Abstract
Hypoxia can induce functional and structural vascular remodeling by changing the expression of trophic factors to promote homeostasis. While most experimental approaches have been focused on functional remodeling, structural remodeling can reflect changes in the abundance and organization of vascular proteins that determine functional remodeling. Better understanding of age-dependent hypoxic macrovascular remodeling processes of the cerebral vasculature and its clinical implications require knowledge of the vasotrophic factors that influence arterial structure and function. Hypoxia can affect the expression of transcription factors, classical receptor tyrosine kinase factors, non-classical G-protein coupled factors, catecholamines, and purines. Hypoxia’s remodeling effects can be mediated by Hypoxia Inducible Factor (HIF) upregulation in most vascular beds, but alterations in the expression of growth factors can also be independent of HIF. PPARγ is another transcription factor involved in hypoxic remodeling. Expression of classical receptor tyrosine kinase ligands, including vascular endothelial growth factor, platelet derived growth factor, fibroblast growth factor and angiopoietins, can be altered by hypoxia which can act simultaneously to affect remodeling. Tyrosine kinase-independent factors, such as transforming growth factor, nitric oxide, endothelin, angiotensin II, catecholamines, and purines also participate in the remodeling process. This adaptation to hypoxic stress can fundamentally change with age, resulting in different responses between fetuses and adults. Overall, these mechanisms integrate to assure that blood flow and metabolic demand are closely matched in all vascular beds and emphasize the view that the vascular wall is a highly dynamic and heterogeneous tissue with multiple cell types undergoing regular phenotypic transformation.
Keywords: Cerebrovascular circulation, chronic hypoxia, fetal maturation, growth factors, receptor tyrosine kinases, smooth muscle phenotype
INTRODUCTION
Vascular remodeling is crucial in maintaining homeostasis during development, exercise, and pregnancy. Blood vessels respond to their constantly changing environment by remodeling to match blood flow to local metabolic demand [1, 2]. Without proper regulation of perfusion, tissues can become ischemic and deprived of oxygen, resulting in cellular apoptosis, organ dysfunction, and eventually necrosis. Over the long term, the vasculature matches supply to demand by inducing capillary angiogenesis and by remodeling larger vessels upstream. A classic example of physiological remodeling is exercise conditioning, in which multiple factors induce long-term increases in maximum blood flow. To match the increased demand for oxygen and enable greater blood flow, existing large vessels undergo macrovascular remodeling while microvascular remodeling increases capillary density at the capillary level [3–6]. Capillary angiogenesis and collateral formation are examples of microvascular remodeling, a process distinctly different from macrovascular remodeling, in which structural changes occur within the walls of arteries and arterioles upstream from the capillaries. The ultimate example of macrovascular adaptation is pregnancy-induced remodeling of the uterine artery, a large conduit vessel that undergoes dramatic functional and structural changes throughout pregnancy [7]. Multiple types of microvascular and macrovascular remodeling are important not only in the mother but also in the developing fetus, especially during the transition from fetal to newborn life [8–10]. Given that the processes governing both microvascular and macrovascular remodeling remain poorly understood, particularly in the fetus and newborn, these processes warrant further research in fetal, newborn and adult arteries.
The principles governing homeostatic vascular remodeling also participate in pathophysiological remodeling in numerous diseases. For example, chronic hypertension can promote hypertrophic arterial remodeling through dynamic mechanisms [11]. Increased intraluminal pressures characteristic of chronic hypertension can alter vascular permeability, wall thickness, composition, and protein abundance [12]. Some of these changes are attributable to genetic factors that enhance inward arteriolar remodeling responses to increased luminal pressure [13] (Fig. 1). This type of remodeling of cerebral arteries can increase distensibility with reduced internal and external diameters [14] and thereby reduce the risk of aneurysms [15]. Other pathologies, such as subarachnoid hemorrhages, also induce cerebrovascular remodeling [16, 17] that is not compensatory but instead compromises flow-metabolism coupling and can even culminate in vasospasm. Clearly, both physiological and pathophysiological patterns of cerebrovascular remodeling are dynamic and regionally heterogeneous multifactorial processes influenced by the expression of numerous genes, receptors, and growth factors [18–20].
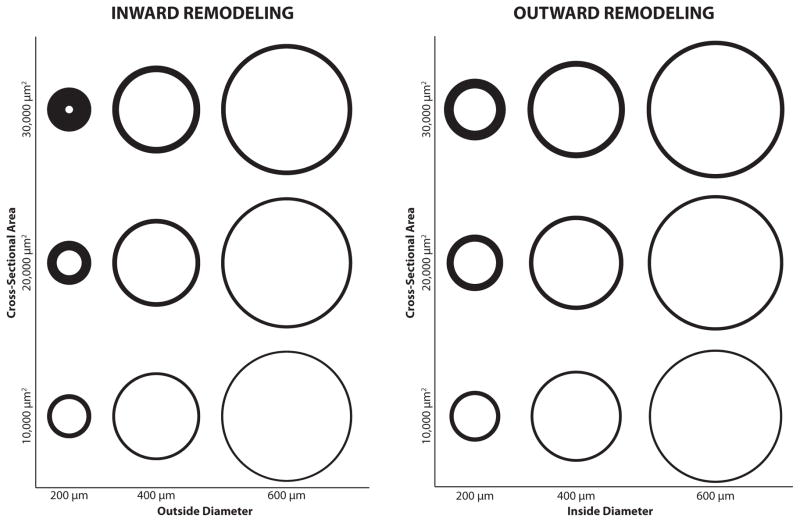
Fig. 1. Categories of Vascular Remodeling.
Remodeling can be hypertrophic, eutrophic, or hypotrophic. In hypertrophic remodeling, the medial cross-sectional area increases. In eutrophic remodeling, total medial cross-sectional area remains unchanged. In hypotrophic remodeling, cross-sectional area decreases. Remodeling that results in a reduction in luminal diameter with constant outside diameter is classified as inward remodeling (left panel). Remodeling that involves an increase in outside arterial diameter with a constant inside diameter is classified as outward remodeling (right panel). In eutrophic remodeling, both inside and outside diameters change. In the above panels, eutrophic remodeling is represented as a change in horizontal position with no vertical change. These combined structural changes profoundly influence the contractile characteristics of the individual smooth muscle cells within the medial layer.
To better understand the mechanisms governing vascular remodeling, it is important to differentiate functional from structural effects. Functional remodeling includes changes in vascular reactivity and contractility, which are fundamentally important for coupling of blood flow to local tissue metabolism. Such changes in function, however, are typically the consequence of changes in artery composition and structure. These structural changes can alter the abundance and organization of adventitial matrix proteins as well as the numbers and composition of individual cell types within the arterial wall [21–23]. Correspondingly, these alterations can increase outer arterial diameter (outward remodeling) or decrease luminal diameter (inward remodeling). In addition, total smooth muscle cell mass per unit length of artery can increase (hypertrophic remodeling) or remain unchanged (eutrophic remodeling) [11, 14, 24]. The extent to which these combined structural effects influence the contractile characteristics of the individual smooth muscle cells in the medial layer defines their functional consequences.
Smooth muscle cells are an integral component of the arterial wall and exhibit a phenotypic heterogeneity that is governed by local mechanical and chemical signals [21, 25, 26]. These cells can be classified as migratory, proliferative, synthetic, or contractile, and any single cell can exhibit mixtures of these and other characteristics [21]. Transitions among these phenotypes can be induced by either receptor tyrosine kinase (RTK) dependent growth factors [27, 28] or by non-classical G-protein coupled receptor (GPCR) ligands [29, 30]. In addition, smooth muscle cells can also undergo apoptosis, which is an important process in vascular remodeling [31]. Ultimately, the integrated effects of changes in the numbers, organization, and individual characteristics of the cellular components that make up the arterial wall determine the net result of remodeling.
Tissue hypoxia is a common feature shared among many types of both physiological and pathophysiological remodeling. This hypoxia drives remodeling to balance oxygen supply and demand at the cellular level through parallel microvascular and macrovascular effects. Most early studies of hypoxic remodeling focused on the pulmonary circulation, due largely to the clinical prevalence of persistent pulmonary hypertension of the newborn [32, 33]. These studies have established that mild chronic hypoxia directly increases pulmonary arterial pressure and promotes changes in vascular structure and function through coordinated actions of multiple vasotrophic factors [34]. Investigations of hypoxic vascular remodeling in other vascular beds are more rare and have focused predominantly on the functional consequences of varying durations of hypoxia, with emphasis on changes in vascular contractility and cardiac output distribution [35]. These effects are particularly prominent in the cerebral circulation, where a wide variety of studies have established that chronic hypoxia stimulate angiogenesis, increase capillary density, and reduce inter-capillary distances within the brain parenchyma [36–42]. The cerebral circulation is also subject to both functional and structural macrovascular remodeling, particularly in response to ischemic insults [43]. Virtually all of these remodeling responses are age-dependent and reflect the integrated action of a broad variety of both classical and non-classical vasotrophic factors [44].
As in the adult, fetal and newborn hypoxia can stimulate an increase in cerebral capillary angiogenesis and permeability [45, 46]. Chronic hypoxia can also compromise autoregulation and the dynamics of blood velocity in fetal and neonatal brains [47–49]. In addition, reactivity to nitric oxide (NO), a primary endogenous vasodilator released from the vascular endothelium, can be depressed by chronic hypoxia through reduced vascular soluble guanylate cyclase (sGC) activity [50]. These functional changes reflect underlying structural remodeling, including increased protein abundance and vascular smooth muscle proliferation in fetal arteries [23, 35, 51, 52]. Not surprisingly, the effects of hypoxia on both functional and structural remodeling vary considerably in fetal and adult arteries [53, 54]. The age-related differences are a consequence of the combined actions of multiple vasotrophic factors whose release and activity vary with age, vascular bed, and intensity of hypoxia.
The roles in vascular remodeling of known vascular growth factors and other non-classical vasotrophic factors remain uncertain, but have the potential to further understanding of vascular pathologies in both the fetal and adult cerebral circulations. To that end, it will be valuable to better appreciate how these factors function not just individually, but in combination in response to common pathophysiological stresses such as hypoxia. The present review therefore explores the main factors known to play a role in vascular remodeling, with emphasis on responses involving the fetal cerebral circulation where possible. Given the relative paucity of results directly related to the fetal cerebral circulation, the review is organized around the three main families of factors that govern overall vascular remodeling. The first of these are the transcription factors that have a global influence on vascular growth and differentiation.
TRANSCRIPTION FACTORS IN HYPOXIC VASCULAR REMODELING
An essential first step in the initiation of hypoxic vascular remodeling is the activation of pathways that can sense and respond to reduced oxygen availability. Low oxygen can function as a trigger, inducing downstream transcriptional and translational events that mechanistically regulate both microvascular and macrovascular remodeling. How hypoxia is detected and translated into changes in gene and protein expression was uncertain for many years prior to the discovery of Hypoxia Inducible Factor (HIF) by Semenza in 1992 [55]. The transcription factor HIF is now recognized as the main signal that activates cellular responses to hypoxia [56] (Fig. 2). It is a heterodimeric protein composed of α and β subunits, both of which are basic-helix-loop-helix (bHLH) proteins classified under the PAS family of transcriptional regulators [57]. Under normoxic conditions, HIF-1β is constitutively expressed whereas HIF-1α is continuously degraded via the ubiquitin-proteosome pathway [58]. Hypoxia inhibits prolyl hydroxylase, which is the oxygen-dependent enzyme governing HIF-1α ubiquitination and degradation [40]. Elevated levels of HIF-1α facilitate the formation of the HIF-1 complex, which then can bind to Hypoxia Responsive Elements (HRE) in the promoter regions of numerous genes and initiate transcription [56].
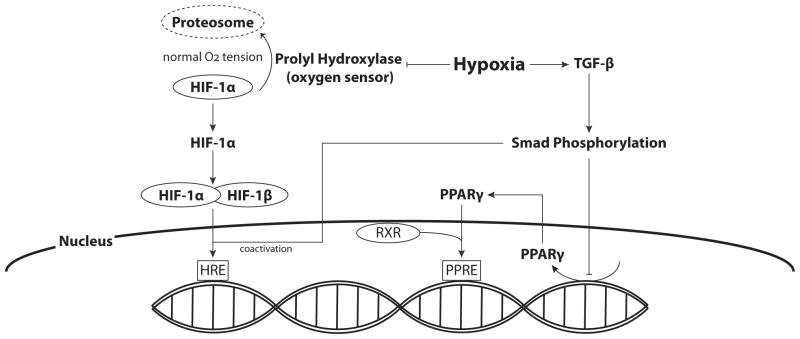
Fig. 2. Hypoxia and Transcription Factors.
Prolyl hydroxylase, the oxygen sensor, is responsible for HIF-1α ubiquitination and degradation under normoxic conditions. Hypoxia inhibits prolyl hydroxylase, leading to elevated levels of HIF-1. Accumulated HIF-1α can then facilitate the formation of the HIF-1 complex with constitutively expressed HIF-1β, which can then translocate to the nucleus where it binds to Hypoxia Responsive Elements (HRE) in the promoter regions of multiple genes and initiates transcription. Hypoxia-induced increases in TGF-β lead to Smad phosphorylation, which can also serve as coactivators for HIF-1α, and decrease PPARγ expression.
The effects of elevated HIF are highly heterogeneous among different tissues. This variability is due, at least in part, to tissue specific factors that influence HIF half-life and degradation. For example, products of HIF-sensitive genes can feedback through tyrosine kinase receptors or G-protein coupled receptors and regulate HIF levels [59, 60]. HIF-1α also can be upregulated by thrombin-α, PDGF-AB, and TGF-β1 in cultured and renal vascular smooth muscle cells [61]. Prostaglandin I2 (PGI2), a vasodilator with vasoprotective and antioxidant properties, can stabilize HIF-1α protein in hypoxic human umbilical vascular endothelial cells (HU-VECs). PGI2 appear to protect HIF-1α via inhibition of NADPH oxidase activity and reduction in levels of reactive oxygen species, which retard HIF-1α degradation [62]. HIF can also be regulated by Chloride Intracellular Channel 4 (CLIC4), which affects the upstream regulation and promotion of HIF and its downstream effectors, therefore influencing active transcription of HIF sensitive genes [63]. Studies of HIF turnover and half-life are a logical area for future research, particularly in situations where revascularization of transplanted tissues is essential for successful surgical outcomes [64, 65].
Another determinant of heterogeneity of local responses to HIF is the compliment of different active genes with HRE in their promoters, and the levels of transcription factors for the other cis-regulatory elements in each promoter region. For example, endothelial cells from various vascular beds differentially respond to hypoxia-induced HIF-1 by variably expressing endothelin-1, inducible nitric oxide synthase (iNOS), Fibulin-5, vascular endothelial growth factor-A (VEGF-A), VEGF receptors, and angiopoietin receptors [66–68]. In arterial smooth muscle, HIF can upregulate expression of low-density lipoprotein receptor-related protein [69]. Elevated levels of HIF-1α can also induce vascular remodeling under normoxic conditions in cultured vascular smooth muscle [70]. This range of effects emphasizes the versatility of diversity of HIF as a mediator of vascular responses to hypoxia.
In the brain, HIF can be used as a marker to identify hypoxia [71, 72]. Although upregulation of HIF-1α and its downstream effectors appear to be involved in vascular remodeling and hypoxic conditioning in both adult and neonatal brains [73–76], there have been no comparisons between adult and fetal HIF levels. Due to the fact that oxygen tensions are dramatically different between fetal and adult tissues, a logical speculation for future studies would be that HIF levels are adapted to the lower tissue oxygen tensions typical of the fetus. In the developing brain, HIF can directly influence proliferation of neuronal precursor cells [77]. HIF can also indirectly promote neuroprotection by stimulating expression of erythropoietin and VEGF [77–79]. Regionally, the effects of HIF are influenced by local conditions that determine whether HIF exerts either neuroprotective effects or neurotoxic effects through stimulation of apoptosis and necrosis [77]. Together, these results reflect the potential of HIF as a mediator of hypoxic vascular remodeling in the brains of both fetuses and adults.
The basic Helix-Loop-Helix structure of HIF-1α is also characteristic of Endothelial PAS protein 1 (EPAS1), a transcription factor closely related to HIF that might also contribute to the hypoxic remodeling response [80]. This transcription factor has had an interesting history owing to its independent discovery by at least three different research groups. Correspondingly, this factor has been named EPAS1 [81], HIF-1α-like factor (HLF) [82], and finally HIF-2α [83]. HIF-2α shares a 48% sequence identity with HIF-1α [84] and can be expressed during embryogenesis [85]. HIF-2α can induce cellular hypertrophy, reduce proliferation, and promote angiogenesis in neuroblastoma cells [86]. HIF-2α might also serve as a biomarker in advanced bladder cancer [87]. Interestingly, both HIF-2α and HIF-1α mRNA are distributed heterogeneously among all tissue and cell types [88] and can be expressed in the heart and lungs of neonates [81]. Both factors are stabilized by hypoxia and bind to HRE in multiple gene promoters [80]. As for HIF-1α, HIF-2α influences angiogenesis through upregulation of VEGF, and stimulates transcription of genes for EPO and the Tie-2 receptor [89–91]. During development, HIF-2α transcripts can be colocalized with HIF-1α transcripts, suggesting redundant roles that extend beyond embryogenesis that could include vascular stabilization and remodeling [92]. In relation to hypoxic remodeling, mutations of the EPAS-1 gene that codes for HIF-2α may have more beneficial effects for high altitude living than mutations of the EPO gene [93]. In addition, HIF-2α can inhibit ROS production by stimulation of antioxidant enzyme production [94]. Unlike HIF-1α, very little research has examined HIF-2α or its role in vascular development, maintenance or remodeling.
Another transcription factor involved in hypoxic remodeling is peroxisome proliferator-activated receptor gamma (PPARγ). Although traditionally associated with lipid metabolism and antioxidant protection during inflammation [95], it also plays a role in hypoxic vascular remodeling. Hypoxia stimulates an increase in TGF-β/Smad signaling that then downregulates PPARγ expression, functionally releasing a “brake” on remodeling [96, 97]. Hypoxic reductions in PPARγ thus promote remodeling and enable functional and structural changes to proceed. In the nucleus, PPARγ heterodimerizes with Retinoid X Receptor α (RXRα) and can then bind to peroxisome proliferator response elements (PPREs) on the promoter region of PPARγ target genes to induce transcription [98, 99]. In contrast to HIF, activation of PPARγ helps maintain vascular myogenic tone and attenuates remodeling [100, 101] by decreasing endothelial-derived ET-1 expression and inhibiting VEGF-induced angiogenesis [102]. PPARγ can also decrease VSMC proliferation and stimulate apoptosis [102].
In the cerebral vasculature, studies of PPARγ are rare but are attracting growing scientific interest. Cerebral arteries from mice with negative mutations in PPARγ exhibited reduced PPARγ levels and underwent both functional and structural remodeling [103]. Functionally, the arteries demonstrated impaired responses to agonist-induced vasodilation, which was attributed to elevated superoxide levels secondary to reduced antioxidant protection by PPARγ. Structural changes included increased distensibility, wall thickness, and cross-sectional area with decreased external diameter, as is typical of hypertrophic inward remodeling. Aside from the well-studied effects of PPARγ on lipid metabolism and inflammation, virtually nothing is known of the influence of hypoxia on PPARγ expression within the fetal cerebrovasculature, making this a promising topic for future investigation.
RECEPTOR TYROSINE KINASE-DEPENDENT VASOTROPHIC FACTORS
Whereas transcription factors exert effects only within the cells where they are synthesized, most growth factors are released into the extracellular space where they act as intercellular messengers. These messenger molecules, of which there are dozens, then activate cell surface receptors in either an autocrine or paracrine manner. One convenient method to classify these factors is according to the receptor type they bind and activate. For vasotrophic factors, the largest single class of receptors is the Receptor Tyrosine Kinase (RTK) family. In turn, the most widely studied vasotrophic factor that acts through RTK receptors is Vascular Endothelial Growth Factor [27].
Vascular Endothelial Growth Factor
VEGF was discovered more than six decades ago as the factor responsible for increased vascular permeability and was originally named Vascular Permeability Factor [104]. Subsequent studies identified VEGF as the main factor responsible for increased vascular permeability in tumors [105] and is now also recognized as the main vascular growth factor mediating angiogenesis [106, 107]. VEGF can also promote angiogenic effects, including tube formation, in cell cultures and can increase vascular endothelial cell proliferation in rat brains [108, 109]. On the other hand, under some conditions endothelial cells do not respond robustly to VEGF stimulation [110], suggesting that the role of the endothelium in remodeling is both heterogeneous and finely regulated. The VEGF family includes seven members (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PIGF), which can act through three known receptor tyrosine kinases, VEGFR-1 (FLT-1), VEGFR-2 (KDR), and VEGFR-3 [111, 112]. Activation of these receptors can initiate highly variable and tissue type-dependent responses. For example, activation of VEGFR-2 can induce cell proliferation in endothelial cells [113], but can modulate contractile protein abundance in vascular smooth muscle [44]. In contrast, VEGFR-3 is expressed predominantly in lymphatic and venous vessels where it regulates lymphangiogenesis and sprouting [114]. Regulation of VEGF reactivity can function in an autocrine loop in which activation of either VEGF-R1 or VEGF-R2 can enhance mRNA and protein expression for VEGF-R1 in either its particulate or soluble form [115]. In turn, expression of VEGF-A, currently the most potent angiogenic protein known [112], can also be induced by TGF-β1 during tumor-induced angiogenesis [116].
A primary physiological stimulus for VEGF synthesis is hypoxia, which acts through HIF-1α to upregulate VEGF and other growth factors to promote homeostatic increases in capillary angiogenesis and vascular remodeling [117]. Hypoxia-induced HIF-1α can increase both VEGF-A and VEGFR-1 expression in endothelial cells derived from multiple different vascular beds [66] (Fig. 3). Hypoxic increases in VEGF within adjacent endothelial cells and pericytes can yield synergistic paracrine effects that enhance cellular growth and proliferation [118]. In some cell types, notably gliomas, hypoxia can also enhance VEGF levels through stabilization of VEGF mRNA [119, 120]. Not surprisingly, the effects of hypoxia on VEGF are highly tissue specific; VEGF levels can be unresponsive to hypoxia in the kidneys [121, 122].
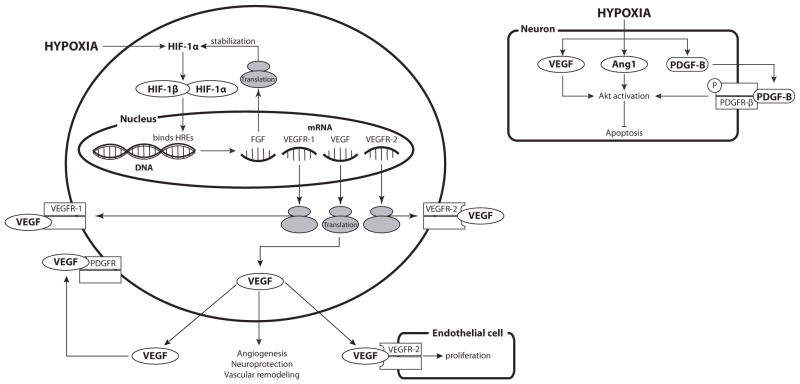
Fig. 3. Effects of Hypoxia on Expression of Receptor Tyrosine Kinase-dependent Vasotrophic Factors.
Hypoxia-induced increases in HIF-1 levels can stimulate the transcription and translation of multiple Receptor Tyrosine Kinase-dependent vasotrophic factors. HIF-induced increases in FGF have been shown to stabilize HIF-1α, effectively enhancing its own synthesis. Increases in VEGF and VEGF receptors can induce endothelial cell proliferation. In addition to having angiogenic effects, VEGF can also be neuroprotective, can induce endothelial cell proliferation and vascular remodeling. VEGF can also activate PDGF receptors. Hypoxia causes an increase in VEGF, Angiopoietin 1 (Ang1), and PDGF-B levels, which activate Akt and inhibit apoptosis, particularly in neurons.
In the brain, the effects of hypoxia on VEGF have been widely studied owing to the potential of VEGF to facilitate recovery from ischemic cerebral insults [123]. These benefits are due not only to the ability of VEGF to stimulate cerebral angiogenesis [40, 41, 124], but are due also to VEGF’s neuroprotective properties in both mature [79, 125] and immature brain [126]. An important component of this overall effect is that hypoxia upregulates expression of VEGF mRNA and protein in the brain [107, 127, 128]. VEGF also can enhance its efficacy in the brain through upregulation of VEGFR-2 mRNA [129]. All cerebral cell types appear to participate in this pattern of responses, including astrocytes, which exhibit increased expression of VEGF following hypoxic exposure [130]. Interestingly, the cellular sources of VEGF are highly age dependent such that VEGF is expressed primarily in neurons of the immature brain, but in both neurons and glial cells of the mature brain [124]. Aside from these differences, sustained hypoxia increases VEGF expression in both neurons and glia, regardless of postnatal age. Based on studies in large arteries [44], these hypoxic increases in VEGF could potentially contribute to hypoxic cerebrovascular remodeling in an age-dependent manner. This hypothesis awaits future experimental confirmation.
Platelet Derived Growth Factor
Platelet-Derived Growth Factor (PDGF) has long been recognized as a major influence on vascular growth and development, particularly in developing tissues [131, 132]. It is a dimeric polypeptide with extensive homology to the peptide sequences of VEGF [133]. One major consequence of this homology is that receptors for PDGF can be activated not only by PDGF, but by VEGF as well [134, 135]. Active PDGF ligands can be composed of any pair of four different isoforms, designated as A, B, C, and D. The most common pairs, biologically, are PDGF-AA, PDGF-AB, and PDGF-BB [136] and thus the A and B forms have been most widely studied. Polypeptides, A and B, are transcribed from different genes but can be dimerized by a disulfide bond [137, 138]. The receptors that bind active PDGF dimers are composed of two different subunits, an α-subunit (PDGFR-α), which can bind both A and B chains, and a β-subunit (PDGFR-β), which can bind B-chain only. These subunits can associate reversibly to bind specific PDGF ligands [139]. Most importantly, different PDGF ligands produce different cellular responses even when acting on a common receptor [140]. PDGF can stimulate mitogenesis in smooth muscle, NO-dependent vasorelaxation in endothelium-intact aortic rings [141], and microvascular angiogenesis in invasive breast cancer [142]. PDGF-BB can transform smooth muscle to a less contractile phenotype, and is crucial for proper lung development of neonatal rats [28, 143].
As for most vasotrophic factors, the levels of PDGF and its receptors in any tissue are subject to regulation by many different influences. PDGFR-α levels can be upregulated by basic fibroblast growth factor (FGF-2), which can facilitate smooth muscle proliferation upon subsequent stimulation with PDGF-AA [144]. Alpha-thrombin can also increase mRNA levels for PDGF-A and simultaneously decrease mRNA for PDGFR-β in smooth muscle [145]. Hypoxia is also an important modulator of PDGF signaling in many different tissues. Although hypoxia has little effect on renal expression of PDGF-A and PDGF-B [121, 122], hypoxia can markedly increase transcription of the PDGF-B gene in HU-VEC cultures [138]. In rat lung parenchyma, hypoxia can transiently increase PDGF-B mRNA levels [146]. In neonatal rat lung, hypoxia increased mRNA for PDGF-B, PDGFR-α and PDGFR-β but decreased the apparent protein abundance of PDGF-A, PDGF-B and PDGFR-α [143], suggesting important hypoxic effects on the stability and translation efficiency of these mRNAs. In pulmonary arterial smooth muscle of neonatal rats, chronic hypoxia increased proliferation and expression of both PDGF-BB and PDGFR-β [147]. Hypoxia also appears to mediate PDGF-dependent hyperphosphorylation of PDGFR-β, and thereby enhance pulmonary artery endothelial and smooth muscle proliferation [148, 149].
Within the central nervous system, PDGF is crucial for recruitment of pericytes involved in brain capillary angiogenesis during embryonic development [136, 150]. Recruited pericytes can produce other vasotrophic factors such as TGF-β and VEGF, and are crucial in initiation, guidance, extension, and maturation of vessels [117]. In areas of focal ischemic cerebral infarct, injured tissue expresses increased levels of mRNA and protein for both PDGF-B and PDGFR-β [136]. More directly, hypoxia can increase mRNA and protein levels for PDGF-B in human glioblastoma cells [151]. In neurons, hypoxia can also increase mRNA and protein for PDGF-B and subsequent phosphorylation of PDGFR-β, leading to Akt activation and attenuation of apoptosis [152]. Effects of hypoxia in the central nervous system also appear to be regionally heterogeneous; chronic hypoxia can depress the abundance of PDGFR-β receptors in the dorsocaudal brainstem and simultaneously increase mRNA levels for PDGF-B and PDGFR-β in the solitary tract nucleus [153–155]. Together, these results demonstrate that, as for VEGF, the effects of hypoxia on PDGF signaling are highly dependent on age and cell type. Similarly, the roles of PDGF in hypoxic cerebrovascular remodeling remain largely unexplored, particularly in the immature brain.
Angiopoietins
Many of the vascular effects of hypoxia are attributable to the factors whose expression is upregulated by the actions of HIF-1α. In addition to VEGF, HIF-1α also drives the expression of angiopoietins, growth factors crucial for vascular maintainance and induction of vessel sprouting [156]. HIF increases angiopoietin-2 (Ang2) levels via a COX-2 dependent increase of prostaglandin E2 [157]. Four types of angio-poietin have been identified, including Ang1, Ang2, Ang3, and Ang4, all of which play a role in vascular and lymphatic remodeling in the adult mice [158, 159]. In endothelial cells, however, Ang1 and Ang3 exhibit few mitogenic effects [160, 161]. Expression of angiopoietins in vascular cell types is also heterogeneous; vascular smooth muscle expresses both Ang1 and Ang2 but endothelial cells primarily express just Ang2 [162]. As for VEGF, angiopoietins can also be anti-apoptotic, particularly in endothelia and mesenchymal stem cells [163, 164]. The receptors for angiopoietins are members of the RTK family and include Tie1 and Tie2 [162, 165]. In combination with these receptors, Ang1 and Ang2 operate in a push-pull manner in which Ang2 destabilizes, and Ang1 stabilizes, vessels undergoing angiogenesis [166–168]. To achieve this effect, Ang2 inhibits binding of Ang1 to Tie2 and thereby destabilizes capillaries and helps initiate microvascular angiogenesis. In addition, Tie1 also reciprocally regulates the binding of Ang1 and Ang2 to the Tie2 receptor to control responses to angiopoietin stimulation [169].
In relation to vascular remodeling, angiopoietins act in concert with VEGF [114]. Together with VEGF, Ang1 promotes increased arterial lumen diameter and Ang2 acts to extend vessel length and increase propagation of sprouting cells [117, 168]. Both VEGF and FGF-2 can increase Ang2 in microvascular endothelial cells, which can antagonize the effects of Ang1 and promote disassembly of the vascular wall and formation of new vessel sprouts [170]. Conversely, TGF-β1 can decrease Ang2 production. Ang1 and Ang2 can also decrease Ang2 production through negative feedback at the mRNA level. Correspondingly, expression of angio-poietin receptors are also subject to physiological regulation through which FGF-2 and VEGF, either alone or in combination, can increase Tie1 expression. Similarly, Tie2 expression can be increased by FGF-2, Ang1, or Ang2 [162]. Clearly, the angiopoietins are another category of important vasotrophic factors whose complex influences are governed by the simultaneous actions of multiple physiological influences.
One key determinant of angiopoietin actions is hypoxia. Hypoxia can upregulate Ang2 mRNA and protein levels in all major categories of cells [171–173]. In endothelial cells, hypoxia-induced increases in HIF-1 produce reciprocal increases in Ang2 and Tie2 expression but decreases in Ang1 expression [66, 173, 174]. Hypoxia also can increase both the transcription and stability of Ang2 mRNA in HUVECs [157]. Hypoxia can regulate Ang2 expression indirectly, at least in HUVEC cultures, through HIF-induced increases in COX-2 and subsequent increases in prostacyclin and prostaglandin E2, which in turn can increase Ang2 levels under either normoxic or hypoxic conditions [157].
Within the central nervous system, angiopoietins and their receptors can be expressed by neurons as well as by cerebrovascular cell types. Ang1 promotes Akt phosphorylation in neurons, and thereby inhibits caspase-3 activation and attenuates apoptosis [175]. In cerebrovascular endothelial cells, hypoxia and ischemia can increase Ang2 mRNA and protein without effects on Ang1 or Tie2 [176, 177]. Cerebral ischemia also can promote transient and region specific changes in Tie1 and Tie2 expression that correspond with regional changes in cerebral blood flow [178]. Most interestingly, regions exhibiting increased angiogenic activity also demonstrated colocalization among Tie2, Ang2, FGF-2 and VEGF, emphasizing the critical role of interactions among factors involved in vascular remodeling [178]. To date, most studies of the roles of angiopoietins in cerebrovascular remodeling have focused on their contribution to responses of the cerebral microcirculation to ischemia [179]; systematic assessments of the effects of hypoxia alone on participation of angiopoietins in cerebrovascular remodeling have yet to be performed. Such studies could be particularly illuminating in regards to control of physiological cerebral angiogenesis and remodeling, particularly in the immature cerebral circulation where low oxygen tension and high prostaglandin concentrations are typical.
Fibroblast Growth Factor
The fibroblast growth factor (FGF) family includes 22 members that can act on any of the four FGF tyrosine kinase receptors [180]. As established mitogens for endothelial cells, basic fibroblast growth factors (FGF-2) can initiate angiogenesis by inducing endothelial cell proliferation and cord formation [181]. As for other angiogenic growth factors, FGFs are synergistic with VEGF and other vasotrophic factors in their ability to promote capillary formation [167]. The production of FGF-2 by capillary endothelial cells can act in an autocrine manner to stimulate further endothelial cell proliferation [182]. In addition to these autocrine effects, FGF-2 can regulate expression of other factors. For example, FGF-2 can upregulate PDGFR-α levels, allowing smooth muscle cells to become more responsive to PDGF-AA stimulation [144]. FGF-2 itself is subject to upregulation by PDGF-BB and TGF-β in VSMCs [183]. In relation to vascular remodeling, a particularly important effect of FGF-2 is its ability to induce morphological, and possibly phenotypic, transformation in aortic smooth muscle [184]. Such effects may be particularly important during hypoxia, in which FGF-2 can increase ROS production, stabilize HIF-1α and other ROS-sensitive transcription factors, and increase its own transcription and translation in an autocrine manner [148, 185, 186]. During episodes of postnatal chronic hypoxia, FGF-2 levels can be increased heterogeneously among different brain regions and are particularly prominent in immature glial cells [187]. In hypoxic neurons, FGF-2 may also improve neuronal survival, contribute to hypoxic conditioning and serve a neuroprotective role [186, 188, 189]. These neuroprotective effects can be observed also in hypoxic-ischemic neonatal rat brain [190]. Interestingly, FGF-2 appears to increase proliferation, retard maturation, and hinder differentiation of neural progenitor cells [191]. How FGF-2 affects vascular smooth muscle progenitor cells is unknown. This raises the untested possibility that a portion of the neuroprotective effects of FGF-2 following an interval of hypoxia may be attributable to potential protective effects on the multiple cell types that make up the arterial wall.
RECEPTOR TYROSINE KINASE-INDEPENDENT VASOTROPHIC FACTORS
The ability of hypoxia to promote vascular remodeling is clearly a consequence of a highly dynamic interplay among multiple vasotrophic factors and physiological influences. As indicated above, growth factors dependent upon tyrosine kinase receptors constitute a major component of this regulation. However, the vasotrophic factors involved in hypoxic remodeling also include many other growth factors that act independently of RTKs. One of the best studied of these RTK-independent vasotrophic factors in Transforming Growth Factor β.
Transforming Growth Factor β
The transforming growth factor beta (TGF-β) superfamily consists of three main isoforms, TGF-β1, TGF-β2, and TGF-β3 [192, 193], all of which can promote angiogenesis or vessel regression in tumors [194, 195]. TGF-β1 can decrease endothelial tube formation and cause capillary-like structures to regress [167]. The receptors for TGF-β molecules are serine-threonine kinases that phosphorylate Smad proteins, leading to their translocation to the nucleus where they alter transcription of numerous genes [196]. In smooth muscle cells, TGF-β1 can promote differentiation but is only one of many factors governing this process [197]. Of particular importance for vascular remodeling are the antagonistic interactions between TGF-β1 and FGF-2. In this context, either decreased FGF-2 or increased TGF-β1 can induce pericyte differentiation and expression of α-smooth muscle actin, leading to differentiation of the contractile smooth muscle phenotype [198].
Hypoxia can increase TGF-β2 mRNA and protein levels and enhance Smad2 and Smad3 phosphorylation in endothelial cells [199] (Fig. 4). Hypoxia-induced HIF-1 also binds Smad proteins, which serve as coactivators and thereby contribute to hypoxic vascular remodeling [200, 201]. Increases in TGF-β expression can induce G protein–coupled receptor kinase 2 (GRK2), a downstream effector of TGF-β, to desensitize G-protein coupled receptors via negative feedback, terminate TGF-β/Smad signaling, and inhibit Ang2-induced proliferation [202]. In the brain, TGF-β1 secreted by microglia and macrophages contribute to cerebrovascular remodeling following a focal ischemic insult [203]. These effects, together with the ability of TGF-β1 to inhibit microglial activation, help explain why TGF-β1 can be neuroprotective following hypoxic-ischemic insults [204–208]. Despite these many effects of TGF-β on vascular development and differentiation, systematic studies of the roles of this growth factor in normal growth and development of the cerebral vasculature have yet to be undertaken, particularly in relation to the vascular effects of hypoxia.
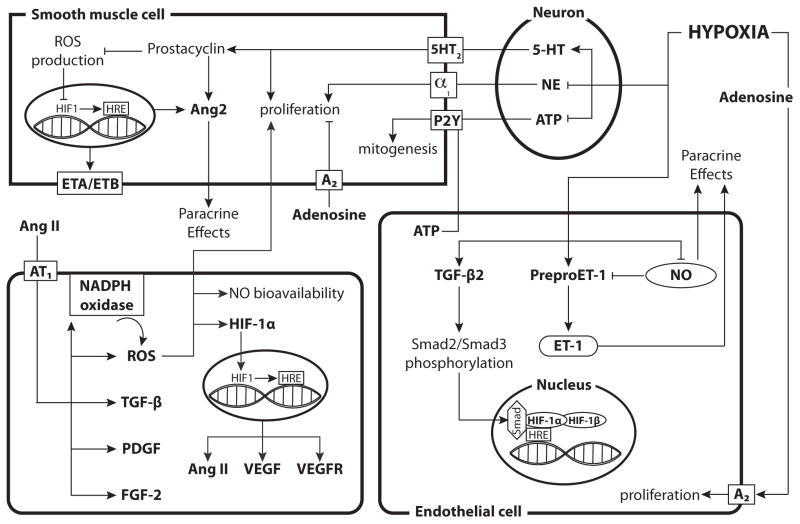
Fig. 4. Hypoxia has heterogeneous effects on Receptor Tyrosine Kinase-independent vasotrophic factor signaling across various cell types.
In perivascular nerves, hypoxia inhibits the synthesis and decreases the content of NE while enhancing serotonin (5-HT) synthesis. Elevated 5-HT levels can then induce proliferation of smooth muscle cells and increase prostacyclin levels, which inhibits ROS production and increase Ang2 production. Adenosine can activate A2 receptors and inhibit proliferation while ATP enhances mitogenesis in SMCs but can also increase endothelial cell proliferation. Hypoxia enhances the expression of TGF-β, preproET-1 and ET-1 while inhibiting NO synthesis in endothelial cells. Hypoxia also enhances expression of both ET receptors in smooth muscle, thereby enhancing the effects of ET-1. Increased TGF-β2 levels enhance Smad2/Smad3 phosphorylation, which can then act as a coactivator for HIF-1. Angiotensin II activates AT1, which leads to an increase in FGF-2, PDGF, TGF-β, and NADPH oxidase. Increased NADPH oxidase leads to enhanced ROS production, which can inhibit NO bioavailability and induce hypertrophy and hyperplasia of smooth muscle cells. ROS can also increase the gene expression of HIF-1α and stabilize the HIF-1α protein. The HIF-1 complex then enters the nucleus, binds HREs, and results in increased transcription of VEGF, VEGF receptors, and Ang II. The diagram includes separate depictions of mechanisms in neurons, smooth muscle cells, and endothelial cells. For reference, a generic (parenchymal) cell is depicted in the lower left corner.
Nitric Oxide
The vascular endothelium plays a critical role in regulating active vascular tone under both normoxic and hypoxic conditions through the release of two main vasoactive factors, nitric oxide (NO) and endothelin (ET), with generally opposing effects on contractility [209, 210]. In addition to their well-documented vasomotor roles, however, both of these factors also exert continuous and opposing trophic influences on adjacent smooth muscle. Given that hypoxia increases endothelin synthesis but decreases NO synthesis and release, both of these factors are important contributors to hypoxic vascular remodeling [66, 211].
The vasorelaxant characteristics of nitric oxide arise largely from its ability to activate soluble guanylate cyclase and increase cGMP synthesis, which activates the serine-threonine kinase Protein Kinase G (PKG) [210, 212, 213]. PKG, in turn, can phosphorylate a broad variety of substrates within smooth muscle, including transcription factors such as CREB that govern smooth muscle phenotype [214, 215]. Aside from smooth muscle, PKG can also play a role in endothelial cell differentiation and tube formation [216]. Apart from PKG, NO can also downregulate expression of other vasotrophic factors, including preproET-1 and PDGF-B [217].
Physiological release of endothelial nitric oxide is governed primarily by fluid shear stress. Not only does shear stress promote the immediate release of NO, it also can upregulate eNOS mRNA and the long-term capacity for NO release [218]. Levels of eNOS are also increased by FGF-2 [216]. Similarly, stimulation of the insulin receptor can activate PI3K and Akt pathways to induce NO production, suggesting that changes in insulin receptor density influence NO release [219]. Through activation of the ETA receptor, endothelin can also upregulate expression of eNOS in pulmonary vascular endothelium [220]. Because oxygen radicals can rapidly inactivate NO [107], any long-term change in anti-oxidant activity also changes NO action on adjacent smooth muscle. Statin treatment can also increase NO bioavailability in fetal sheep, most probably through an increased capacity for NO synthesis [221]. Equally important, the capacity for NO synthesis and release in most vascular beds increases with developmental age [222, 223], which helps explain certain age-related differences in reactivity to endothelium-dependent vasodilators [224] but also predicts that the role of NO in vascular remodeling strengthens with advancing postnatal age.
Under conditions of hypoxia, changes in NO production are highly heterogeneous and depend on the duration and intensity of hypoxia in an organ specific manner. In the hypoxic lung, NO can promote angiogenesis and ameliorate hypoxic pulmonary hypertension [225]. Hypoxia also increases eNOS mRNA in the pulmonary vasculature, which helps attenuate pulmonary remodeling and hypertrophy [220, 226]. In contrast, in the cerebral and femoral vasculatures, NO production is depressed, which compromises NO-dependent stabilization of contractility and promotes remodeling [227, 228]. Attenuation of the capacity for NO release by chronic hypoxia is further enhanced by simultaneous reductions in sGC activity in both fetal and adult arteries [50]. In parallel, chronic hypoxia enhanced neuronal NOS expression in fetal brain homogenates [229] but depressed nNOS expression in the perivascular nerves innervating middle cerebral arteries [230], suggesting that hypoxia exerts opposite and tissue specific effects on NO production within the brain. Most interestingly, cerebral expression of eNOS, nNOS and iNOS were all increased following recovery from hypoxia, demonstrating that overall regulation of NO production is very tightly controlled. Altogether, these findings emphasize that NO stabilizes the contractile phenotype but is only one of many factors that contribute to the highly integrated, multifactorial processes determining vascular differentiation and remodeling, particularly during sustained hypoxia.
Endothelins
The discovery that vascular endothelium mediates acetylcholine-induced vasodilatation [231] through the release of NO [232] motivated numerous follow-up studies of other possible endothelium-derived vasoactive factors. In 1988, Yanagisawa reported that in addition to NO, the endothelium also releases endothelin (ET), one of the most potent endogenous vasoconstrictors ever discovered [233]. Three isoforms of endothelin have been identified (ET-1, ET-2, and ET-3) and these activate two separate endothelin receptors (ETA and ETB) [234]. The two ET receptors display distinct affinities for each ET subtype and often exhibit opposing actions; ETA can induce vasoconstriction whereas ETB can stimulate vasodilation, depending on the location and distribution of each receptor type [235, 236]. In some situations, ET can also induce release of vasodilators [237, 238].
Endothelin is implicated in many diseases, especially in hypertension-induced remodeling [239]. ET appears involved in hypertension-induced hypertrophy of cerebral arteries without changing their distensibility [240]. Diabetic mice can also display increased ET receptor levels and ET-1 dependent matrix metalloproteinase activation, which can facilitate cerebrovascular remodeling, especially after hypoxic exposure [241, 242]. Through binding to ETA receptor, increased ET levels can activate the transcription factor Nuclear Factor of Activated T cells, isoform 3 (NFAT3), resulting in hypertension and vascular remodeling. In smooth muscle, NFAT3 can increase smooth muscle α-actin mRNA and contribute to increased cross-sectional wall thickness in mesenteric arteries [243].
Expression and release of ETs are regulated by a broad variety of influences. Importantly, ETs can be produced by non-endothelial cell types, including vascular smooth muscle, although at a much lower rate than by endothelial cells [244]. Levels of ET mRNA in cultured human vascular smooth muscle can be enhanced by numerous vasotrophic factors including Ang2, TGF-β, and PDGF-AA [245]. In pulmonary artery smooth muscle, TGF-β can directly enhance expression of mRNA for preproET-1 (ET-1 precursor) and thereby increase ET-1 expression [246, 247]. In feedback fashion, hypoxia-induced increases in RTK-dependent growth factors (FGF-1, FGF-2, and PDGF-BB), but not G-protein coupled vasotrophic factors (Angiotensin-II and ET-1) can upregulate ETA expression in cultured pulmonary artery smooth muscle [248].
Hypoxia is a particularly important regulator of ET expression in many vascular beds. In the rat kidney, hypoxia increases ET-1 expression [121]. In the rat pulmonary circulation, hypoxia can increase both pulmonary and plasma ET expression [249]. In mouse and human pulmonary artery endothelial cells, hypoxia can increase expression of not only ET-1, but also Endothelin Converting Enzyme-1, ETA, and ETB [250]. Chronic hypoxia can also increase mRNA levels for preproET-1 and ET-1 protein in pulmonary smooth muscle and epithelium together with increased medial thickness of bronchiolar arteries [251]. In turn, hypoxic increases in ET-1 can be attenuated by PPARγ activation [250]. Interactions between NO and ET, both of which are modulated by hypoxia, also affect the hypoxic remodeling response. In this manner, endothelium derived NO can attenuate hypoxic remodeling and medial hypertrophy secondary to increased ET-1 levels [226]. NO can also downregulate ET-1 levels and this effect can be strong enough to abrogate hypoxia-induced increases in ET-1 mRNA and protein in endothelial cells [217]. In feedback fashion, hypoxic increases in ET-1 can act through the ETA and ETB receptors to elevate eNOS mRNA in the pulmonary vasculature while also increasing circulating hematocrit and ET-1 levels. These increased ET-1 levels promote thickening of the medial layer in pulmonary arteries [220].
In the normoxic central nervous system, neurons and endothelial cells express preproET-1, and neurons also express both ETA and ETB receptors [252, 253]. Following a hypoxic-ischemic insult, ET-1 expression is upregulated primarily in endothelial and glial cells [253, 254]. Hypoxia-ischemia can also upregulate ETA and ETB receptors in cerebral arteries [255]. Such changes in ET-1 signaling pathways can have important consequences for post-ischemic recovery, given that ET-1 can reduce cerebral perfusion under normoxic, hypoxic, and hypercapnic conditions, such as those typical of the post-ischemic brain [256]. Consistent with this possibility, overexpression of ET-1 can compromise blood-brain-barrier integrity and enhance edema following an ischemic cerebral insult [257]. In addition, by virtue of its properties as an endogenous ET antagonist [258], the hormone relaxin has the potential to ameliorate ET-induced cerebrovascular remodeling [259]. This hypothesis awaits direct experimental confirmation, as does the more general hypothesis that age-dependent hypoxic cerebrovascular remodeling is mediated, at least in part, by increased ET-1 effects on cerebral arteries.
Angiotensin II
The renin-angiotensin system is best known for its critical roles in regulation of salt and water balance, and how dysfunction of this regulation can lead to hypertension. Hypertension associated with elevated production of angiotension, in turn, can also lead to secondary changes in vascular structure and function [260, 261]. Some of this remodeling, however, may be due to direct vasotrophic effects of angiotensin II on vascular smooth muscle [262, 263]. Correspondingly, any perturbations that alter the levels or activity of Angiotensin Converting Enzyme (ACE), responsible for the conversion of angiotensin I to angiotensin II, also play a role in hypertensive remodeling and atherosclerosis [264].
Angiotensins include four main molecules (-I, -II, -III, and -IV) that bind and activate two isoforms of G-protein coupled receptors, the AT1 and AT2 [265]. AT1 receptors can be further sub-classified as AT1A or AT1B, each with a unique tissue distribution [266, 267]. The AT1 receptor appears to induce vascular remodeling when activated by angiotensin II [262, 268]. The AT2 receptor is typically less abundant than the AT1 except in developing tissues [268]. Stimulation of AT2 receptors can inhibit proliferation and induce cellular differentiation [269]. The AT2 receptor also can stimulate NO production and cGMP increases in the kidneys, especially during sodium depletion [270]. Tissue distributions of AT1 and AT2 receptors are highly heterogeneous, but both receptors can be expressed on vascular endothelia where they generally exert opposing effects [271]. Similarly, the AT1 and AT2 receptors also have opposing actions on angiotensin II mediated regulation of circulating blood volume and pressure [268, 272]. These effects can involve interactions among angiotensin II receptors, and the mineralocorticoid receptors that bind and respond to aldosterone [273]. Local inflammation can enhance the ability of angiotensin II to induce vascular remodeling [274]. Typically, angiotensin II stimulates expression of PDGF and TGF-β through activation of AT1 receptors [275], and can increase eNOS and NO release in fetoplacental artery endothelial cells [276]. Angiotensin II can also transactivate certain tyrosine kinase receptors, including those that mediate responses to PDGF [262, 277].
One of the most important effects of AT1 activation is increased formation of reactive oxygen species (ROS) [278]. These ROS molecules, which may originate from membrane-bound NADPH oxidase or mitochondrial synthesis [279], can induce vascular smooth muscle hypertrophy, hyperplasia, and migration [280, 281]. Activation of AT1 by angiotensin II can increase the expression and activity of membrane-bound NADPH oxidase, and thereby stimulate ROS production [275, 278]. Increases in ROS can, in turn, have many effects including reaction with NO leading to decreased NO bioavailability. In turn, loss of NO can enhance the effects of angiotensin II on smooth muscle growth by upregulating AT1 receptors, and can increase expression of endothelial ACE and ET-1 [282]. Angiotensin II can also increase HIF-1α gene expression and protein stability via a ROS-dependent mechanism [283, 284].
Numerous physiological and pathological perturbations influence the levels and cardiovascular effects of the angiotensins. Angiotensin II can be induced by VEGF, resulting in a positive feedback loop, in which the increased angiotensin II activates AT1 receptors that further increase expression of HIF-1, VEGF, and VEGF receptors leading to additional increases in angiotensin II [285]. Hypoxia can alter AT1 expression through mechanisms that appear highly sensitive to history and context; hypoxia has been reported both to increase [286] and decrease [287] AT1 expression in vascular smooth muscle. During hypertension, the effects of angiotension II can be modulated by the simultaneous actions of FGF-2, resulting in enhanced stimulation of smooth muscle hypertrophy, proliferation and remodeling in cerebral but not extracerebral arteries. Conversely, angiotensin II can stimulate FGF-2 synthesis, and thereby amplify its effects on hypertension-induced cerebrovascular remodeling [288]. How angiotensin II contributes to hypoxic cerebrovascular remodeling remains unstudied, particularly in the immature cerebral circulation.
Catecholamines
Catecholamines serve important roles as neurotransmitters in both the central and peripheral nervous systems [289]. Aside from their well-documented effects on post-synaptic G-protein coupled receptors, both norepinephrine (NE) and serotonin (5-HT) can exert trophic effects on smooth muscle. These effects were recognized for NE in the late 1970s when it was observed that sympathetic denervation caused a relative atrophy and thinning of rabbit cerebral arteries [290, 291]. Subsequent studies furthered these findings and documented the ability of adrenergic perivascular nerves to stimulate phenotypic transformation in vascular smooth muscle [292] through activation of α1A adrenergic receptors by NE [293]. Because chronic hypoxia can depress NE content and stimulation-evoked release [230, 294], chronic hypoxia should also attenuate the trophic influence of NE on cerebrovascular smooth muscle growth and differentiation. In addition, chronic hypoxia appears to depress NO release by perivascular nitrergic nerves [230], which should further compromise adrenergic vasotrophic stimulation of cerebrovascular growth and differentiation. Given that the adrenergic neuroeffector apparatus is functionally immature in fetal cerebral arteries [295], these results raise the possibility that cerebrovascular maturation relies on increasing trophic support from the adrenergic perivascular innervation. In turn, if chronic hypoxia inhibits the functional maturation of the adrenergic perivascular innervation, then the functional effects should be similar to adrenergic denervation in the fetal cerebral circulation. This hypothesis awaits experimental evaluation.
The other main neurotransmitter catecholamine with trophic effects is serotonin. This molecule can act through a broad variety of G-protein-coupled receptors [296, 297] that are heterogeneously expressed by both the smooth muscle and endothelium of virtually all blood vessel types [298]. In the pulmonary circulation, 5-HT can increase vascular permeability and induce smooth muscle proliferation. These effects appear to be mediated through activation of 5-HT1B receptors and subsequent stimulation of ROS production [299]. Pathological increases in the expression of serotonin transporters (5-HTT or SERT) appear to enhance the proliferative, ROS-dependent effects of 5-HT on pulmonary smooth muscle [296, 300]. Some mitogenic effects of 5-HT, however, may be attributable to increased prostaglandin synthesis [297]. For example, 5-HT can stimulate prostacyclin production in aortic smooth muscle [301]. Prostacyclin, in turn, can stabilize HIF-1 through attenuation of ROS production [62] and both prostacyclin and PGE2 can increase expression of Ang2 [157]. Stimulation of prostacyclin receptors can upregulate smooth muscle cell contractile markers reflecting a shift from synthetic to contractile phenotype [30]. These effects of 5-HT may be more pronounced in older individuals [302]. Owing to the ability of estradiol to potentiate the proliferative effects of 5-HT, these effects can be more pronounced in females than in males and may contribute to the higher incidence of pulmonary arterial hypertension observed in women [303].
In relation to hypoxic vascular remodeling, hypoxia can increase mRNA for 5-HT and thereby enhance smooth muscle proliferation [304]. Adenosine, whose concentrations are elevated by hypoxia, can potentiate the proliferative effects of 5-HT by enhancing expression of the 5-HT transporter. This effect leads to internalization of 5-HT and increased ROS production, which contributes to the mitogenic effects of 5-HT on smooth muscle [304]. In contrast to other vasotrophic factors, hypoxia appears to have little effect on the expression of 5-HT receptors and their artery-size dependent patterns of expression [305]. It remains possible, however, that the perivascular serotonergic cerebrovascular innervation could be modulated by chronic hypoxia, as suggested for the adrenergic innervation. Because the serotonergic innervation is completely intracranial [306], it is not surgically possible to perform a denervation and observe the resulting effects on cerebovascular growth, differentiation, and function. Confirmation of a vasotrophic role for the serotonergic cerebrovascular innervation must await the development of alternative experimental approaches.
Purines
As a class, the purines couple tissue metabolic activity to vascular growth, proliferation, and contraction through actions on three main classes of G-protein coupled purinergic receptors (P1, P2X, and P2Y) [307]. Adenosine can activate four P1 receptors (A1, A2A, A2B, and A3) and also the P2X1 receptor. ADP can activate both P2X and P2Y receptors [308]. ATP can bind and activate P2X1 and P2Y receptors [308–311]. Together, the purines help regulate endothelial and smooth muscle proliferation, migration, and apoptosis and thereby contribute significantly to many patterns of vascular remodeling [310]. ATP, released by perivascular nerves and endothelial cells, can promote mitogenesis in vascular smooth muscle [312]. In relation to regulation of smooth muscle phenotype, synthetic smooth muscle tends to express P2Y1 and P2Y2 receptors more than P2X1, whereas in contractile smooth muscle P2X1 abundance predominates over that of the P2Y isoforms [29, 312]. This pattern raises the important question: are patterns of P2X and P2Y receptor expression a cause, or a consequence, of phenotypic transformation in smooth muscle? ADP can also induce proliferation and migration of endothelial cells, and can activate A2 receptors to inhibit proliferation of smooth muscle cells [312]. In addition, ADP acts synergistically with PDGF, TGF-β, among others to induce VSM proliferation [310, 312]. Extracellular adenosine can contribute to pulmonary vascular remodeling via A2A receptors, and extracellular actions of both ATP and adenosine can stimulate endothelial apoptosis and act through A2 receptor, a P1 receptor subtype, to inhibit SMC proliferation [312, 313]. Hypoxia can inhibit ATP production due to decreased oxygen availability. On the other hand, hypoxia increases adenosine levels and thereby amplifies the proliferative effects of adenosine. For example, hypoxic increases in adenosine activate endothelial A2A and A2B receptors and stimulate EC proliferation [311, 312]. Importantly, A2B receptor stimulation can also increase VEGF mRNA to promote angiogenesis [312]. Through activation of P2 and A2A receptors, adenosine can also promote NO release and thereby activate NO-dependent influences on smooth muscle growth and differentiation [310, 311]. Hypoxia further potentiates these effects of adenosine by inhibiting the abundance and activity of adenosine kinase, the enzyme responsible for recycling of adenosine through conversion into AMP [314]. This effect is mediated by HIF-1α binding to HREs, which depresses transcription of the adenosine kinase gene [314]. As a group, the purines are important mediators of the coupling between oxygen dependent metabolic activity and vascular function. The importance of these mechanisms in the cerebral circulation remains largely unstudied in all age groups. Owing to the common therapeutic use of agents such as dipyridamole that alter circulating purine levels and actions [315], the potential vasotrophic effects of such treatments urge caution.
FUTURE DIRECTIONS
The past decade has ushered in a revolution in the understanding of vascular biology. The classical view of blood vessels as static, homogeneous structures has slowly yielded to the more contemporary view of the vascular wall as a highly dynamic and heterogeneous tissue with multiple cell types undergoing regular phenotypic transformation. The extent and character of these transformations are governed by a growing list of vasotrophic factors that continuously modulate vessel structure and function to support tissue growth and metabolic demand. The vasotrophic factors involved include not only the classical receptor tyrosine kinase ligands such as VEGF, PDGF, angiopoietins and FGF, but also a diverse category of smaller multifunctional molecules that influence smooth muscle growth and proliferation independent of receptor tyrosine kinases. This category includes TGF-β, nitric oxide, endothelin, angiotensin II, catecholamines, and purines. These non-classical vasotrophic factors appear to help fine-tune vascular composition and reactivity to meet the demands of tissue growth, development, and physiological stress. As seen repeatedly, the expression of these vasotrophic factors can be heterogeneous among various tissue types and vascular beds to ensure a close coupling between metabolic supply and demand. These fundamental differences in oxygen requirements for metabolic homeostasis among various tissues imply different susceptibilities to hypoxic insults. Consequently, both functional and structural adaptations of the vasculature are also organ specific. These mechanisms integrate to assure that blood flow and metabolic demand are closely matched in all vascular beds, especially under environmental stresses such as hypoxia. From this perspective, one of the most promising future endeavors will be to better understand the basic principle of “excitation-transcription coupling”, as introduced by Wamhoff [316]. This idea advances the notion that the same calcium transients that initiate muscle contraction simultaneously help activate key transcription factors, such as myocardin [317, 318], that drive expression of genes coding for critical proteins required for contraction. In this manner, contractile stimuli produce both short-term and long-term effects that serve to “condition” the blood vessels involved. How these signals integrate with other vasotrophic signals, growth factors, and pathogenic stimuli remains an exciting arena for future investigation.
Footnotes
Send Orders for Reprints to reprints@benthamscience.net
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Kubis N, Checoury A, Tedgui A, Levy BI. Adaptive common carotid arteries remodeling after unilateral internal carotid artery occlusion in adult patients. Cardiovascular research. 2001;50(3):597–602. doi: 10.1016/s0008-6363(01)00206-1. Epub 2001/05/30. [DOI] [PubMed] [Google Scholar]
- 2.Masuda H, Sugita A, Zhuang YJ. Pathology of the arteries in the central nervous system with special reference to their dilatation: blood flow. Neuropathology: official journal of the Japanese Society of Neuropathology. 2000;20(1):98–103. doi: 10.1046/j.1440-1789.2000.00274.x. Epub 2000/08/10. [DOI] [PubMed] [Google Scholar]
- 3.Bloor CM. Angiogenesis during exercise and training. Angiogenesis. 2005;8(3):263–71. doi: 10.1007/s10456-005-9013-x. Epub 2005/12/06. [DOI] [PubMed] [Google Scholar]
- 4.Amaral SL, Papanek PE, Greene AS. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am J Physiol Heart Circ Physiol. 2001;281(3):H1163–9. doi: 10.1152/ajpheart.2001.281.3.H1163. Epub 2001/08/22. [DOI] [PubMed] [Google Scholar]
- 5.White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol. 1998;85(3):1160–8. doi: 10.1152/jappl.1998.85.3.1160. Epub 1998/09/08. [DOI] [PubMed] [Google Scholar]
- 6.Zoeller RF, Angelopoulos TJ, Thompson BC, et al. Vascular remodeling in response to 12 wk of upper arm unilateral resistance training. Medicine and science in sports and exercise. 2009;41(11):2003–8. doi: 10.1249/MSS.0b013e3181a70707. Epub 2009/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. Epub 2009/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connors G, Hunse C, Gagnon R, Richardson B, Han V, Rosenberg H. Perinatal assessment of cerebral flow velocity wave forms in the human fetus and neonate. Pediatric research. 1992;31(6):649–52. doi: 10.1203/00006450-199206000-00022. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 9.Wang DB, Blocher NC, Spence ME, Rovainen CM, Woolsey TA. Development and remodeling of cerebral blood vessels and their flow in postnatal mice observed with in vivo videomicroscopy. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1992;12(6):935–46. doi: 10.1038/jcbfm.1992.130. Epub 1992/11/01. [DOI] [PubMed] [Google Scholar]
- 10.Hutanu C, Cox BE, DeSpain K, Liu XT, Rosenfeld CR. Vascular development in early ovine gestation: carotid smooth muscle function, phenotype, and biochemical markers. American journal of physiology Regulatory, integrative and comparative physiology. 2007;293(1):R323–33. doi: 10.1152/ajpregu.00851.2006. Epub 2007/05/04. [DOI] [PubMed] [Google Scholar]
- 11.Baumbach GL, Heistad DD. Drug-induced changes in mechanics and structure of cerebral arterioles. Journal of hypertension Supplement: official journal of the International Society of Hypertension. 1992;10(6):S137–40. Epub 1992/08/01. [PubMed] [Google Scholar]
- 12.Nag S. Immunohistochemical localization of extracellular matrix proteins in cerebral vessels in chronic hypertension. Journal of neuropathology and experimental neurology. 1996;55(3):381–8. doi: 10.1097/00005072-199603000-00014. Epub 1996/03/01. [DOI] [PubMed] [Google Scholar]
- 13.Chillon JM, Baumbach GL. Effects of chronic nitric oxide synthase inhibition on cerebral arterioles in Wistar-Kyoto rats. Journal of hypertension. 2004;22(3):529–34. doi: 10.1097/00004872-200403000-00015. Epub 2004/04/13. [DOI] [PubMed] [Google Scholar]
- 14.Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21(6 Pt 1):816–26. doi: 10.1161/01.hyp.21.6.816. Epub 1993/06/01. [DOI] [PubMed] [Google Scholar]
- 15.Heistad DD, Baumbach GL. Cerebral vascular changes during chronic hypertension: good guys and bad guys. Journal of hypertension Supplement: official journal of the International Society of Hypertension. 1992;10(7):S71–5. Epub 1992/12/01. [PubMed] [Google Scholar]
- 16.Zhang ZD, Macdonald RL. Contribution of the remodeling response to cerebral vasospasm. Neurological research. 2006;28(7):713–20. doi: 10.1179/016164106X151990. Epub 2006/12/14. [DOI] [PubMed] [Google Scholar]
- 17.Humphrey JD. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell biochemistry and biophysics. 2008;50(2):53–78. doi: 10.1007/s12013-007-9002-3. Epub 2008/01/23. [DOI] [PubMed] [Google Scholar]
- 18.Coutard M, Osborne-Pellegrin M, Fontaine V, Jacob MP, Michel JB. High-flow-induced arterial remodeling in rats with different susceptibilities to cerebral aneurysms. Journal of vascular research. 2006;43(3):217–28. doi: 10.1159/000091101. Epub 2006/01/24. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Tian Y, Wei HJ, Chen J, Dong JF, Zacharek A, et al. Erythropoietin increases circulating endothelial progenitor cells and reduces the formation and progression of cerebral aneurysm in rats. Neuroscience. 2011;181:292–9. doi: 10.1016/j.neuroscience.2011.02.051. Epub 2011/03/08. [DOI] [PubMed] [Google Scholar]
- 20.Vikman P, Beg S, Khurana TS, Hansen-Schwartz J, Edvinsson L. Gene expression and molecular changes in cerebral arteries following subarachnoid hemorrhage in the rat. J Neurosurg. 2006;105(3):438–44. doi: 10.3171/jns.2006.105.3.438. Epub 2006/09/12. [DOI] [PubMed] [Google Scholar]
- 21.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi: 10.1152/physrev.00041.2003. Epub 2004/07/23. [DOI] [PubMed] [Google Scholar]
- 22.Rensen SS, Doevendans PA, van Eys GJ. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands heart journal: monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2007;15(3):100–8. doi: 10.1007/BF03085963. Epub 2007/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams JM, Pearce WJ. Age-dependent modulation of endothelium-dependent vasodilatation by chronic hypoxia in ovine cranial arteries. J Appl Physiol. 2006;100(1):225–32. doi: 10.1152/japplphysiol.00221.2005. Epub 2005/09/24. [DOI] [PubMed] [Google Scholar]
- 24.Dupuis F, Atkinson J, Liminana P, Chillon JM. Comparative effects of the angiotensin II receptor blocker, telmisartan, and the angiotensin-converting enzyme inhibitor, ramipril, on cerebrovascular structure in spontaneously hypertensive rats. Journal of hypertension. 2005;23(5):1061–6. doi: 10.1097/01.hjh.0000166848.95592.a5. Epub 2005/04/19. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima N, Nagahiro S, Sano T, Satomi J, Satoh K. Phenotypic modulation of smooth muscle cells in human cerebral aneurysmal walls. Acta neuropathologica. 2000;100(5):475–80. doi: 10.1007/s004010000220. Epub 2000/10/25. [DOI] [PubMed] [Google Scholar]
- 26.Ohkuma H, Suzuki S, Ogane K. Phenotypic modulation of smooth muscle cells and vascular remodeling in intraparenchymal small cerebral arteries after canine experimental subarachnoid hemorrhage. Neurosci Lett. 2003;344(3):193–6. doi: 10.1016/s0304-3940(03)00464-6. [DOI] [PubMed] [Google Scholar]
- 27.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacological reviews. 2004;56(4):549–80. doi: 10.1124/pr.56.4.3. Epub 2004/12/17. [DOI] [PubMed] [Google Scholar]
- 28.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284(6):3728–38. doi: 10.1074/jbc.M808788200. Epub 2008/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erlinge D, Hou M, Webb TE, Barnard EA, Moller S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem Biophys Res Commun. 1998;248(3):864–70. doi: 10.1006/bbrc.1998.9083. Epub 1998/08/15. [DOI] [PubMed] [Google Scholar]
- 30.Fetalvero KM, Shyu M, Nomikos AP, et al. The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol. 2006;290(4):H1337–46. doi: 10.1152/ajpheart.00936.2005. Epub 2006/01/10. [DOI] [PubMed] [Google Scholar]
- 31.Takagi Y, Hattori I, Nozaki K, Ishikawa M, Hashimoto N. DNA fragmentation in central nervous system vascular malformations. Acta neurochirurgica. 2000;142(9):987–94. doi: 10.1007/s007010070053. Epub 2000/11/22. [DOI] [PubMed] [Google Scholar]
- 32.Greenough A, Khetriwal B. Pulmonary hypertension in the newborn. Paediatric respiratory reviews. 2005;6(2):111–6. doi: 10.1016/j.prrv.2005.03.005. Epub 2005/05/25. [DOI] [PubMed] [Google Scholar]
- 33.Therese P. Persistent pulmonary hypertension of the newborn. Paediatric respiratory reviews. 2006;7 (Suppl 1):S175–6. doi: 10.1016/j.prrv.2006.04.211. Epub 2006/06/27. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Chen SJ, Chen YF, Meng QC, Durand J, Oparil S, et al. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. Journal of applied physiology. 1994;77(3):1451–9. doi: 10.1152/jappl.1994.77.3.1451. Epub 1994/09/01. [DOI] [PubMed] [Google Scholar]
- 35.Pearce W. Hypoxic regulation of the fetal cerebral circulation. Journal of applied physiology. 2006;100(2):731–8. doi: 10.1152/japplphysiol.00990.2005. Epub 2006/01/20. [DOI] [PubMed] [Google Scholar]
- 36.Xu K, Lamanna JC. Chronic hypoxia and the cerebral circulation. Journal of applied physiology. 2006;100(2):725–30. doi: 10.1152/japplphysiol.00940.2005. Epub 2006/01/20. [DOI] [PubMed] [Google Scholar]
- 37.Patt S, Sampaolo S, Theallier-Janko A, Tschairkin I, Cervos-Navarro J. Cerebral angiogenesis triggered by severe chronic hypoxia displays regional differences. J Cereb Blood Flow Metab. 1997;17(7):801–6. doi: 10.1097/00004647-199707000-00010. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 38.Boero JA, Ascher J, Arregui A, Rovainen C, Woolsey TA. Increased brain capillaries in chronic hypoxia. Journal of applied physiology. 1999;86(4):1211–9. doi: 10.1152/jappl.1999.86.4.1211. Epub 1999/04/08. [DOI] [PubMed] [Google Scholar]
- 39.Milner R, Hung S, Erokwu B, Dore-Duffy P, LaManna JC, del Zoppo GJ. Increased expression of fibronectin and the alpha 5 beta 1 integrin in angiogenic cerebral blood vessels of mice subject to hypobaric hypoxia. Mol Cell Neurosci. 2008;38(1):43–52. doi: 10.1016/j.mcn.2008.01.013. Epub 2008/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207(Pt 18):3163–9. doi: 10.1242/jeb.00976. Epub 2004/08/10. [DOI] [PubMed] [Google Scholar]
- 41.LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. Journal of applied physiology. 1992;72(6):2238–43. doi: 10.1152/jappl.1992.72.6.2238. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Welser JV, Milner R. Absence of the alpha v beta 3 integrin dictates the time-course of angiogenesis in the hypoxic central nervous system: accelerated endothelial proliferation correlates with compensatory increases in alpha 5 beta 1 integrin expression. J Cereb Blood Flow Metab. 2010;30(5):1031–43. doi: 10.1038/jcbfm.2009.276. Epub 2010/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coulson RJ, Chesler NC, Vitullo L, Cipolla MJ. Effects of ischemia and myogenic activity on active and passive mechanical properties of rat cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283(6):H2268–75. doi: 10.1152/ajpheart.00542.2002. Epub 2002/10/22. [DOI] [PubMed] [Google Scholar]
- 44.Butler SM, Abrassart JM, Hubbell MC, et al. Contributions of VEGF to age-dependent transmural gradients in contractile protein expression in ovine carotid arteries. Am J Physiol Cell Physiol. 2011;301(3):C653–66. doi: 10.1152/ajpcell.00413.2010. Epub 2011/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanaan A, Farahani R, Douglas RM, Lamanna JC, Haddad GG. Effect of chronic continuous or intermittent hypoxia and reoxygenation on cerebral capillary density and myelination. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R1105–14. doi: 10.1152/ajpregu.00535.2005. Epub 2005/12/03. [DOI] [PubMed] [Google Scholar]
- 46.Ment LR, Stewart WB, Fronc R, et al. Vascular endothelial growth factor mediates reactive angiogenesis in the postnatal developing brain. Brain Res Dev Brain Res. 1997;100(1):52–61. doi: 10.1016/s0165-3806(97)00012-6. Epub 1997/05/20. [DOI] [PubMed] [Google Scholar]
- 47.Tweed WA, Cote J, Pash M, Lou H. Arterial oxygenation determines autoregulation of cerebral blood flow in the fetal lamb. Pediatr Res. 1983;17(4):246–9. doi: 10.1203/00006450-198304000-00002. Epub 1983/04/01. [DOI] [PubMed] [Google Scholar]
- 48.Dubiel M, Gunnarsson GO, Gudmundsson S. Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet Gynecol. 2002;20(2):117–21. doi: 10.1046/j.1469-0705.2002.00758.x. Epub 2002/08/03. [DOI] [PubMed] [Google Scholar]
- 49.Salihagic-Kadic A, Medic M, Jugovic D, et al. Fetal cerebrovascular response to chronic hypoxia--implications for the prevention of brain damage. J Matern Fetal Neonatal Med. 2006;19(7):387–96. doi: 10.1080/14767050600637861. Epub 2006/08/23. [DOI] [PubMed] [Google Scholar]
- 50.Pearce WJ, Williams JM, White CR, Lincoln TM. Effects of chronic hypoxia on soluble guanylate cyclase activity in fetal and adult ovine cerebral arteries. Journal of applied physiology. 2009;107(1):192–9. doi: 10.1152/japplphysiol.00233.2009. Epub 2009/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Longo LD, Hull AD, Long DM, Pearce WJ. Cerebrovascular adaptations to high-altitude hypoxemia in fetal and adult sheep. Am J Physiol. 1993;264(1 Pt 2):R65–72. doi: 10.1152/ajpregu.1993.264.1.R65. [DOI] [PubMed] [Google Scholar]
- 52.Longo LD, Pearce WJ. Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: a model for prenatal programming of adult disease? Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R16–24. doi: 10.1152/ajpregu.00462.2004. Epub 2004/12/14. [DOI] [PubMed] [Google Scholar]
- 53.Nauli SM, Williams JM, Gerthoffer WT, Pearce WJ. Chronic hypoxia modulates relations among calcium, myosin light chain phosphorylation, and force differently in fetal and adult ovine basilar arteries. Journal of applied physiology. 2005;99(1):120–7. doi: 10.1152/japplphysiol.01131.2004. Epub 2005/07/23. [DOI] [PubMed] [Google Scholar]
- 54.Buchholz J, Duckles SP. Chronic hypoxia alters prejunctional alpha(2)-receptor function in vascular adrenergic nerves of adult and fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281(3):R926–34. doi: 10.1152/ajpregu.2001.281.3.R926. Epub 2001/08/17. [DOI] [PubMed] [Google Scholar]
- 55.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Molecular and cellular biology. 1992;12(12):5447–54. doi: 10.1128/mcb.12.12.5447. Epub 1992/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends in molecular medicine. 2001;7(8):345–50. doi: 10.1016/s1471-4914(01)02090-1. Epub 2001/08/23. [DOI] [PubMed] [Google Scholar]
- 57.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92(12):5510–4. doi: 10.1073/pnas.92.12.5510. Epub 1995/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95(14):7987–92. doi: 10.1073/pnas.95.14.7987. Epub 1998/07/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schultz K, Fanburg BL, Beasley D. Hypoxia and hypoxia-inducible factor-1alpha promote growth factor-induced proliferation of human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;290(6):H2528–34. doi: 10.1152/ajpheart.01077.2005. Epub 2006/01/10. [DOI] [PubMed] [Google Scholar]
- 60.Lauzier MC, Page EL, Michaud MD, Richard DE. Differential regulation of hypoxia-inducible factor-1 through receptor tyrosine kinase transactivation in vascular smooth muscle cells. Endocrinology. 2007;148(8):4023–31. doi: 10.1210/en.2007-0285. Epub 2007/05/19. [DOI] [PubMed] [Google Scholar]
- 61.Gorlach A, Diebold I, Schini-Kerth VB, et al. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Circulation research. 2001;89(1):47–54. doi: 10.1161/hh1301.092678. Epub 2001/07/07. [DOI] [PubMed] [Google Scholar]
- 62.Chang TC, Huang CJ, Tam K, et al. Stabilization of hypoxia-inducible factor-1{alpha} by prostacyclin under prolonged hypoxia via reducing reactive oxygen species level in endothelial cells. J Biol Chem. 2005;280(44):36567–74. doi: 10.1074/jbc.M504280200. Epub 2005/08/24. [DOI] [PubMed] [Google Scholar]
- 63.Chalothorn D, Zhang H, Smith JE, Edwards JC, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009;105(1):89–98. doi: 10.1161/CIRCRESAHA.109.197145. Epub 2009/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marce M, Semenza GL. Adenoviral transfer of HIF-1alpha enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci U S A. 2009;106(44):18769–74. doi: 10.1073/pnas.0910561106. Epub 2009/10/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vural E, Berbee M, Acott A, Blagg R, Fan CY, Hauer-Jensen M. Skin graft take rates, granulation, and epithelialization: dependence on myeloid cell hypoxia-inducible factor 1alpha. Archives of otolaryngology--head & neck surgery. 2010;136(7):720–3. doi: 10.1001/archoto.2010.103. Epub 2010/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nilsson I, Shibuya M, Wennstrom S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Experimental cell research. 2004;299(2):476–85. doi: 10.1016/j.yexcr.2004.06.005. Epub 2004/09/08. [DOI] [PubMed] [Google Scholar]
- 67.Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. 2006;107(7):2705–12. doi: 10.1182/blood-2005-09-3541. Epub 2005/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guadall A, Orriols M, Rodriguez-Calvo R, et al. Fibulin-5 is up-regulated by hypoxia in endothelial cells through a hypoxia-inducible factor-1 (HIF-1alpha)-dependent mechanism. J Biol Chem. 2011;286(9):7093–103. doi: 10.1074/jbc.M110.162917. Epub 2011/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castellano J, Aledo R, Sendra J, et al. Hypoxia stimulates low-density lipoprotein receptor-related protein-1 expression through hypoxia-inducible factor-1alpha in human vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(6):1411–20. doi: 10.1161/ATVBAHA.111.225490. Epub 2011/04/02. [DOI] [PubMed] [Google Scholar]
- 70.Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem. 2000;275(35):26765–71. doi: 10.1074/jbc.M003325200. Epub 2000/06/06. [DOI] [PubMed] [Google Scholar]
- 71.Trollmann R, Gassmann M. The role of hypoxia-inducible transcription factors in the hypoxic neonatal brain. Brain & development. 2009;31(7):503–9. doi: 10.1016/j.braindev.2009.03.007. Epub 2009/04/29. [DOI] [PubMed] [Google Scholar]
- 72.Trollmann R, Strasser K, Keller S, et al. Placental HIFs as markers of cerebral hypoxic distress in fetal mice. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1973–81. doi: 10.1152/ajpregu.00053.2008. Epub 2008/10/04. [DOI] [PubMed] [Google Scholar]
- 73.Bani Hashemi S, Braun J, Bernhardt WM, Rascher W, Dotsch J, Trollmann R. HIF-1alpha subunit and vasoactive HIF-1-dependent genes are involved in carbon monoxide-induced cerebral hypoxic stress response. Eur J Appl Physiol. 2008;104(1):95–102. doi: 10.1007/s00421-008-0776-9. Epub 2008/06/19. [DOI] [PubMed] [Google Scholar]
- 74.Chavez JC, Agani F, Pichiule P, LaManna JC. Expression of hypoxia-inducible factor-1alpha in the brain of rats during chronic hypoxia. J Appl Physiol. 2000;89(5):1937–42. doi: 10.1152/jappl.2000.89.5.1937. Epub 2000/10/29. [DOI] [PubMed] [Google Scholar]
- 75.Bergeron M, Gidday JM, Yu AY, Semenza GL, Ferriero DM, Sharp FR. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Annals of neurology. 2000;48(3):285–96. Epub 2000/09/08. [PubMed] [Google Scholar]
- 76.Sharp FR, Ran R, Lu A, et al. Hypoxic preconditioning protects against ischemic brain injury. NeuroRx: the journal of the American Society for Experimental NeuroTherapeutics. 2004;1(1):26–35. doi: 10.1602/neurorx.1.1.26. Epub 2005/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan X, Heijnen CJ, van der Kooij MA, Groenendaal F, van Bel F. The role and regulation of hypoxia-inducible factor-1alpha expression in brain development and neonatal hypoxic-ischemic brain injury. Brain Res Rev. 2009;62(1):99–108. doi: 10.1016/j.brainresrev.2009.09.006. Epub 2009/09/30. [DOI] [PubMed] [Google Scholar]
- 78.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207(Pt 18):3233–42. doi: 10.1242/jeb.01049. Epub 2004/08/10. [DOI] [PubMed] [Google Scholar]
- 79.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. BioEssays: news and reviews in molecular, cellular and developmental biology. 2004;26(9):943–54. doi: 10.1002/bies.20092. Epub 2004/09/08. [DOI] [PubMed] [Google Scholar]
- 80.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors--similar but not identical. Molecules and cells. 2010;29(5):435–42. doi: 10.1007/s10059-010-0067-2. Epub 2010/04/17. [DOI] [PubMed] [Google Scholar]
- 81.Ladoux A, Frelin C. Cardiac expressions of HIF-1 alpha and HLF/EPAS, two basic loop helix/PAS domain transcription factors involved in adaptative responses to hypoxic stresses. Biochem Biophys Res Commun. 1997;240(3):552–6. doi: 10.1006/bbrc.1997.7708. Epub 1997/12/17. [DOI] [PubMed] [Google Scholar]
- 82.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94(9):4273–8. doi: 10.1073/pnas.94.9.4273. Epub 1997/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flamme I, Krieg M, Plate KH. Up-regulation of vascular endothelial growth factor in stromal cells of hemangioblastomas is correlated with up-regulation of the transcription factor HRF/HIF-2alpha. The American journal of pathology. 1998;153(1):25–9. doi: 10.1016/s0002-9440(10)65541-1. Epub 1998/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes & development. 1997;11(1):72–82. doi: 10.1101/gad.11.1.72. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 85.Ota K, Nagai H, Sheng G. Expression and hypoxic regulation of hif1alpha and hif2alpha during early blood and endothelial cell differentiation in chick. Gene expression patterns: GEP. 2007;7(7):761–6. doi: 10.1016/j.modgep.2007.05.007. Epub 2007/07/13. [DOI] [PubMed] [Google Scholar]
- 86.Favier J, Lapointe S, Maliba R, Sirois MG. HIF2 alpha reduces growth rate but promotes angiogenesis in a mouse model of neuroblastoma. BMC cancer. 2007;7:139. doi: 10.1186/1471-2407-7-139. Epub 2007/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ji P, Xuan JW, Onita T, Sakai H, Kanetake H, Gabril MY, et al. Correlation study showing no concordance between EPAS-1/HIF-2alpha mRNA and protein expression in transitional cellcancer of the bladder. Urology. 2003;61(4):851–7. doi: 10.1016/s0090-4295(02)02405-6. Epub 2003/04/03. [DOI] [PubMed] [Google Scholar]
- 88.Wiesener MS, Turley H, Allen WE, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92(7):2260–8. Epub 1998/09/25. [PubMed] [Google Scholar]
- 89.Warnecke C, Zaborowska Z, Kurreck J, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18(12):1462–4. doi: 10.1096/fj.04-1640fje. Epub 2004/07/09. [DOI] [PubMed] [Google Scholar]
- 90.Duan LJ, Zhang-Benoit Y, Fong GH. Endothelium-intrinsic requirement for Hif-2alpha during vascular development. Circulation. 2005;111(17):2227–32. doi: 10.1161/01.CIR.0000163580.98098.A3. Epub 2005/04/27. [DOI] [PubMed] [Google Scholar]
- 91.Chavez JC, Baranova O, Lin J, Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(37):9471–81. doi: 10.1523/JNEUROSCI.2838-06.2006. Epub 2006/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Favier J, Kempf H, Corvol P, Gasc JM. Coexpression of endothelial PAS protein 1 with essential angiogenic factors suggests its involvement in human vascular development. Developmental dynamics: an official publication of the American Association of Anatomists. 2001;222(3):377–88. doi: 10.1002/dvdy.1207. Epub 2001/12/18. [DOI] [PubMed] [Google Scholar]
- 93.van Patot MC, Gassmann M. Hypoxia: adapting to high altitude by mutating EPAS-1, the gene encoding HIF-2alpha. High altitude medicine & biology. 2011;12(2):157–67. doi: 10.1089/ham.2010.1099. Epub 2011/07/02. [DOI] [PubMed] [Google Scholar]
- 94.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Molecular cell. 2010;40(2):294–309. doi: 10.1016/j.molcel.2010.09.022. Epub 2010/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405(6785):421–4. doi: 10.1038/35013000. Epub 2000/06/06. [DOI] [PubMed] [Google Scholar]
- 96.Nicola T, Ambalavanan N, Zhang W, et al. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. American journal of physiology Lung cellular and molecular physiology. 2011;301(1):L125–34. doi: 10.1152/ajplung.00074.2011. Epub 2011/05/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gong K, Xing D, Li P, et al. Hypoxia induces downregulation of PPAR-gamma in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-beta signaling. American journal of physiology Lung cellular and molecular physiology. 2011;301(6):L899–907. doi: 10.1152/ajplung.00062.2011. Epub 2011/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Germain P, Chambon P, Eichele G, et al. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacological reviews. 2006;58(4):760–72. doi: 10.1124/pr.58.4.7. Epub 2006/11/30. [DOI] [PubMed] [Google Scholar]
- 99.Qi C, Zhu Y, Reddy JK. Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell biochemistry and biophysics. 2000 Spring;32:187–204. doi: 10.1385/cbb:32:1-3:187. Epub 2001/05/02. [DOI] [PubMed] [Google Scholar]
- 100.Cipolla MJ, Bishop N, Vinke RS, Godfrey JA. PPAR{gamma} activation prevents hypertensive remodeling of cerebral arteries and improves vascular function in female rats. Stroke. 2010;41(6):1266–70. doi: 10.1161/STROKEAHA.109.576942. Epub 2010/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Halabi CM, Beyer AM, de Lange WJ, et al. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell metabolism. 2008;7(3):215–26. doi: 10.1016/j.cmet.2007.12.008. Epub 2008/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamblin M, Chang L, Fan Y, Zhang J, Chen YE. PPARs and the cardiovascular system. Antioxid Redox Signal. 2009;11(6):1415–52. doi: 10.1089/ars.2008.2280. Epub 2008/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beyer AM, Baumbach GL, Halabi CM, et al. Interference with PPARgamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51(4):867–71. doi: 10.1161/HYPERTENSIONAHA.107.103648. Epub 2008/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilhelm DL, Miles AA, Mackay ME. Enzyme-like globulins from serum reproducing the vascular phenomena of inflammation. II. Isolation and properties of the permeability factor and its inhibitor. British journal of experimental pathology. 1955;36(1):82–104. Epub 1955/02/01. [PMC free article] [PubMed] [Google Scholar]
- 105.Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer research. 1986;46(11):5629–32. Epub 1986/11/01. [PubMed] [Google Scholar]
- 106.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. The Journal of clinical investigation. 1989;84(5):1470–8. doi: 10.1172/JCI114322. Epub 1989/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276(4 Pt 1):C812–20. doi: 10.1152/ajpcell.1999.276.4.C812. Epub 1999/04/13. [DOI] [PubMed] [Google Scholar]
- 108.Krum JM, Mani N, Rosenstein JM. Roles of the endogenous VEGF receptors flt-1 and flk-1 in astroglial and vascular remodeling after brain injury. Experimental neurology. 2008;212(1):108–17. doi: 10.1016/j.expneurol.2008.03.019. Epub 2008/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chow J, Ogunshola O, Fan SY, Li Y, Ment LR, Madri JA. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain Res Dev Brain Res. 2001;130(1):123–32. doi: 10.1016/s0165-3806(01)00220-6. Epub 2001/09/15. [DOI] [PubMed] [Google Scholar]
- 110.Gerber SA, Pober JS. IFN-alpha induces transcription of hypoxia-inducible factor-1alpha to inhibit proliferation of human endothelial cells. J Immunol. 2008;181(2):1052–62. doi: 10.4049/jimmunol.181.2.1052. Epub 2008/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Molecular diversity. 2006;10(4):515–27. doi: 10.1007/s11030-006-9027-3. Epub 2006/09/15. [DOI] [PubMed] [Google Scholar]
- 112.Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: review. Blood cells, molecules & diseases. 2007;38(3):258–68. doi: 10.1016/j.bcmd.2006.12.003. Epub 2007/03/09. [DOI] [PubMed] [Google Scholar]
- 113.Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, Chen H, et al. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J Biol Chem. 1996;271(10):5638–46. doi: 10.1074/jbc.271.10.5638. Epub 1996/03/08. [DOI] [PubMed] [Google Scholar]
- 114.Saharinen P, Bry M, Alitalo K. How do angiopoietins Tie in with vascular endothelial growth factors? Current opinion in hematology. 2010;17(3):198–205. doi: 10.1097/MOH.0b013e3283386673. Epub 2010/04/09. [DOI] [PubMed] [Google Scholar]
- 115.Barleon B, Siemeister G, Martiny-Baron G, Weindel K, Herzog C, Marme D. Vascular endothelial growth factor up-regulates its receptor fms-like tyrosine kinase 1 (FLT-1) and a soluble variant of FLT-1 in human vascular endothelial cells. Cancer research. 1997;57(23):5421–5. Epub 1997/12/11. [PubMed] [Google Scholar]
- 116.Breier G, Blum S, Peli J, et al. Transforming growth factor-beta and Ras regulate the VEGF/VEGF-receptor system during tumor angiogenesis. International journal of cancer Journal international du cancer. 2002;97(2):142–8. doi: 10.1002/ijc.1599. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 117.Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal. 2007;9(9):1363–71. doi: 10.1089/ars.2007.1713. Epub 2007/07/14. [DOI] [PubMed] [Google Scholar]
- 118.Nomura M, Yamagishi S, Harada S, et al. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270(47):28316–24. doi: 10.1074/jbc.270.47.28316. Epub 1995/11/24. [DOI] [PubMed] [Google Scholar]
- 119.Ikeda E, Achen MG, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270(34):19761–6. doi: 10.1074/jbc.270.34.19761. Epub 1995/08/25. [DOI] [PubMed] [Google Scholar]
- 120.Finkenzeller G, Technau A, Marme D. Hypoxia-induced transcription of the vascular endothelial growth factor gene is independent of functional AP-1 transcription factor. Biochem Biophys Res Commun. 1995;208(1):432–9. doi: 10.1006/bbrc.1995.1356. Epub 1995/03/08. [DOI] [PubMed] [Google Scholar]
- 121.Kramer BK, Bucher M, Sandner P, et al. Effects of hypoxia on growth factor expression in the rat kidney in vivo. Kidney international. 1997;51(2):444–7. doi: 10.1038/ki.1997.59. Epub 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 122.Schweda F, Blumberg FC, Schweda A, et al. Effects of chronic hypoxia on renal PDGF-A, PDGF-B, and VEGF gene expression in rats. Nephron. 2000;86(2):161–6. doi: 10.1159/000045735. Epub 2000/10/03. [DOI] [PubMed] [Google Scholar]
- 123.Ma Y, Qu Y, Fei Z. Vascular endothelial growth factor in cerebral ischemia. Journal of neuroscience research. 2011;89(7):969–78. doi: 10.1002/jnr.22628. Epub 2011/04/07. [DOI] [PubMed] [Google Scholar]
- 124.Ogunshola OO, Stewart WB, Mihalcik V, Solli T, Madri JA, Ment LR. Neuronal VEGF expression correlates with angiogenesis in postnatal developing rat brain. Brain Res Dev Brain Res. 2000;119(1):139–53. doi: 10.1016/s0165-3806(99)00125-x. Epub 2000/01/29. [DOI] [PubMed] [Google Scholar]
- 125.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(18):10242–7. doi: 10.1073/pnas.97.18.10242. Epub 2000/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feng Y, Rhodes PG, Bhatt AJ. Neuroprotective effects of vascular endothelial growth factor following hypoxic ischemic brain injury in neonatal rats. Pediatric research. 2008;64(4):370–4. doi: 10.1203/PDR.0b013e318180ebe6. Epub 2008/06/07. [DOI] [PubMed] [Google Scholar]
- 127.Patt S, Danner S, Theallier-Janko A, et al. Upregulation of vascular endothelial growth factor in severe chronic brain hypoxia of the rat. Neurosci Lett. 1998;252(3):199–202. doi: 10.1016/s0304-3940(98)00582-5. Epub 1998/09/18. [DOI] [PubMed] [Google Scholar]
- 128.LaManna JC, Kuo NT, Lust WD. Hypoxia-induced brain angiogenesis. Signals and consequences. Adv Exp Med Biol. 1998;454:287–93. doi: 10.1007/978-1-4615-4863-8_34. Epub 1999/01/16. [DOI] [PubMed] [Google Scholar]
- 129.Kremer C, Breier G, Risau W, Plate KH. Up-regulation of flk-1/vascular endothelial growth factor receptor 2 by its ligand in a cerebral slice culture system. Cancer research. 1997;57(17):3852–9. Epub 1997/09/01. [PubMed] [Google Scholar]
- 130.Perrin JS, Araneda S, Catteau J, Autran S, Denavit-Saubie M, Pequignot JM. Glial vascular endothelial growth factor overexpression in rat brainstem under tolerable hypoxia: evidence for a central chemosensitivity. Journal of neuroscience research. 2009;87(1):79–85. doi: 10.1002/jnr.21837. Epub 2008/08/30. [DOI] [PubMed] [Google Scholar]
- 131.Chabrier PE. Growth factors and vascular wall. Int Angiol. 1996;15(2):100–3. Epub 1996/06/01. [PubMed] [Google Scholar]
- 132.Betsholtz C, Raines EW. Platelet-derived growth factor: a key regulator of connective tissue cells in embryogenesis and pathogenesis. Kidney international. 1997;51(5):1361–9. doi: 10.1038/ki.1997.186. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 133.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome biology. 2005;6(2):209. doi: 10.1186/gb-2005-6-2-209. Epub 2005/02/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ball SG, Shuttleworth CA, Kielty CM. Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors. J Cell Mol Med. 2007;11(5):1012–30. doi: 10.1111/j.1582-4934.2007.00120.x. Epub 2007/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J Cell Biol. 2007;177(3):489–500. doi: 10.1083/jcb.200608093. Epub 2007/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Renner O, Tsimpas A, Kostin S, Valable S, Petit E, Schaper W, et al. Time- and cell type-specific induction of platelet-derived growth factor receptor-beta during cerebral ischemia. Brain research Molecular brain research. 2003;113(1–2):44–51. doi: 10.1016/s0169-328x(03)00085-8. Epub 2003/05/17. [DOI] [PubMed] [Google Scholar]
- 137.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79(4):1283–316. doi: 10.1152/physrev.1999.79.4.1283. Epub 1999/10/03. [DOI] [PubMed] [Google Scholar]
- 138.Kourembanas S, Hannan RL, Faller DV. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990;86(2):670–4. doi: 10.1172/JCI114759. Epub 1990/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seifert RA, Hart CE, Phillips PE, et al. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989;264(15):8771–8. Epub 1989/05/25. [PubMed] [Google Scholar]
- 140.Hosang M, Rouge M. Human vascular smooth muscle cells have at least two distinct PDGF receptors and can secrete PDGF-AA. J Cardiovasc Pharmacol. 1989;14 (Suppl 6):S22–6. Epub 1989/01/01. [PubMed] [Google Scholar]
- 141.Cunningham LD, Brecher P, Cohen RA. Platelet-derived growth factor receptors on macrovascular endothelial cells mediate relaxation via nitric oxide in rat aorta. J Clin Invest. 1992;89(3):878–82. doi: 10.1172/JCI115667. Epub 1992/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bos R, van Diest PJ, de Jong JS, van der Groep P, van der Valk P, van der Wall E. Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology. 2005;46(1):31–6. doi: 10.1111/j.1365-2559.2005.02045.x. Epub 2005/01/20. [DOI] [PubMed] [Google Scholar]
- 143.Buch S, Han RN, Cabacungan J, et al. Changes in expression of platelet-derived growth factor and its receptors in the lungs of newborn rats exposed to air or 60% O(2) Pediatric research. 2000;48(4):423–33. doi: 10.1203/00006450-200010000-00003. Epub 2000/09/27. [DOI] [PubMed] [Google Scholar]
- 144.Schollmann C, Grugel R, Tatje D, et al. Basic fibroblast growth factor modulates the mitogenic potency of the platelet-derived growth factor (PDGF) isoforms by specific upregulation of the PDGF alpha receptor in vascular smooth muscle cells. J Biol Chem. 1992;267(25):18032–9. Epub 1992/09/05. [PubMed] [Google Scholar]
- 145.Okazaki H, Majesky MW, Harker LA, Schwartz SM. Regulation of platelet-derived growth factor ligand and receptor gene expression by alpha-thrombin in vascular smooth muscle cells. Circulation research. 1992;71(6):1285–93. doi: 10.1161/01.res.71.6.1285. Epub 1992/12/01. [DOI] [PubMed] [Google Scholar]
- 146.Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. American journal of respiratory and critical care medicine. 1998;158(6):1920–8. doi: 10.1164/ajrccm.158.6.9804076. Epub 1998/12/16. [DOI] [PubMed] [Google Scholar]
- 147.Ziino AJ, Ivanovska J, Belcastro R, et al. Effects of rho-kinase inhibition on pulmonary hypertension, lung growth, and structure in neonatal rats chronically exposed to hypoxia. Pediatr Res. 2010;67(2):177–82. doi: 10.1203/PDR.0b013e3181c6e5a7. Epub 2009/10/28. [DOI] [PubMed] [Google Scholar]
- 148.Kuwabara K, Ogawa S, Matsumoto M, et al. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(10):4606–10. doi: 10.1073/pnas.92.10.4606. Epub 1995/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.ten Freyhaus H, Dagnell M, Leuchs M, et al. Hypoxia enhances platelet-derived growth factor signaling in the pulmonary vasculature by down-regulation of protein tyrosine phosphatases. American journal of respiratory and critical care medicine. 2011;183(8):1092–102. doi: 10.1164/rccm.200911-1663OC. Epub 2010/12/24. [DOI] [PubMed] [Google Scholar]
- 150.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126(14):3047–55. doi: 10.1242/dev.126.14.3047. Epub 1999/06/22. [DOI] [PubMed] [Google Scholar]
- 151.Yoshida D, Kim K, Noha M, Teramoto A. Hypoxia inducible factor 1-alpha regulates of platelet derived growth factor-B in human glioblastoma cells. Journal of neuro-oncology. 2006;76(1):13–21. doi: 10.1007/s11060-005-3279-0. Epub 2005/09/02. [DOI] [PubMed] [Google Scholar]
- 152.Zhang SX, Gozal D, Sachleben LR, Jr, Rane M, Klein JB, Gozal E. Hypoxia induces an autocrine-paracrine survival pathway via platelet-derived growth factor (PDGF)-B/PDGF-beta receptor/phosphatidylinositol 3-kinase/Akt signaling in RN46A neuronal cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17(12):1709–11. doi: 10.1096/fj.02-1111fje. Epub 2003/09/06. [DOI] [PubMed] [Google Scholar]
- 153.Alea OA, Czapla MA, Lasky JA, Simakajornboon N, Gozal E, Gozal D. PDGF-beta receptor expression and ventilatory acclimatization to hypoxia in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):R1625–33. doi: 10.1152/ajpregu.2000.279.5.R1625. Epub 2000/10/26. [DOI] [PubMed] [Google Scholar]
- 154.Vlasic V, Simakajornboon N, Gozal E, Gozal D. PDGF-beta receptor expression in the dorsocaudal brainstem parallels hypoxic ventilatory depression in the developing rat. Pediatr Res. 2001;50(2):236–41. doi: 10.1203/00006450-200108000-00012. Epub 2001/07/31. [DOI] [PubMed] [Google Scholar]
- 155.Gozal D, Simakajornboon N, Czapla MA, et al. Brainstem activation of platelet-derived growth factor-beta receptor modulates the late phase of the hypoxic ventilatory response. Journal of neurochemistry. 2000;74(1):310–9. doi: 10.1046/j.1471-4159.2000.0740310.x. Epub 2000/01/05. [DOI] [PubMed] [Google Scholar]
- 156.Reiss Y. Angiopoietins. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2010;180:3–13. doi: 10.1007/978-3-540-78281-0_2. Epub 2009/12/25. [DOI] [PubMed] [Google Scholar]
- 157.Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J Biol Chem. 2004;279(13):12171–80. doi: 10.1074/jbc.M305146200. Epub 2004/01/02. [DOI] [PubMed] [Google Scholar]
- 158.Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12(2):125–37. doi: 10.1007/s10456-009-9147-3. Epub 2009/05/19. [DOI] [PubMed] [Google Scholar]
- 159.Kim KE, Cho CH, Kim HZ, Baluk P, McDonald DM, Koh GY. In vivo actions of angiopoietins on quiescent and remodeling blood and lymphatic vessels in mouse airways and skin. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(3):564–70. doi: 10.1161/01.ATV.0000256458.82320.be. Epub 2006/12/30. [DOI] [PubMed] [Google Scholar]
- 160.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7):1161–9. doi: 10.1016/s0092-8674(00)81812-7. Epub 1996/12/27. [DOI] [PubMed] [Google Scholar]
- 161.Kim I, Kwak HJ, Ahn JE, So JN, Liu M, Koh KN, et al. Molecular cloning and characterization of a novel angiopoietin family protein, angiopoietin-3. FEBS letters. 1999;443(3):353–6. doi: 10.1016/s0014-5793(99)00008-3. Epub 1999/02/20. [DOI] [PubMed] [Google Scholar]
- 162.Mandriota SJ, Pepper MS. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circulation research. 1998;83(8):852–9. doi: 10.1161/01.res.83.8.852. Epub 1998/10/20. [DOI] [PubMed] [Google Scholar]
- 163.Liu XB, Jiang J, Gui C, Hu XY, Xiang MX, Wang JA. Angiopoietin-1 protects mesenchymal stem cells against serum deprivation and hypoxia-induced apoptosis through the PI3K/Akt pathway. Acta pharmacologica Sinica. 2008;29(7):815–22. doi: 10.1111/j.1745-7254.2008.00811.x. Epub 2008/06/21. [DOI] [PubMed] [Google Scholar]
- 164.Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS letters. 1999;448(2–3):249–53. doi: 10.1016/s0014-5793(99)00378-6. Epub 1999/04/28. [DOI] [PubMed] [Google Scholar]
- 165.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171–80. doi: 10.1016/s0092-8674(00)81813-9. Epub 1996/12/27. [DOI] [PubMed] [Google Scholar]
- 166.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends in immunology. 2006;27(12):552–8. doi: 10.1016/j.it.2006.10.004. Epub 2006/10/19. [DOI] [PubMed] [Google Scholar]
- 167.Ramsauer M, D’Amore PA. Contextual role for angiopoietins and TGFbeta1 in blood vessel stabilization. Journal of cell science. 2007;120(Pt 10):1810–7. doi: 10.1242/jcs.003533. Epub 2007/05/16. [DOI] [PubMed] [Google Scholar]
- 168.Asahara T, Chen D, Takahashi T, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circulation research. 1998;83(3):233–40. doi: 10.1161/01.res.83.3.233. Epub 1998/08/26. [DOI] [PubMed] [Google Scholar]
- 169.Hansen TM, Singh H, Tahir TA, Brindle NP. Effects of angiopoietins-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cellular signalling. 2010;22(3):527–32. doi: 10.1016/j.cellsig.2009.11.007. Epub 2009/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Distler JH, Hirth A, Kurowska-Stolarska M, Gay RE, Gay S, Distler O. Angiogenic and angiostatic factors in the molecular control of angiogenesis. Q J Nucl Med. 2003;47(3):149–61. [PubMed] [Google Scholar]
- 171.Sivakumar V, Zhang Y, Ling EA, Foulds WS, Kaur C. Insulin-like growth factors, angiopoietin-2, and pigment epithelium-derived growth factor in the hypoxic retina. Journal of neuroscience research. 2008;86(3):702–11. doi: 10.1002/jnr.21519. Epub 2007/10/19. [DOI] [PubMed] [Google Scholar]
- 172.Yuan HT, Yang SP, Woolf AS. Hypoxia up-regulates angiopoietin-2, a Tie-2 ligand, in mouse mesangial cells. Kidney international. 2000;58(5):1912–9. doi: 10.1111/j.1523-1755.2000.00363.x. Epub 2000/10/24. [DOI] [PubMed] [Google Scholar]
- 173.Zhang EG, Smith SK, Baker PN, Charnock-Jones DS. The regulation and localization of angiopoietin-1, -2, and their receptor Tie2 in normal and pathologic human placentae. Mol Med. 2001;7(9):624–35. Epub 2002/01/10. [PMC free article] [PubMed] [Google Scholar]
- 174.Willam C, Koehne P, Jurgensen JS, et al. Tie2 receptor expression is stimulated by hypoxia and proinflammatory cytokines in human endothelial cells. Circulation research. 2000;87(5):370–7. doi: 10.1161/01.res.87.5.370. Epub 2000/09/02. [DOI] [PubMed] [Google Scholar]
- 175.Valable S, Bellail A, Lesne S, et al. Angiopoietin-1-induced PI3-kinase activation prevents neuronal apoptosis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2003;17(3):443–5. doi: 10.1096/fj.02-0372fje. Epub 2003/01/07. [DOI] [PubMed] [Google Scholar]
- 176.Pichiule P, LaManna JC. Angiopoietin-2 and rat brain capillary remodeling during adaptation and deadaptation to prolonged mild hypoxia. Journal of applied physiology. 2002;93(3):1131–9. doi: 10.1152/japplphysiol.00318.2002. Epub 2002/08/17. [DOI] [PubMed] [Google Scholar]
- 177.Beck H, Acker T, Wiessner C, Allegrini PR, Plate KH. Expression of angiopoietin-1, angiopoietin-2, and tie receptors after middle cerebral artery occlusion in the rat. The American journal of pathology. 2000;157(5):1473–83. doi: 10.1016/S0002-9440(10)64786-4. Epub 2000/11/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin TN, Nian GM, Chen SF, et al. Induction of Tie-1 and Tie-2 receptor protein expression after cerebral ischemia-reperfusion. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21(6):690–701. doi: 10.1097/00004647-200106000-00007. Epub 2001/08/08. [DOI] [PubMed] [Google Scholar]
- 179.Lee HS, Han J, Bai HJ, Kim KW. Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. The FEBS journal. 2009;276(17):4622–35. doi: 10.1111/j.1742-4658.2009.07174.x. Epub 2009/08/12. [DOI] [PubMed] [Google Scholar]
- 180.Ornitz DM, Itoh N. Fibroblast growth factors. Genome biology. 2001;2(3):REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. Epub 2001/03/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA. 1986;83(19):7297–301. doi: 10.1073/pnas.83.19.7297. Epub 1986/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325(6101):257–9. doi: 10.1038/325257a0. Epub 1987/01/15. [DOI] [PubMed] [Google Scholar]
- 183.Brogi E, Wu T, Namiki A, Isner JM. Indirect angiogenic cytokines upregulate VEGF and bFGF gene expression in vascular smooth muscle cells, whereas hypoxia upregulates VEGF expression only. Circulation. 1994;90(2):649–52. doi: 10.1161/01.cir.90.2.649. Epub 1994/08/01. [DOI] [PubMed] [Google Scholar]
- 184.Saltis J, Thomas AC, Agrotis A, Campbell JH, Campbell GR, Bobik A. Expression of growth factor receptors in arterial smooth muscle cells. Dependency on cell phenotype and serum factors. Atherosclerosis. 1995;118(1):77–87. doi: 10.1016/0021-9150(95)05595-n. Epub 1995/11/01. [DOI] [PubMed] [Google Scholar]
- 185.Black SM, DeVol JM, Wedgwood S. Regulation of fibroblast growth factor-2 expression in pulmonary arterial smooth muscle cells involves increased reactive oxygen species generation. American journal of physiology Cell physiology. 2008;294(1):C345–54. doi: 10.1152/ajpcell.00216.2007. Epub 2007/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sakaki T, Yamada K, Otsuki H, Yuguchi T, Kohmura E, Hayakawa T. Brief exposure to hypoxia induces bFGF mRNA and protein and protects rat cortical neurons from prolonged hypoxic stress. Neuroscience research. 1995;23(3):289–96. doi: 10.1016/0168-0102(95)00954-x. Epub 1995/10/01. [DOI] [PubMed] [Google Scholar]
- 187.Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM. Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience. 2002;112(4):977–91. doi: 10.1016/s0306-4522(02)00060-x. Epub 2002/06/29. [DOI] [PubMed] [Google Scholar]
- 188.Morrison RS, Sharma A, de Vellis J, Bradshaw RA. Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc Natl Acad Sci U S A. 1986;83(19):7537–41. doi: 10.1073/pnas.83.19.7537. Epub 1986/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Song BW, Vinters HV, Wu D, Pardridge WM. Enhanced neuroprotective effects of basic fibroblast growth factor in regional brain ischemia after conjugation to a blood-brain barrier delivery vector. J Pharmacol Exp Ther. 2002;301(2):605–10. doi: 10.1124/jpet.301.2.605. Epub 2002/04/19. [DOI] [PubMed] [Google Scholar]
- 190.Nozaki K, Finklestein SP, Beal MF. Basic fibroblast growth factor protects against hypoxia-ischemia and NMDA neurotoxicity in neonatal rats. J Cereb Blood Flow Metab. 1993;13(2):221–8. doi: 10.1038/jcbfm.1993.27. Epub 1993/03/01. [DOI] [PubMed] [Google Scholar]
- 191.Dayer AG, Jenny B, Sauvain MO, et al. Expression of FGF-2 in neural progenitor cells enhances their potential for cellular brain repair in the rodent cortex. Brain: a journal of neurology. 2007;130(Pt 11):2962–76. doi: 10.1093/brain/awm200. Epub 2007/08/31. [DOI] [PubMed] [Google Scholar]
- 192.Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes & development. 1994;8(2):133–46. doi: 10.1101/gad.8.2.133. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 193.Clark DA, Coker R. Transforming growth factor-beta (TGF-beta) The international journal of biochemistry & cell biology. 1998;30(3):293–8. doi: 10.1016/s1357-2725(97)00128-3. Epub 1998/06/05. [DOI] [PubMed] [Google Scholar]
- 194.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. Epub 2000/01/27. [DOI] [PubMed] [Google Scholar]
- 195.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235(4787):442–7. doi: 10.1126/science.2432664. Epub 1987/01/23. [DOI] [PubMed] [Google Scholar]
- 196.Miyazono K, ten Dijke P, Heldin CH. TGF-beta signaling by Smad proteins. Advances in immunology. 2000;75:115–57. doi: 10.1016/s0065-2776(00)75003-6. Epub 2000/07/06. [DOI] [PubMed] [Google Scholar]
- 197.King-Briggs KE, Shanahan CM. TGF-beta superfamily members do not promote smooth muscle-specific alternative splicing, a late marker of vascular smooth muscle cell differentiation. Differentiation; research in biological diversity. 2000;66(1):43–8. doi: 10.1046/j.1432-0436.2000.066001043.x. Epub 2000/09/21. [DOI] [PubMed] [Google Scholar]
- 198.Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Investigative ophthalmology & visual science. 2003;44(11):4994–5005. doi: 10.1167/iovs.03-0291. Epub 2003/10/28. [DOI] [PubMed] [Google Scholar]
- 199.Zhang H, Akman HO, Smith EL, Zhao J, Murphy-Ullrich JE, Batuman OA. Cellular response to hypoxia involves signaling via Smad proteins. Blood. 2003;101(6):2253–60. doi: 10.1182/blood-2002-02-0629. Epub 2002/11/02. [DOI] [PubMed] [Google Scholar]
- 200.Akman HO, Zhang H, Siddiqui MA, Solomon W, Smith EL, Batuman OA. Response to hypoxia involves transforming growth factor-beta2 and Smad proteins in human endothelial cells. Blood. 2001;98(12):3324–31. doi: 10.1182/blood.v98.12.3324. Epub 2001/11/24. [DOI] [PubMed] [Google Scholar]
- 201.Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem. 2002;277(46):43799–808. doi: 10.1074/jbc.M207160200. Epub 2002/09/14. [DOI] [PubMed] [Google Scholar]
- 202.Guo J, Chen H, Ho J, Mancini J, Sontag T, Laporte SA, et al. TGFbeta-induced GRK2 expression attenuates AngII-regulated vascular smooth muscle cell proliferation and migration. Cellular signalling. 2009;21(6):899–905. doi: 10.1016/j.cellsig.2009.01.037. Epub 2009/04/23. [DOI] [PubMed] [Google Scholar]
- 203.Lehrmann E, Kiefer R, Christensen T, et al. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia. 1998;24(4):437–48. doi: 10.1002/(sici)1098-1136(199812)24:4<437::aid-glia9>3.0.co;2-x. Epub 1998/11/14. [DOI] [PubMed] [Google Scholar]
- 204.McNeill H, Williams C, Guan J, et al. Neuronal rescue with transforming growth factor-beta 1 after hypoxic-ischaemic brain injury. Neuroreport. 1994;5(8):901–4. doi: 10.1097/00001756-199404000-00012. Epub 1994/04/14. [DOI] [PubMed] [Google Scholar]
- 205.Williams C, Guan J, Miller O, et al. The role of the growth factors IGF-1 and TGF beta 1 after hypoxic-ischemic brain injury. Annals of the New York Academy of Sciences. 1995;765:306–7. doi: 10.1111/j.1749-6632.1995.tb16592.x. Epub 1995/09/15. [DOI] [PubMed] [Google Scholar]
- 206.Dhandapani KM, Brann DW. Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell biochemistry and biophysics. 2003;39(1):13–22. doi: 10.1385/CBB:39:1:13. Epub 2003/07/02. [DOI] [PubMed] [Google Scholar]
- 207.Guan J, Miller OT, Waugh KM, McCarthy DC, Gluckman PD, Gunn AJ. TGF beta-1 and neurological function after hypoxia-ischemia in adult rats. Neuroreport. 2004;15(6):961–4. doi: 10.1097/00001756-200404290-00006. Epub 2004/04/13. [DOI] [PubMed] [Google Scholar]
- 208.Lin CH, Cheng FC, Lu YZ, Chu LF, Wang CH, Hsueh CM. Protection of ischemic brain cells is dependent on astrocyte-derived growth factors and their receptors. Experimental neurology. 2006;201(1):225–33. doi: 10.1016/j.expneurol.2006.04.014. Epub 2006/06/13. [DOI] [PubMed] [Google Scholar]
- 209.Giuffrida R, Bellomo M, Polizzi G, Malatino LS. Ischemia-induced changes in the immunoreactivity for endothelin and other vasoactive peptides in the brain of the Mongolian gerbil. J Cardiovasc Pharmacol. 1992;20 (Suppl 12):S41–4. doi: 10.1097/00005344-199204002-00013. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 210.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1989;3(9):2007–18. Epub 1989/07/01. [PubMed] [Google Scholar]
- 211.Pearce WJ, Williams JM, Hamade MW, Chang MM, White CR. Chronic hypoxia modulates endothelium-dependent vasorelaxation through multiple independent mechanisms in ovine cranial arteries. Adv Exp Med Biol. 2006;578:87–92. doi: 10.1007/0-387-29540-2_14. [DOI] [PubMed] [Google Scholar]
- 212.Tomita T, Tsuyama S, Imai Y, Kitagawa T. Purification of bovine soluble guanylate cyclase and ADP-ribosylation on its small subunit by bacterial toxins. Journal of biochemistry. 1997;122(3):531–6. doi: 10.1093/oxfordjournals.jbchem.a021785. Epub 1997/11/05. [DOI] [PubMed] [Google Scholar]
- 213.Schlossmann J, Desch M. IRAG and novel PKG targeting in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2011;301(3):H672–82. doi: 10.1152/ajpheart.00198.2011. Epub 2011/06/15. [DOI] [PubMed] [Google Scholar]
- 214.Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Frontiers in bioscience: a journal and virtual library. 2006;11:356–67. doi: 10.2741/1803. Epub 2005/09/09. [DOI] [PubMed] [Google Scholar]
- 215.Klemm DJ, Watson PA, Frid MG, et al. cAMP response element-binding protein content is a molecular determinant of smooth muscle cell proliferation and migration. J Biol Chem. 2001;276(49):46132–41. doi: 10.1074/jbc.M104769200. Epub 2001/09/19. [DOI] [PubMed] [Google Scholar]
- 216.Babaei S, Teichert-Kuliszewska K, Monge JC, Mohamed F, Bendeck MP, Stewart DJ. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circulation research. 1998;82(9):1007–15. doi: 10.1161/01.res.82.9.1007. Epub 1998/05/23. [DOI] [PubMed] [Google Scholar]
- 217.Kourembanas S, McQuillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Invest. 1993;92(1):99–104. doi: 10.1172/JCI116604. Epub 1993/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Malek AM, Izumo S, Alper SL. Modulation by pathophysiological stimuli of the shear stress-induced up-regulation of endothelial nitric oxide synthase expression in endothelial cells. Neurosurgery. 1999;45(2):334–44. doi: 10.1097/00006123-199908000-00028. discussion 44-5. Epub 1999/08/17. [DOI] [PubMed] [Google Scholar]
- 219.Zeng G, Nystrom FH, Ravichandran LV, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101(13):1539–45. doi: 10.1161/01.cir.101.13.1539. Epub 2000/04/04. [DOI] [PubMed] [Google Scholar]
- 220.Blumberg FC, Wolf K, Arzt M, Lorenz C, Riegger GA, Pfeifer M. Effects of ET-A receptor blockade on eNOS gene expression in chronic hypoxic rat lungs. Journal of applied physiology. 2003;94(2):446–52. doi: 10.1152/japplphysiol.00239.2002. Epub 2002/10/23. [DOI] [PubMed] [Google Scholar]
- 221.Kane AD, Herrera EA, Hansell JA, Giussani DA. Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol. 2012;590(Pt 2):323–34. doi: 10.1113/jphysiol.2011.217968. Epub 2011/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Williams JM, Hull AD, Pearce WJ. Maturational modulation of endothelium-dependent vasodilatation in ovine cerebral arteries. American journal of physiology Regulatory, integrative and comparative physiology. 2005;288(1):R149–57. doi: 10.1152/ajpregu.00427.2004. Epub 2004/09/11. [DOI] [PubMed] [Google Scholar]
- 223.White CR, Hamade MW, Siami K, et al. Maturation enhances fluid shear-induced activation of eNOS in perfused ovine carotid arteries. Am J Physiol Heart Circ Physiol. 2005;289(5):H2220–7. doi: 10.1152/ajpheart.01013.2004. Epub 2005/06/01. [DOI] [PubMed] [Google Scholar]
- 224.Harris AP, Helou S, Gleason CA, Traystman RJ, Koehler RC. Fetal cerebral and peripheral circulatory responses to hypoxia after nitric oxide synthase inhibition. Am J Physiol Regul Integr Comp Physiol. 2001;281(2):R381–90. doi: 10.1152/ajpregu.2001.281.2.R381. Epub 2001/07/13. [DOI] [PubMed] [Google Scholar]
- 225.Howell K, Costello CM, Sands M, Dooley I, McLoughlin P. L-Arginine promotes angiogenesis in the chronically hypoxic lung: a novel mechanism ameliorating pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L1042–50. doi: 10.1152/ajplung.90327.2008. Epub 2009/04/07. [DOI] [PubMed] [Google Scholar]
- 226.Ozaki M, Kawashima S, Yamashita T, et al. Reduced hypoxic pulmonary vascular remodeling by nitric oxide from the endothelium. Hypertension. 2001;37(2):322–7. doi: 10.1161/01.hyp.37.2.322. Epub 2001/03/07. [DOI] [PubMed] [Google Scholar]
- 227.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol. 2004;286(1):H388–93. doi: 10.1152/ajpheart.00683.2003. Epub 2003/09/27. [DOI] [PubMed] [Google Scholar]
- 228.Ruijtenbeek K, Kessels LC, De Mey JG, Blanco CE. Chronic moderate hypoxia and protein malnutrition both induce growth retardation, but have distinct effects on arterial endothelium-dependent reactivity in the chicken embryo. Pediatr Res. 2003;53(4):573–9. doi: 10.1203/01.PDR.0000055770.07236.98. Epub 2003/03/04. [DOI] [PubMed] [Google Scholar]
- 229.Aguan K, Murotsuki J, Gagnon R, Thompson LP, Weiner CP. Effect of chronic hypoxemia on the regulation of nitric-oxide synthase in the fetal sheep brain. Brain Res Dev Brain Res. 1998;111(2):271–7. doi: 10.1016/s0165-3806(98)00145-x. Epub 1998/12/05. [DOI] [PubMed] [Google Scholar]
- 230.Mbaku EM, Zhang L, Pearce WJ, Duckles SP, Buchholz J. Chronic hypoxia alters the function of NOS nerves in cerebral arteries of near-term fetal and adult sheep. Journal of applied physiology. 2003;94(2):724–32. doi: 10.1152/japplphysiol.00771.2002. Epub 2002/11/16. [DOI] [PubMed] [Google Scholar]
- 231.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6. doi: 10.1038/288373a0. Epub 1980/11/27. [DOI] [PubMed] [Google Scholar]
- 232.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–6. doi: 10.1038/327524a0. Epub 1987/06/11. [DOI] [PubMed] [Google Scholar]
- 233.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–5. doi: 10.1038/332411a0. Epub 1988/03/31. [DOI] [PubMed] [Google Scholar]
- 234.Davenport AP International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacological reviews. 2002;54(2):219–26. doi: 10.1124/pr.54.2.219. Epub 2002/05/31. [DOI] [PubMed] [Google Scholar]
- 235.Masuda Y, Miyazaki H, Kondoh M, et al. Two different forms of endothelin receptors in rat lung. FEBS letters. 1989;257(2):208–10. doi: 10.1016/0014-5793(89)81535-2. Epub 1989/11/06. [DOI] [PubMed] [Google Scholar]
- 236.Bigaud M, Pelton JT. Discrimination between ETA- and ETB-receptor-mediated effects of endothelin-1 and [Ala1,3,11,15]endothelin-1 by BQ-123 in the anaesthetized rat. British journal of pharmacology. 1992;107(4):912–8. doi: 10.1111/j.1476-5381.1992.tb13385.x. Epub 1992/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.de Nucci G, Thomas R, D’Orleans-Juste P, et al. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci USA. 1988;85(24):9797–800. doi: 10.1073/pnas.85.24.9797. Epub 1988/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Warner TD, Mitchell JA, de Nucci G, Vane JR. Endothelin-1 and endothelin-3 release EDRF from isolated perfused arterial vessels of the rat and rabbit. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S85–8. doi: 10.1097/00005344-198900135-00021. discussion S102. Epub 1989/01/01. [DOI] [PubMed] [Google Scholar]
- 239.Dao HH, Essalihi R, Graillon JF, Lariviere R, De Champlain J, Moreau P. Pharmacological prevention and regression of arterial remodeling in a rat model of isolated systolic hypertension. Journal of hypertension. 2002;20(8):1597–606. doi: 10.1097/00004872-200208000-00023. Epub 2002/08/13. [DOI] [PubMed] [Google Scholar]
- 240.Chillon JM, Heistad DD, Baumbach GL. Effects of endothelin receptor inhibition on cerebral arterioles in hypertensive rats. Hypertension. 1996;27(3 Pt 2):794–8. doi: 10.1161/01.hyp.27.3.794. Epub 1996/03/01. [DOI] [PubMed] [Google Scholar]
- 241.Kelly-Cobbs AI, Harris AK, Elgebaly MM, et al. Endothelial endothelin B receptor-mediated prevention of cerebrovascular remodeling is attenuated in diabetes because of up-regulation of smooth muscle endothelin receptors. The Journal of pharmacology and experimental therapeutics. 2011;337(1):9–15. doi: 10.1124/jpet.110.175380. Epub 2011/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li W, Kelly-Cobbs AI, Mezzetti EM, Fagan SC, Ergul A. Endothelin-1-mediated cerebrovascular remodeling is not associated with increased ischemic brain injury in diabetes. Can J Physiol Pharmacol. 2010;88(8):788–95. doi: 10.1139/Y10-040. Epub 2010/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.de Frutos S, Caldwell E, Nitta CH, et al. NFATc3 contributes to intermittent hypoxia-induced arterial remodeling in mice. Am J Physiol Heart Circ Physiol. 2010;299(2):H356–63. doi: 10.1152/ajpheart.00341.2010. Epub 2010/05/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kanse SM, Takahashi K, Warren JB, et al. Production of endothelin by vascular smooth muscle cells. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S113–6. doi: 10.1097/00005344-199100177-00029. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 245.Resink TJ, Hahn AW, Scott-Burden T, Powell J, Weber E, Buhler FR. Inducible endothelin mRNA expression and peptide secretion in cultured human vascular smooth muscle cells. Biochem Biophys Res Commun. 1990;168(3):1303–10. doi: 10.1016/0006-291x(90)91171-n. Epub 1990/05/16. [DOI] [PubMed] [Google Scholar]
- 246.Markewitz BA, Farrukh IS, Chen Y, Li Y, Michael JR. Regulation of endothelin-1 synthesis in human pulmonary arterial smooth muscle cells. Effects of transforming growth factor-beta and hypoxia. Cardiovascular research. 2001;49(1):200–6. doi: 10.1016/s0008-6363(00)00221-2. Epub 2000/12/21. [DOI] [PubMed] [Google Scholar]
- 247.Olave N, Nicola T, Zhang W, Bulger A, James ML, Oparil S, et al. Transforming growth factor-beta regulates endothelin-1 signaling in the newborn mouse lung during hypoxia exposure. Am J Physiol Lung Cell Mol Physiol. 2012 doi: 10.1152/ajplung.00258.2011. Epub 2012/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Li P, Oparil S, Sun JZ, Thompson JA, Chen YF. Fibroblast growth factor mediates hypoxia-induced endothelin-- a receptor expression in lung artery smooth muscle cells. Journal of applied physiology. 2003;95(2):643–51. doi: 10.1152/japplphysiol.00652.2002. discussion 863. Epub 2003/07/10. [DOI] [PubMed] [Google Scholar]
- 249.Elton TS, Oparil S, Taylor GR, Hicks PH, Yang RH, Jin H, et al. Normobaric hypoxia stimulates endothelin-1 gene expression in the rat. Am J Physiol. 1992;263(6 Pt 2):R1260–4. doi: 10.1152/ajpregu.1992.263.6.R1260. Epub 1992/12/01. [DOI] [PubMed] [Google Scholar]
- 250.Kang BY, Kleinhenz JM, Murphy TC, Hart CM. The PPARgamma ligand rosiglitazone attenuates hypoxia-induced endothelin signaling in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2011;301(6):L881–91. doi: 10.1152/ajplung.00195.2011. Epub 2011/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Aguirre JI, Morrell NW, Long L, et al. Vascular remodeling and ET-1 expression in rat strains with different responses to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2000;278(5):L981–7. doi: 10.1152/ajplung.2000.278.5.L981. Epub 2000/04/27. [DOI] [PubMed] [Google Scholar]
- 252.Wilkes LC, Boarder MR. Characterization of endothelin receptors on a human neuroblastoma cell line: evidence for the ETA subtype. British journal of pharmacology. 1991;104(3):750–4. doi: 10.1111/j.1476-5381.1991.tb12499.x. Epub 1991/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tsang MC, Lo AC, Cheung PT, Chung SS, Chung SK. Perinatal hypoxia-/ischemia-induced endothelin-1 mRNA in astrocyte-like and endothelial cells. Neuroreport. 2001;12(10):2265–70. doi: 10.1097/00001756-200107200-00044. Epub 2001/07/12. [DOI] [PubMed] [Google Scholar]
- 254.Barone FC, Globus MY, Price WJ, et al. Endothelin levels increase in rat focal and global ischemia. J Cereb Blood Flow Metab. 1994;14(2):337–42. doi: 10.1038/jcbfm.1994.41. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 255.Stenman E, Malmsjo M, Uddman E, Gido G, Wieloch T, Edvinsson L. Cerebral ischemia upregulates vascular endothelin ET(B) receptors in rat. Stroke. 2002;33(9):2311–6. doi: 10.1161/01.str.0000028183.04277.32. Epub 2002/09/07. [DOI] [PubMed] [Google Scholar]
- 256.Aranda JV, Monin P, Beharry K, Bairam A, Vert P. The effect of endothelin-1 on the cerebrovascular response to hypoxia and hypercapnia in the newborn. Semin Perinatol. 1992;16(3):196–9. Epub 1992/06/01. [PubMed] [Google Scholar]
- 257.Lo AC, Chen AY, Hung VK, Yaw LP, Fung MK, Ho MC, et al. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25(8):998–1011. doi: 10.1038/sj.jcbfm.9600108. Epub 2005/04/09. [DOI] [PubMed] [Google Scholar]
- 258.Dschietzig T, Bartsch C, Richter C, Laule M, Baumann G, Stangl K. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist: attenuation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-kappaB. Circ Res. 2003;92(1):32–40. doi: 10.1161/01.res.0000051884.27117.7e. Epub 2003/01/11. [DOI] [PubMed] [Google Scholar]
- 259.Chan SL, Cipolla MJ. Relaxin causes selective outward remodeling of brain parenchymal arterioles via activation of peroxisome proliferator-activated receptor-gamma. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25(9):3229–39. doi: 10.1096/fj.10-175471. Epub 2011/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41(1):50–5. doi: 10.1161/01.hyp.0000042427.05390.5c. Epub 2003/01/04. [DOI] [PubMed] [Google Scholar]
- 261.Dupuis F, Atkinson J, Liminana P, Chillon JM. Captopril improves cerebrovascular structure and function in old hypertensive rats. British journal of pharmacology. 2005;144(3):349–56. doi: 10.1038/sj.bjp.0706001. Epub 2005/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clinical science (London, England: 1979) 2007;112(8):417–28. doi: 10.1042/CS20060342. Epub 2007/03/10. [DOI] [PubMed] [Google Scholar]
- 263.Touyz RM. The role of angiotensin II in regulating vascular structural and functional changes in hypertension. Curr Hypertens Rep. 2003;5(2):155–64. doi: 10.1007/s11906-003-0073-2. Epub 2003/03/19. [DOI] [PubMed] [Google Scholar]
- 264.Heeneman S, Sluimer JC, Daemen MJ. Angiotensin-converting enzyme and vascular remodeling. Circ Res. 2007;101(5):441–54. doi: 10.1161/CIRCRESAHA.107.148338. Epub 2007/09/01. [DOI] [PubMed] [Google Scholar]
- 265.Russell MJ, Klemmer AM, Olson KR. Angiotensin signaling and receptor types in teleost fish. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2001;128(1):41–51. doi: 10.1016/s1095-6433(00)00296-8. Epub 2001/01/04. [DOI] [PubMed] [Google Scholar]
- 266.Griendling KK, Lassegue B, Alexander RW. Angiotensin receptors and their therapeutic implications. Annual review of pharmacology and toxicology. 1996;36:281–306. doi: 10.1146/annurev.pa.36.040196.001433. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 267.Kai H, Alexander RW, Ushio-Fukai M, Lyons PR, Akers M, Griendling KK. G-Protein binding domains of the angiotensin II AT1A receptors mapped with synthetic peptides selected from the receptor sequence. The Biochemical journal. 1998;332 ( Pt 3):781–7. doi: 10.1042/bj3320781. Epub 1998/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lemarie CA, Schiffrin EL. The angiotensin II type 2 receptor in cardiovascular disease. Journal of the renin-angiotensin-aldosterone system: JRAAS. 2010;11(1):19–31. doi: 10.1177/1470320309347785. Epub 2009/10/29. [DOI] [PubMed] [Google Scholar]
- 269.Wolf G, Harendza S, Schroeder R, et al. Angiotensin II’s antiproliferative effects mediated through AT2-receptors depend on down-regulation of SM-20. Laboratory investigation; a journal of technical methods and pathology. 2002;82(10):1305–17. doi: 10.1097/01.lab.0000029207.92039.2f. Epub 2002/10/16. [DOI] [PubMed] [Google Scholar]
- 270.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100(2):264–9. doi: 10.1172/JCI119531. Epub 1997/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Ardaillou R. Angiotensin II receptors. Journal of the American Society of Nephrology: JASN. 1999;10 (Suppl 11):S30–9. Epub 1999/01/19. [PubMed] [Google Scholar]
- 272.Ruiz-Ortega M, Ruperez M, Esteban V, Egido J. Molecular mechanisms of angiotensin II-induced vascular injury. Curr Hypertens Rep. 2003;5(1):73–9. doi: 10.1007/s11906-003-0014-0. Epub 2003/01/18. [DOI] [PubMed] [Google Scholar]
- 273.Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76(9):834–9. doi: 10.1016/j.steroids.2011.02.015. Epub 2011/03/05. [DOI] [PubMed] [Google Scholar]
- 274.Silva PM. From endothelial dysfunction to vascular occlusion: role of the renin-angiotensin system. Revista portuguesa de cardiologia: orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology: an official journal of the Portuguese Society of Cardiology. 2010;29(5):801–24. Epub 2010/09/28. [PubMed] [Google Scholar]
- 275.de Gasparo M. Angiotensin II and nitric oxide interaction. Heart failure reviews. 2002;7(4):347–58. doi: 10.1023/a:1020714518246. Epub 2002/10/16. [DOI] [PubMed] [Google Scholar]
- 276.Zheng J, Bird IM, Chen DB, Magness RR. Angiotensin II regulation of ovine fetoplacental artery endothelial functions: interactions with nitric oxide. The Journal of physiology. 2005;565(Pt 1):59–69. doi: 10.1113/jphysiol.2004.082420. Epub 2005/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Touyz RM, Berry C. Recent advances in angiotensin II signaling. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 2002;35(9):1001–15. doi: 10.1590/s0100-879x2002000900001. Epub 2002/09/10. [DOI] [PubMed] [Google Scholar]
- 278.Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda) 2006;21:269–80. doi: 10.1152/physiol.00004.2006. Epub 2006/07/27. [DOI] [PubMed] [Google Scholar]
- 279.Patten DA, Lafleur VN, Robitaille GA, Chan DA, Giaccia AJ, Richard DE. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrial-derived reactive oxygen species. Molecular biology of the cell. 2010;21(18):3247–57. doi: 10.1091/mbc.E10-01-0025. Epub 2010/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 2004;37(8):1263–73. doi: 10.1590/s0100-879x2004000800018. Epub 2004/07/27. [DOI] [PubMed] [Google Scholar]
- 281.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovascular research. 2006;71(2):216–25. doi: 10.1016/j.cardiores.2006.02.033. Epub 2006/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhou MS, Schulman IH, Raij L. Nitric oxide, angiotensin II, and hypertension. Seminars in nephrology. 2004;24(4):366–78. doi: 10.1016/j.semnephrol.2004.04.008. Epub 2004/07/15. [DOI] [PubMed] [Google Scholar]
- 283.Page EL, Robitaille GA, Pouyssegur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. The Journal of biological chemistry. 2002;277(50):48403–9. doi: 10.1074/jbc.M209114200. Epub 2002/10/16. [DOI] [PubMed] [Google Scholar]
- 284.Wang Z, Tang L, Zhu Q, et al. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney international. 2011;79(3):300–10. doi: 10.1038/ki.2010.326. Epub 2010/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zhao Q, Ishibashi M, Hiasa K, Tan C, Takeshita A, Egashira K. Essential role of vascular endothelial growth factor in angiotensin II-induced vascular inflammation and remodeling. Hypertension. 2004;44(3):264–70. doi: 10.1161/01.HYP.0000138688.78906.6b. Epub 2004/07/21. [DOI] [PubMed] [Google Scholar]
- 286.Sodhi CP, Kanwar YS, Sahai A. Hypoxia and high glucose upregulate AT1 receptor expression and potentiate ANG II-induced proliferation in VSM cells. Am J Physiol Heart Circ Physiol. 2003;284(3):H846–52. doi: 10.1152/ajpheart.00625.2002. Epub 2002/11/16. [DOI] [PubMed] [Google Scholar]
- 287.Matsuura H, Ichiki T, Ikeda J, et al. Inhibition of prolyl hydroxylase domain-containing protein downregulates vascular angiotensin II type 1 receptor. Hypertension. 2011;58(3):386–93. doi: 10.1161/HYPERTENSIONAHA.110.167106. Epub 2011/08/10. [DOI] [PubMed] [Google Scholar]
- 288.Wang Z, Rao PJ, Shillcutt SD, Newman WH. Angiotensin II induces proliferation of human cerebral artery smooth muscle cells through a basic fibroblast growth factor (bFGF) dependent mechanism. Neurosci Lett. 2005;373(1):38–41. doi: 10.1016/j.neulet.2004.09.068. Epub 2004/11/24. [DOI] [PubMed] [Google Scholar]
- 289.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. British journal of pharmacology. 2011;164 (Suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. Epub 2011/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bevan RD, Tsuru H. Long-term denervation of vascular smooth muscle causes not only functional but structural change. Blood vessels. 1979;16(2):109–12. Epub 1979/01/01. [PubMed] [Google Scholar]
- 291.Bevan RD, Tsuru H. Functional and structural changes in the rabbit ear artery after sympathetic denervation. Circ Res. 1981;49(2):478–85. doi: 10.1161/01.res.49.2.478. Epub 1981/08/01. [DOI] [PubMed] [Google Scholar]
- 292.Damon DH. Sympathetic innervation promotes vascular smooth muscle differentiation. Am J Physiol Heart Circ Physiol. 2005;288(6):H2785–91. doi: 10.1152/ajpheart.00354.2004. Epub 2005/01/25. [DOI] [PubMed] [Google Scholar]
- 293.Teeters JC, Erami C, Zhang H, Faber JE. Systemic alpha 1A-adrenoceptor antagonist inhibits neointimal growth after balloon injury of rat carotid artery. Am J Physiol Heart Circ Physiol. 2003;284(1):H385–92. doi: 10.1152/ajpheart.00658.2002. Epub 2002/10/22. [DOI] [PubMed] [Google Scholar]
- 294.Buchholz J, Edwards-Teunissen K, Duckles SP. Impact of development and chronic hypoxia on NE release from adrenergic nerves in sheep arteries. Am J Physiol. 1999;276(3 Pt 2):R799–808. doi: 10.1152/ajpregu.1999.276.3.R799. Epub 1999/03/10. [DOI] [PubMed] [Google Scholar]
- 295.Pearce WJ, Duckles SP, Buchholz J. Effects of maturation on adrenergic neurotransmission in ovine cerebral arteries. Am J Physiol. 1999;277(4 Pt 2):R931–7. doi: 10.1152/ajpregu.1999.277.4.R931. Epub 1999/10/12. [DOI] [PubMed] [Google Scholar]
- 296.Maclean MR, Dempsie Y. The serotonin hypothesis of pulmonary hypertension revisited. Adv Exp Med Biol. 2010;661:309–22. doi: 10.1007/978-1-60761-500-2_20. Epub 2010/03/06. [DOI] [PubMed] [Google Scholar]
- 297.Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol. 1997;272(5 Pt 1):L795–806. doi: 10.1152/ajplung.1997.272.5.L795. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 298.Ullmer C, Schmuck K, Kalkman HO, Lubbert H. Expression of serotonin receptor mRNAs in blood vessels. FEBS letters. 1995;370(3):215–21. doi: 10.1016/0014-5793(95)00828-w. Epub 1995/08/21. [DOI] [PubMed] [Google Scholar]
- 299.MacLean MR, Dempsie Y. Serotonin and pulmonary hypertension--from bench to bedside? Current opinion in pharmacology. 2009;9(3):281–6. doi: 10.1016/j.coph.2009.02.005. Epub 2009/03/17. [DOI] [PubMed] [Google Scholar]
- 300.Watts SW. 5-HT in systemic hypertension: foe, friend or fantasy? Clinical science (London, England: 1979) 2005;108(5):399–412. doi: 10.1042/CS20040364. Epub 2005/04/16. [DOI] [PubMed] [Google Scholar]
- 301.Demolle D, Van Coevorden A, Boeynaems JM. Stimulation of aortic smooth muscle prostacyclin by serotonin: role of distinct receptors in contractile and synthetic states. Circulation research. 1989;64(4):806–13. doi: 10.1161/01.res.64.4.806. Epub 1989/04/01. [DOI] [PubMed] [Google Scholar]
- 302.Wiernsperger NF. Serotonin, 5-HT2 receptors, and their blockade by naftidrofuryl: a targeted therapy of vascular diseases. Journal of cardiovascular pharmacology. 1994;23 (Suppl 3):S37–43. Epub 1994/01/01. [PubMed] [Google Scholar]
- 303.White K, Dempsie Y, Nilsen M, Wright AF, Loughlin L, MacLean MR. The serotonin transporter, gender, and 17beta oestradiol in the development of pulmonary arterial hypertension. Cardiovascular research. 2011;90(2):373–82. doi: 10.1093/cvr/cvq408. Epub 2010/12/24. [DOI] [PubMed] [Google Scholar]
- 304.Eddahibi S, Raffestin B, Hamon M, Adnot S. Is the serotonin transporter involved in the pathogenesis of pulmonary hypertension? The Journal of laboratory and clinical medicine. 2002;139(4):194–201. doi: 10.1067/mlc.2002.122181. Epub 2002/05/25. [DOI] [PubMed] [Google Scholar]
- 305.Teng GQ, Williams J, Zhang L, Purdy R, Pearce WJ. Effects of maturation, artery size, and chronic hypoxia on 5-HT receptor type in ovine cranial arteries. Am J Physiol. 1998;275(3 Pt 2):R742–53. doi: 10.1152/ajpregu.1998.275.3.R742. Epub 1998/09/05. [DOI] [PubMed] [Google Scholar]
- 306.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Progress in neurobiology. 1996;50(4):335–62. doi: 10.1016/s0301-0082(96)00033-0. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 307.Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, et al. Towards a revised nomenclature for P1 and P2 receptors. Trends in pharmacological sciences. 1997;18(3):79–82. doi: 10.1016/s0165-6147(96)01038-3. Epub 1997/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Boeynaems JM, Communi D, Gonzalez NS, Robaye B. Overview of the P2 receptors. Seminars in thrombosis and hemostasis. 2005;31(2):139–49. doi: 10.1055/s-2005-869519. Epub 2005/04/27. [DOI] [PubMed] [Google Scholar]
- 309.Fredholm BB, APIJ, Jacobson KA, Klotz KN, Linden J International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological reviews. 2001;53(4):527–52. Epub 2001/12/06. [PMC free article] [PubMed] [Google Scholar]
- 310.Burnstock G. Purinergic regulation of vascular tone and remodelling. Autonomic & autacoid pharmacology. 2009;29(3):63–72. doi: 10.1111/j.1474-8673.2009.00435.x. Epub 2009/07/02. [DOI] [PubMed] [Google Scholar]
- 311.Borowiec A, Lechward K, Tkacz-Stachowska K, Skladanowski AC. Adenosine as a metabolic regulator of tissue function: production of adenosine by cytoplasmic 5′-nucleotidases. Acta biochimica Polonica. 2006;53(2):269–78. Epub 2006/06/14. [PubMed] [Google Scholar]
- 312.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(3):364–73. doi: 10.1161/hq0302.105360. Epub 2002/03/09. [DOI] [PubMed] [Google Scholar]
- 313.Xu MH, Gong YS, Su MS, Dai ZY, Dai SS, Bao SZ, et al. Absence of the adenosine A2A receptor confers pulmonary arterial hypertension and increased pulmonary vascular remodeling in mice. Journal of vascular research. 2011;48(2):171–83. doi: 10.1159/000316935. Epub 2010/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111(12):5571–80. doi: 10.1182/blood-2007-11-126763. Epub 2008/03/01. [DOI] [PubMed] [Google Scholar]
- 315.Zhao L, Gray L, Leonardi-Bee J, Weaver CS, Heptinstall S, Bath PM. Effect of aspirin, clopidogrel and dipyridamole on soluble markers of vascular function in normal volunteers and patients with prior ischaemic stroke. Platelets. 2006;17(2):100–4. doi: 10.1080/09537100500235966. Epub 2006/01/20. [DOI] [PubMed] [Google Scholar]
- 316.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circulation research. 2006;98(7):868–78. doi: 10.1161/01.RES.0000216596.73005.3c. Epub 2006/04/15. [DOI] [PubMed] [Google Scholar]
- 317.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100(5):633–44. doi: 10.1161/01.RES.0000259563.61091.e8. Epub 2007/03/17. [DOI] [PubMed] [Google Scholar]
- 318.Jie W, Guo J, Shen Z, Wang X, Zheng S, Wang G, et al. Contribution of myocardin in the hypoxia-induced phenotypic switching of rat pulmonary arterial smooth muscle cells. Experimental and molecular pathology. 2010;89(3):301–6. doi: 10.1016/j.yexmp.2010.06.010. Epub 2010/07/14. [DOI] [PubMed] [Google Scholar]