Tinzaparin is typically dosed subcutaneously at 4500 U daily or 75 U/kg daily58.
4.5. Prophylaxis in Special Clinical Scenarios
4.5.1. Patients with a Central Venous Catheter
The role of anticoagulation in the prevention of central venous catheter (cvc)–related thrombosis has been investigated39,59. Although anticoagulants have been shown not to increase the risk of bleeding, three systematic reviews and a meta-analysis failed to show that they lower the incidence of symptomatic cvc-related thrombosis60–63. Thromboprophylaxis for a cvc is therefore not recommended14–17.
4.5.2. Patients with Renal Insufficiency
In patients with impaired renal function (creatinine clearance ≤ 30 mL/min), lmwh can accumulate as a result of reduced excretion, which could result in an increased risk of bleeding. Prophylactic lmwh should therefore be used with caution in patients with renal impairment64. Tinzaparin has the highest average molecular weight (6500 Da) of the available lmwhs, followed by dalteparin (6000 Da). Enoxaparin (4500 Da) is the smallest and most renal-dependent lmwh. In contrast, by virtue of its larger size, tinzaparin appears to be less dependent on renal clearance and more dependent on the reticuloendothelial system65. Tinzaparin might therefore be preferable in patients with renal insufficiency.
4.5.3. Elderly Patients
There are limited data and no rcts comparing tinzaparin, enoxaparin, and dalteparin with each other or with ufh or coumadin in elderly patients with cancer. The direct study included critically ill patients (n = 138) with a creatinine clearance less than 30 mL/min given dalteparin (5000 IU daily) in the prophylactic setting. Bioaccumulation (that is, a factor Xa level > 0.40 IU/mL) was observed in no patients (0%; 95% ci: 0% to 3.0%) and the median trough level of factor Xa was below the detectable limit (<0.10 IU/mL)66. A rct that enrolled patients with a median creatinine clearance of 34.7 ± 11.4 mL/min randomized to enoxaparin (40 mg) or to tinzaparin once daily in the prophylactic setting found that factor Xa did not accumulate significantly for tinzaparin, but did accumulate for enoxaparin (p < 0.0001)67. None of the available data are cancer-specific.
4.5.4. Patients with Thrombocytopenia
No trials have evaluated bleeding risk relative to platelet count in any population of patients requiring prophylactic anticoagulation. In general, thrombocytopenic patients are excluded from anticoagulation trials. Expert opinion would suggest that the bleeding risk is negligible in patients with an isolated thrombocytopenia when platelets number 50,000/μL or more; however, spontaneous bleeding, including fatal intracranial hemorrhage, is typically felt to be more relevant in patients with a platelet count below 20,000/μL. The American Society of Clinical Oncology guidelines do not recommend prophylaxis with anticoagulants in patients with a platelet count below 50,000/μL14, but many experts in the field suggest that prophylactic anticoagulation can rationally be used for specific patients with platelet counts as low as 20,000/μL68–70. In such situations, we recommend consultation with thrombosis experts.
4.5.5. Patients with Central Nervous System Malignancy
Anticoagulation is acceptable in patients with central nervous system malignancies, but should be provided at the discretion of the treating physician. The prodige trial randomized 186 adults with malignant glioma to subcutaneous dalteparin (5000 U) or to placebo once daily and reported no difference in the incidence of vte in the first 6 months (9.1% vs. 14.9% respectively; hazard ratio: 0.51; 95% ci: 0.19 to 1.4; p = 0.29). Major bleeding at 12 months was also not significantly different (5.1% vs. 1.2%, p = 0.22)25. A retrospective series of 40 newly diagnosed patients with grade 3 or 4 malignant glioma initiated on a prophylactic dose of daily tinzaparin between 48 hours and 4 weeks postoperatively for a planned duration of 12 months reported grade 4 or 5 central nervous system hemorrhages or grade 2 or greater systemic hemorrhages. Of the 40 patients, 1 developed a dvt while taking tinzaparin, and 3 developed thromboembolic complications while off tinzaparin58. Similar findings from retrospective reviews of bleeding events have been reported elsewhere in patients with central nervous system malignancy receiving prophylactic anticoagulation71,72.
4.5.6. Obese Patients
Patients with a body mass index greater than 30 kg/m2 are considered to be obese73, and the anticoagulation needs of most patients in that range will exceed the highest prefilled syringes of lmwh. A prospective comparison of three enoxaparin dosing regimens for dvt prophylaxis in 531 medically ill patients with extreme obesity (>40 kg/m2) showed that peak factor Xa levels were significantly higher in the higher weight-based dose group (enoxaparin 0.5 mg/kg daily) than in the lower weight-based dose group (enoxaparin 0.4 mg/kg daily) or in the fixed-dose group (40 mg daily). Compared with the lower weight-based dose or fixed-dose groups, the higher weight-based dose group more frequently achieved target factor Xa levels (p < 0.05). No adverse event (bleeding, thrombosis) occurred in any group74.
Weight-adjusted tinzaparin and dalteparin dosing showed a predictable response regardless of body weight or body mass index, without a need for maximal absolute dose capping75,76. Given that no available data suggest increased bleeding rates or other toxicities at higher lmwh doses in obese patients, the consensus recommendation is to avoid dose-capping or the use of ideal body weight for dose calculations; for all lmwhs, use the actual weight-based dose.
4.6. New Oral Anticoagulants
Novel direct oral anticoagulants that have been developed include factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) and thrombin inhibitors (dabigatran). Some of these agents have completed phase iii trials in dvt prophylaxis after hip and knee replacement surgery and for medically ill patients77–79. However, data for cancer-specific populations in those studies are lacking. Data are limited mostly to post hoc analyses of subgroups, which typically constituted only about 5% of the total study population. In addition, variable definitions of cancer were used in each trial, many of which did not reflect accepted definitions of active cancer.
Cancer subgroups from the dvt prophylaxis trials showed a concerning trend toward no efficacy, but increased rates of major bleeding. Compared with a parenteral agent, oral agents could be disadvantaged in patients at increased risk of gastrointestinal dys-function (because of nausea, vomiting, and so on), altered absorption patterns, and drug interactions. All of those limitations highlight the need for more high-quality cancer-specific clinical trials before novel direct oral anticoagulants can be endorsed. Use of such agents is not recommended for cancer-associated thromboprophylaxis.
4.7. Anticoagulation to Improve Overall Survival
It is not recommended that anticoagulation be used to extend survival in patients with cancer in the absence of other indications for anticoagulation14. However, there are interesting data to suggest that a survival benefit might accrue to the use of lmwh in cancer patients. A meta-analysis of 3343 patients from eleven studies of ufh (one trial), lmwh (six trials), and warfarin (four trials) compared anticoagulation treatment with no anticoagulation or placebo for primary vte prophylaxis. The results showed a lower risk of mortality that was statistically significant for lmwh (p = 0.015), but not for ufh (p = 0.095) or warfarin (p = 0.239). With all anticoagulant options, the rate of major bleeding was observed to be increased (p < 0.0001), although the increase was not significant for lmwh on its own (p = 0.128)80. Notably, more than half the included trials had been published more than 15 years earlier.
Two Cochrane reviews of clinical trials assessed cancer survival with the addition of oral anticoagulation (six trials with warfarin, one trial with apixaban)81 or parenteral anticoagulation (one trial with ufh, eight trials with lmwh; 2857 participants in total)82 compared with no anticoagulation or placebo. At 1 year, no mortality benefit was observed for ufh and lmwh; however, at 2 years, a statistically significant mortality benefit emerged (rr: 0.79; 95% ci: 0.67 to 0.93). Major bleeding was increased (rr: 1.30; 95% ci: 0.59 to 2.88), and the difference was statistically significant. Despite those interesting observations, many of the available studies were published more than 15 years ago, included small numbers of patients or represented subgroup or post hoc analyses, or did not focus on survival as the primary outcome. The cancer types studied were also limited, and so results might not apply broadly. Finally, major bleeding rates were uniformly elevated. Until more rigorous randomized trials using modern cancer therapies and anticoagulation regimens are conducted in future, anticoagulation to extend survival in patients with cancer is not recommended83.
5. SUMMARY
This is the first national Canadian guideline on the prophylaxis of vte in cancer patients. Patients with cancer are at increased risk of vte. Prophylactic antithrombotic therapy with lmwh can greatly reduce the risk of vte, particularly for hospitalized cancer patients. Some subgroups of patients with cancer, including those with thrombocytopenia, renal insufficiency, and obesity might require modifications of the anticoagulant regimen. Use of direct oral anticoagulants for prophylaxis is not supported at the present time.
6. ACKNOWLEDGMENTS
Three companies were approached for funding to complete this work. Sanofi and Leo Pharma both provided unrestricted educational grants.
7. CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: JCE has received honoraria from Leo Pharma, Sanofi, and Pfizer. CMJW has received honoraria from Leo Pharma and Pfizer. PK has received educational grants from Sanofi and Leo Pharma and has also served on the Leo Pharma advisory board. SS has received honoraria from Leo Pharma and Pfizer. VT has received honoraria and unrestricted grants from Sanofi and Pfizer. HJL has received honoraria from Sanofi. No other author had a conflict to declare.
8. REFERENCES
- 1.Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125:490–3. doi: 10.1016/j.thromres.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruf W. Hemostasis and angiogenesis. In: Khorana AA, Francis CW, editors. Cancer-Associated Thrombosis: New Findings in Translational Science, Prevention, and Treatment. New York, NY: Informa Healthcare USA; 2007. pp. 17–34. [DOI] [Google Scholar]
- 3.Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275. doi: 10.1371/journal.pmed.1001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–47. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickmann B, Ahlbrecht J, Ay C, et al. Regional lymph node metastases are a strong risk factor for venous thromboembolism: results from the Vienna Cancer and Thrombosis Study. Haematologica. 2013;98:1309–14. doi: 10.3324/haematol.2012.073338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 7.Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer—a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–13. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Khorana AA. Risk assessment and prophylaxis for vte in cancer patients. J Natl Compr Canc Netw. 2011;9:789–97. doi: 10.6004/jnccn.2011.0064. [DOI] [PubMed] [Google Scholar]
- 9.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AY, Levine MN. Thromboembolism and cancer: risks and outcomes. Circulation. 2003;107:1–17. doi: 10.1161/01.CIR.0000078466.72504.AC. [DOI] [PubMed] [Google Scholar]
- 11.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–4. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 12.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–5. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH, Khorana AA, Kuderer NM, et al. on behalf of the American Society of Clinical Oncology Clinical Practice Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 15.Shea–Budgell MA, Wu CM, Easaw JC. Evidence-based guidance on venous thromboembolism in patients with solid tumours. Curr Oncol. 2014;21:e504–14. doi: 10.3747/co.21.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streiff MB, Bockenstedt PL, Cataland SR, et al. on behalf of the National Comprehensive Cancer Network Venous thromboembolic disease. J Natl Compr Canc Netw. 2013;11:1402–29. doi: 10.6004/jnccn.2013.0163. [DOI] [PubMed] [Google Scholar]
- 17.Mandalà M, Falanga A, Roila F, on behalf of the esmo Guidelines Working Group Management of venous thromboembolism (vte) in cancer patients: esmo clinical practice guidelines. Ann Oncol. 2011;22(suppl 6):vi85–92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 18.Kearon C, Akl EA, Comerota AJ, et al. on behalf of the American College of Chest Physicians Antithrombotic therapy for vte disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl):e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.K. National Institute for Health and Clinical Excellence (nice) How NICE Clinical Guidelines Are Developed: An Overview for Stakeholders, the Public and the NHS. 4th ed. London, U.K.: NICE; 2009. [Google Scholar]
- 20.Cummings P, Rivara FP. Reviewing manuscripts for Archives of Pediatrics & Adolescent Medicine. Arch Pediatr Adolesc Med. 2002;156:11–13. doi: 10.1001/archpedi.156.1.11. [DOI] [PubMed] [Google Scholar]
- 21.agree Collaboration . Appraisal of guidelines for research and evaluation (agreeii) instrument. Toronto, ON: AGREE Collaboration; n.d. [Downloadable from: http://www.agreetrust.org; cited July 15, 2014] [Google Scholar]
- 22.University of Oxford, Centre for Evidence-based Medicine (cebm) Centre for Evidence-based Medicine—Levels of Evidence (March 2009) [Web page] Oxford UK: CEBM; 2009. [Available at: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009; cited July 15, 2014] [Google Scholar]
- 23.Agnelli G, George DJ, Kakkar AK, et al. on behalf of the save-onco investigators Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601–9. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- 24.Maraveyas A, Waters J, Roy R, et al. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48:1283–92. doi: 10.1016/j.ejca.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Perry JR, Julian JA, Laperriere NJ, et al. prodige: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010;8:1959–65. doi: 10.1111/j.1538-7836.2010.03973.x. [DOI] [PubMed] [Google Scholar]
- 26.Karthaus M, Kretzschmar A, Kröning H, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase iii trial. Ann Oncol. 2006;17:289–96. doi: 10.1093/annonc/mdj059. [DOI] [PubMed] [Google Scholar]
- 27.Kakkar AK, Levine MN, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin, and survival in advanced cancer: the Fragmin Advanced Malignancy Outcome Study (famous) J Clin Oncol. 2004;22:1944–8. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Haas S, Schellong SM, Tebbe U, et al. Heparin based prophylaxis to prevent venous thromboembolic events and death in patients with cancer—a subgroup analysis of certify. BMC Cancer. 2011;11:316. doi: 10.1186/1471-2407-11-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakkar VV, Balibrea JL. Martínez–González J, Prandoni P on behalf of the canbesure study group. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the canbesure randomized study. J Thromb Haemost. 2010;8:1223–9. doi: 10.1111/j.1538-7836.2010.03892.x. [DOI] [PubMed] [Google Scholar]
- 30.Agnelli G, Gussoni G, Bianchini C, on behalf of the protecht investigators Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–9. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 31.Simonneau G, Laporte S, Mismetti P, on behalf of the FX140 study investigators A randomized study comparing the efficacy and safety of nadroparin 2850 IU (0.3 mL) vs. enoxaparin 4000 IU (40 mg) in the prevention of venous thromboembolism after colorectal surgery for cancer. J Thromb Haemost. 2006;4:1693–700. doi: 10.1111/j.1538-7836.2006.02083.x. [DOI] [PubMed] [Google Scholar]
- 32.Minnema MC, Breitkreutz I, Auwerda JJ, et al. Prevention of venous thromboembolism with low molecular-weight heparin in patients with multiple myeloma treated with thalidomide and chemotherapy. Leukemia. 2004;18:2044–6. doi: 10.1038/sj.leu.2403533. [DOI] [PubMed] [Google Scholar]
- 33.Mismetti P, Mille D, Laporte S, on behalf of the cip study group Low-molecular-weight heparin (nadroparin) and very low doses of warfarin in the prevention of upper extremity thrombosis in cancer patients with indwelling long-term central venous catheters: a pilot randomized trial. Haematologica. 2003;88:67–73. [PubMed] [Google Scholar]
- 34.Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119:933–9. doi: 10.1182/blood-2011-03-344333. [DOI] [PubMed] [Google Scholar]
- 35.Kakkar AK, Cimminiello C, Goldhaber SZ, Parakh R, Wang C, Bergmann JF, on behalf of the lifenox investigators Low-molecular-weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:2463–72. doi: 10.1056/NEJMoa1111288. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase iii, open-label, randomized trial. J Clin Oncol. 2011;29:986–93. doi: 10.1200/JCO.2010.31.6844. [DOI] [PubMed] [Google Scholar]
- 37.Hull RD, Schellong SM, Tapson VF, et al. on behalf of the exclaim study Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18. doi: 10.7326/0003-4819-153-1-201007060-00004. [DOI] [PubMed] [Google Scholar]
- 38.Riess H, Pelzer U, Hilbig A, et al. Rationale and design of prospect-conko 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy. BMC Cancer. 2008;8:361. doi: 10.1186/1471-2407-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verso M, Agnelli G, Bertoglio S, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. J Clin Oncol. 2005;23:4057–62. doi: 10.1200/JCO.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 40.Young AM, Billingham LJ, Begum G, et al. on behalf of the warp Collaborative Group U.K. Warfarin thromboprophylaxis in cancer patients with central venous catheters (warp): an open-label randomised trial. Lancet. 2009;373:567–74. doi: 10.1016/S0140-6736(09)60205-1. [DOI] [PubMed] [Google Scholar]
- 41.Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23:4063–9. doi: 10.1200/JCO.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 42.Leizorovicz A, Siguret V, Mottier D, et al. on behalf of the Innohep in Renal Insufficiency Study Steering Committee Safety profile of tinzaparin versus subcutaneous unfractionated heparin in elderly patients with impaired renal function treated for acute deep vein thrombosis: the Innohep in Renal Insufficiency Study (iris) Thromb Res. 2011;128:27–34. doi: 10.1016/j.thromres.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Di Nisio M, Porreca E, Otten HM, Rutjes AW. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2014;(8):CD008500. doi: 10.1002/14651858.CD008500.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Kanaan AO, Silva MA, Donovan JL, Roy T, Al-Homsi AS. Meta-analysis of venous thromboembolism prophylaxis in medically ill patients. Clin Ther. 2007;29:2395–405. doi: 10.1016/j.clinthera.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Turpie AG. Thrombosis prophylaxis in the acutely ill medical patient: insights from the prophylaxis in Medical Patients with Enoxaparin (medenox) trial. Am J Cardiol. 2000;86:48M–52M. doi: 10.1016/S0002-9149(00)01481-8. [DOI] [PubMed] [Google Scholar]
- 46.Riess H, Haas S, Tebbe U, et al. A randomized, double-blind study of certoparin vs. unfractionated heparin to prevent venous thromboembolic events in acutely ill, non-surgical patients: certify study. J Thromb Haemost. 2010;8:1209–15. doi: 10.1111/j.1538-7836.2010.03848.x. [DOI] [PubMed] [Google Scholar]
- 47.Schellong SM, Haas S, Greinacher A, et al. An open-label comparison of the efficacy and safety of certoparin versus unfractionated heparin for the prevention of thromboembolic complications in acutely ill medical patients: certain. Expert Opin Pharmacother. 2010;11:2953–61. doi: 10.1517/14656566.2010.521498. [DOI] [PubMed] [Google Scholar]
- 48.Wells PS, Anderson DR, Rodger MA, et al. A randomized trial comparing 2 low-molecular-weight heparins for the outpatient treatment of deep vein thrombosis and pulmonary embolism. Arch Intern Med. 2005;165:733–8. doi: 10.1001/archinte.165.7.733. [DOI] [PubMed] [Google Scholar]
- 49.Rodger MA, Ramsay T, MacKinnon M, et al. Tinzaparin versus dalteparin for periprocedure prophylaxis of thromboembolic events in hemodialysis patients: a randomized trial. Am J Kidney Dis. 2012;60:427–34. doi: 10.1053/j.ajkd.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Planès A, Samama MM, Lensing AW, et al. Prevention of deep vein thrombosis after hip replacement—comparison between two low-molecular heparins, tinzaparin and enoxaparin. Thromb Haemost. 1999;81:22–5. [PubMed] [Google Scholar]
- 51.Chiou–Tan FY, Garza H, Chan KT, et al. Comparison of dalteparin and enoxaparin for deep venous thrombosis prophylaxis in patients with spinal cord injury. Am J Phys Med Rehabil. 2003;82:678–85. doi: 10.1097/01.PHM.0000083671.27501.47. [DOI] [PubMed] [Google Scholar]
- 52.Carrier M, Khorana AA, Moretto P, Le Gal G, Karp R, Zwicker JI. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am J Med. 2014;127:82–6. doi: 10.1016/j.amjmed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 53.Akl EA, Vasireddi SR, Gunukula S, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2011:CD006649. doi: 10.1002/14651858.CD006649.pub4. [DOI] [PubMed] [Google Scholar]
- 54.Bottaro FJ, Elizondo MC, Doti C, et al. Efficacy of extended thromboprophylaxis in major abdominal surgery: what does the evidence show? A meta-analysis. Thromb Haemost. 2008;99:1104–11. doi: 10.1160/TH07-12-0759. [DOI] [PubMed] [Google Scholar]
- 55.Rasmussen MS, Jørgensen LN, Wille–Jørgensen P. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. 2009:CD004318. doi: 10.1002/14651858.CD004318.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Pannucci CJ, Shanks A, Moote MJ, et al. Identifying patients at high risk for venous thromboembolism requiring treatment after outpatient surgery. Ann Surg. 2012;255:1093–9. doi: 10.1097/SLA.0b013e3182519ccf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorevska N, Amoateng–Adjepong Y, Sabahi R, et al. Anticoagulation in hospitalized patients with renal insufficiency: a comparison of bleeding rates with unfractionated heparin vs enoxaparin. Chest. 2004;125:856–63. doi: 10.1378/chest.125.3.856. [DOI] [PubMed] [Google Scholar]
- 58.Perry SL, Bohlin C, Reardon DA, et al. Tinzaparin prophylaxis against venous thromboembolic complications in brain tumor patients. J Neurooncol. 2009;95:129–34. doi: 10.1007/s11060-009-9911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Cicco M, Matovic M, Balestreri L, et al. Early and short-term acenocumarine or dalteparin for the prevention of central vein catheter-related thrombosis in cancer patients: a randomized controlled study based on serial venographies. Ann Oncol. 2009;20:1936–42. doi: 10.1093/annonc/mdp235. [DOI] [PubMed] [Google Scholar]
- 60.Cunningham MS, White B, Hollywood D, O’Donnell J. Primary thromboprophylaxis for cancer patients with central venous catheters: a reappraisal of the evidence. Br J Cancer. 2006;94:189–94. doi: 10.1038/sj.bjc.6602917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan A, Iannucci A, Dager WE. Systemic anticoagulant prophylaxis for central catheter-associated venous thrombosis in cancer patients. Ann Pharmacother. 2007;41:635–41. doi: 10.1345/aph.1G714. [DOI] [PubMed] [Google Scholar]
- 62.Akl EA, Karmath G, Yosuico V, et al. Anticoagulation for thrombosis prophylaxis in cancer patients with central venous catheters. Cochrane Database Syst Rev. 2007:CD006468. doi: 10.1002/14651858.CD006468.pub2. [DOI] [PubMed] [Google Scholar]
- 63.Rawson KM, Newburn–Cook CV. The use of low-dose warfarin as prophylaxis for central venous catheter thrombosis in patients with cancer: a meta-analysis. Oncol Nurs Forum. 2007;34:1037–43. doi: 10.1188/07.ONF.1037-1043. [DOI] [PubMed] [Google Scholar]
- 64.Siguret V, Pautas E, Février M, et al. Elderly patients treated with tinzaparin (Innohep) administered once daily (175 anti-Xa IU/kg): anti-Xa and anti-IIa activities over 10 days. Thromb Haemost. 2000;84:800–4. [PubMed] [Google Scholar]
- 65.Lim WL, Cook DJ, Crowther MA. Safety and efficacy of low molecular weight heparins for hemodialysis in patients with end-stage renal failure: a meta-analysis of randomized trials. J Am Soc Nephrol. 2004;15:3192–206. doi: 10.1097/01.ASN.0000145014.80714.35. [DOI] [PubMed] [Google Scholar]
- 66.Douketis J, Cook D, Meade M, et al. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the direct study. Arch Intern Med. 2008;168:1805–12. doi: 10.1001/archinte.168.16.1805. [DOI] [PubMed] [Google Scholar]
- 67.Mahe I, Gouin–Thibault I, Drouet L, et al. Elderly medical patients treated with prophylactic dosages of enoxaparin: influence of renal function on anti-Xa activity level. Drugs Aging. 2007;24:63–71. doi: 10.2165/00002512-200724010-00005. [DOI] [PubMed] [Google Scholar]
- 68.Carrier M, Khorana AA, Zwicker J, et al. Management of challenging cases of patient with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the ssc of the isth. J Thromb Haemost. 2013;11:1760–5. doi: 10.1111/jth.12338. [DOI] [PubMed] [Google Scholar]
- 69.Herishanu Y, Misgav M, Kirgner I, Ben-Tal O, Eldor A, Naparstek E. Enoxaparin can be used safely in patients with severe thrombocytopenia due to intensive chemotherapy regimens. Leuk Lymphoma. 2004;45:1407–11. doi: 10.1080/10428190410001663671. [DOI] [PubMed] [Google Scholar]
- 70.Lee AY, Peterson EA. Treatment of cancer-associated thrombosis. Blood. 2013;122:2310–17. doi: 10.1182/blood-2013-04-460162. [DOI] [PubMed] [Google Scholar]
- 71.Alvarado G, Noor R, Bassett R, et al. Risk of intracranial hemorrhage with anticoagulation therapy in melanoma patients with brain metastases. Melanoma Res. 2012;22:310–15. doi: 10.1097/CMR.0b013e328353efd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vitale FV, Rotondo S, Sessa E, et al. Low molecular weight heparin administration in cancer patients with hypercoagulability-related complications and carrying brain metastases: a case series study. J Oncol Pharm Pract. 2012;18:10–16. doi: 10.1177/1078155210390254. [DOI] [PubMed] [Google Scholar]
- 73.World Health Organization(who) Global Database on Body Mass Index [Web page] Geneva, Switzerland: WHO; 2006. [Available online at: http://www.assessmentpsychology.com/icbmi.htm; cited February 28, 2015] [Google Scholar]
- 74.Freeman A, Horner T, Pendleton RC, Rondina MT. Prospective comparison of three enoxaparin dosing regimens to achieve target anti-factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87:740–3. doi: 10.1002/ajh.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hainer JW, Barrett JS, Assaid CA, et al. Dosing in heavyweight/obese patients with the lmwh, tinzaparin: a pharmacodynamic study. Thromb Haemost. 2002;87:817–23. [PubMed] [Google Scholar]
- 76.Al-Yaseen E, Wells PS, Anderson J, Martin J, Kovacs MJ. The safety of dosing dalteparin based on actual body weight for the treatment of acute venous thromboembolism in obese patients. J Thromb Haemost. 2005;3:100–2. doi: 10.1111/j.1538-7836.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 77.Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. on behalf of the adopt trial investigators Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–77. doi: 10.1056/NEJMoa1110899. [DOI] [PubMed] [Google Scholar]
- 78.Cohen AT, Spiro TE, Spyropoulos AC, on behalf of the magellan Steering Committee Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:1945–6. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 79.Levine MN, Gu C, Liebeman HA, et al. A randomized phase ii trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost. 2012;10:807–14. doi: 10.1111/j.1538-7836.2012.04693.x. [DOI] [PubMed] [Google Scholar]
- 80.Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110:1149–61. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 81.Akl EA, Kahale L, Terrenato I, et al. Oral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2014;(7):CD006466. doi: 10.1002/14651858.CD006466.pub4. [DOI] [PubMed] [Google Scholar]
- 82.Akl EA, Gunukula S, Barba M, et al. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2011:CD006652. doi: 10.1002/14651858.CD006652.pub3. [DOI] [PubMed] [Google Scholar]
- 83.Maddali S, Biring T, Bluhm J, on behalf of the Institute for Clinical Systems Improvement (icsi) Antithrombotic Therapy Supplement. Bloomington, MN: ICSI; 2012. [Google Scholar]


