Abstract
STUDY QUESTION
Are STAT3 signaling molecules differentially expressed in endometriosis?
SUMMARY ANSWER
Levels of phospho-STAT3 and HIF1A, its downstream signaling molecule, are significantly higher in eutopic endometrium from women with endometriosis when compared with women without the disease.
WHAT IS KNOWN ALREADY
Endometriosis is an estrogen-dependent inflammatory condition. Interleukin 6 (IL-6) is an inflammatory survival cytokine known to induce prolonged activation of STAT3 via association with the IL-6 receptor.
STUDY DESIGN, SIZE, DURATION
Cross-sectional measurements of STAT3 and HIF1A protein levels in eutopic endometrium from women with endometriosis versus those without.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Levels of phospho-STAT3 (pSTAT3) and HIF1A were examined in the endometrium of patients with and without endometriosis as well as in a non-human primate animal model using western blot and immunohistochemical analysis.
MAIN RESULTS AND THE ROLE OF CHANCE
Levels of pSTAT3 were significantly higher in the eutopic endometrium from women with endometriosis when compared with women without the disease in both the proliferative and secretory phases. HIF1A is known to be stabilized by STAT3 and IL-6. Our immunohistochemistry results show abundant HIF1A expression within the eutopic endometrial epithelial cells of women with endometriosis. Furthermore, pSTAT3 and HIF1A proteins are co-localized in endometriosis. This aberrant activation of pSTAT3 and HIF1A is confirmed by sequential analysis of eutopic endometrium using a baboon animal model of induced endometriosis. Lastly, we confirmed this IL-6 induction of both STAT3 phosphorylation and HIF1A mRNA expression in Ishikawa human endometrial adenocarcinoma cell line.
LIMITATIONS, REASONS FOR CAUTION
Ishikawa cancer cell line was used to study a benign disease. The peritoneal fluid contains various inflammatory cytokines in addition to IL-6 and so it is possible that other cytokines may affect the activity and expression of STAT3 signaling molecules.
WIDER IMPLICATIONS OF THE FINDINGS
Our results imply that aberrant activation of STAT3 signaling plays an important role in the pathogenesis of endometriosis. Our findings could progress in our understanding of the etiology and pathophysiology of endometriosis and potential therapeutic interventions by targeted pharmacological.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by NIH R01 HD067721 (to S.L.Y and B.A.L) and NIH R01 HD057873 and American Cancer Society Research Grant RSG-12-084-01-TBG (to J.-W.J.). There are no conflicts of interest.
Keywords: endometriosis, STAT3, HIF1A, IL6
Introduction
Endometriosis is a major cause of infertility and chronic pain, affecting 1 in 10 women of reproductive age and 35–50% of infertile women (Bulun, 2009; May et al., 2011). With an estimated annual societal cost of $22 billion, the economic impact of this disease is profound in the USA (Xiong et al., 2013). Because of a dearth of reliable biomarkers and considerable symptom overlap with other gynecological pathologies, this disease is difficult to diagnose. Time from onset to clinical diagnosis averages 8–11 years (Bulun, 2009). Surgical resection of ectopic lesions and hormonal suppression are currently the current gold standards for treatment, but both approaches are associated with a high incidence of relapse and adverse side effects. Although several theories have been proposed for causality, the etiology of this disease is still unclear. Research examining the molecular mechanisms which permit the establishment of this disease at onset will enable earlier and more effective treatment interventions.
Endometriosis is an estrogen-dependent inflammatory condition associated with elevated tissue-, peripheral- and peritoneal-cytokines (Bulun, 2009). Interleukin-6 (IL-6) is a well-characterized pro-inflammatory cytokine previously implicated with this disease (Heinrich et al., 2003). Recent in vitro studies have shown that levels of IL-6 are greater in human endometrial stromal cells (hESCs) derived from the endometrial biopsies of women with endometriosis when compared with women without the disease (Tsudo et al., 2000). IL-6 is known to signal via the Signal Transducer and Signal transducer and activator of transcription (STAT) family of transcription factors, specifically STAT3 (Wang et al., 2013).
The transcription factor, STAT3 is localized in the cytoplasm until activated by phosphorylation. A variety of cytokines which are leukemia inhibitory factor (LIF), IL-6, IL-11, and epidermal growth factor (EGF) can activate STAT3 (Zhong et al., 1994). After STAT3 activation, it translocates to the nucleus with the formation of homodimers or heterodimers and binds to promoter regions for target gene expression (Darnell, 1997). Using an oligodeoxynucleotide transferred in utero, implantation and decidualization are interrupted by transient and local suppression of STAT3 activity (Nakamura et al., 2006), and also phosphorylation of STAT3 is certainly required for embryonic development (Takeda et al., 1997). Phosphorylated STAT3 is connected to a number of tumor-promoting processes including maintenance of the stem cell, block differentiation, promoting growth and angiogenesis, and regulating the immune response and tumor microenvironment (Carro et al., 2010).
Hypoxia has a pathophysiological effect through the process of disease and regulation of gene expression (Semenza, 2000a,b). Hypoxia-inducible factors (HIFs) are a family of transcription factors implicated in cellular adaptation to low oxygen levels via transcriptional regulation. HIF1 is the master transcription factor and is composed of HIF1A and HIF1B subunits (Bulun et al., 2000). HIF1B subunit is constitutively expressed, whereas HIF1A levels vary in response to hypoxia. HIF1A is normally present only under hypoxic conditions, but can be stabilized by STAT3, nitric oxide (NO) and IL-6 via the epidermal growth factor receptor (EGFR), Ras, mechanistic target of rapamycin (mTOR) and Phosphatidylinositol-3-Kinase and Protein Kinase B (PI3K/AKT) pathways (O'Donnell et al., 2006).
Phosphorylated STAT3 induces HIF1A expression (Couto et al., 2012) while targeting STAT3 blocks HIF1A and VEGF expression (Xu et al., 2005). However, there are currently no studies investigating the correlation between STAT3 and HIF1A-specific signaling in the context of endometriosis. In the present study, we investigated levels of total and activated STAT3 and HIF1A in the endometrium from women with and without endometriosis. Our findings provide new insight into the etiology of endometriosis and provide a new molecular framework useful for the design of new therapeutic strategies.
Materials and Methods
Human endometrium samples
The study has been reviewed and approved by the Institutional Review Boards of Michigan State University, Spectrum Health Medical System (Grand Rapids, MI), Greenville Health System (Greenville, SC), and the University of North Carolina (Chapel Hill, NC). Written informed consent was obtained from all human subjects. Human endometrial samples were obtained through the Michigan State University's Center for Women's Health Research Female Reproductive Tract Biorepository, the Greenville Hospital System, and the University of North Carolina. To compare protein expression patterns of eutopic endometrium with and without endometriosis, endometrial biopsies were obtained at the time of surgery from 43 regularly cycling women between the age of 18 and 45. For control eutopic endometrium, five samples were collected from the proliferative phase, seven were from the early secretory phase, four were from the mid secretory phase, and four were late secretory. For endometriosis eutopic endometrium, 10 samples were collected from the proliferative phase, and 13 were from the secretory phase. The presence or absence of disease was confirmed during surgery. Women laparoscopically negative for this disease were placed into the control group, whereas women laparoscopically positive for this disease were placed in the endometriosis group. The endometriosis patients consisted of 6 stage I, 9 stage II, 5 stage III and 3 stage IV of endometriosis. Use of an IUD or hormonal therapies in the 3 months preceding surgery was exclusionary. Histologic dating of endometrial samples was performed by board certified pathologist and subsequently confirmed by an experienced Fertility specialist (B.A.L.).
Baboon endometrium samples
Use of this animal model was reviewed and approved by the Institutional Animal Care and Use Committees (IACUCs) of both the University of Illinois at Chicago and Michigan State University. Endometriosis was induced by intraperitoneal inoculation of menstrual endometrium on two consecutive menstrual cycles as previously described (Afshar et al., 2013). For baboon endometrium, eutopic endometrial tissues were collected from five early secretory phase baboons at pre-inoculation, and 3, 6, and 9 months of post-inoculation.
Cell culture and treatment
The uterine endometrial epithelial cell line, Ishikawa (endometrial adenocarcinoma) were grown in Dulbecco's Modified Eagle's Medium with F12 (Invitrogen, Grand Island, NY, USA), supplemented with 10% (v/v) fetal bovine serum (Invitrogen), 50 units/ml penicillin and 50 µg/ml streptomycin (Invitrogen) in an atmosphere of 5% CO2 and 95% air at 37°C. To determine the effects of IL-6, E2+Medroxyprogesterone Acetate (MPA), and WP1066 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) on STAT3 and HIF1A activation, these reagents and inhibitors were directly added to sub-confluent Ishikawa cells and then incubated for the indicated time. All experiments were performed in triplicate with independent protein lysates.
Western blot analysis
Endometrial tissue was lysed using a lysis buffer (10 mM Tris–HCl (pH 7.4), 150 mM NaCl, 2.5 mM EDTA, and 0.125% (v/v) Nonidet P-40 supplemented with both a protease inhibitor cocktail (Roche, Indianapolis, IN, USA) and a phosphatase inhibitor cocktail (Sigma Aldrich, St. Louis, MO, USA). Thirty μg of total protein was separated on 8% SDS–PAGE gels and transferred into a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA, USA). After blocking with 0.5% (w/v) casein for 2 h in phosphate-buffered saline (PBS) with 0.1% (v/v) Tween 20 (Sigma Aldrich), membranes were incubated with either anti-STAT3 (Cell Signaling, Danvers, MA, USA), anti-phospho-STAT3 (Cell Signaling), or anti-HIF1A (BD Bioscience, San Jose, CA, USA) antibodies. Total Actin (Santa Cruz Biotechnology, Inc.) levels were examined for loading controls. Following incubation with primary antibody, membranes were exposed to a horse-radish peroxidase-linked (HRP−) secondary antibody and positive immunostained observed using an enhanced chemiluminescence HRP substrate. All experiments were performed in triplicate with independent protein lysates. The band intensity was determined by relative densitometry using ImageJ (National Institute of Health, USA), and normalized against the bands obtained for actin or STAT3.
Immunohistochemical and immunofluorescence analyses
Uterine sections were blocked with 10% normal goat serum in PBS (pH 7.5) for immunohistochemistry (IHC) and 10% normal goat serum in PBST (0.01% Triton X-100 in PBS) for immunofluorescence (IF). Sections were exposed to appropriate primary antibody ([anti-STAT3, Cell Signaling], [anti-phospho-STAT3, Cell Signaling] or [anti-HIF1A, BD Bioscience]) in 10% normal goat serum in PBS (pH 7.5) overnight at 4°C. For IHC, sections were incubated with the appropriate secondary antibody (Vector Laboratories, Burlingame, CA, USA). Following exposure to the horseradish peroxidase-conjugated streptavidin substrate, positive immunoreactivity (brown precipitate) was detected using the Vectastain Elite DAB kit (Vector Laboratories). A semiquantitative grading system (H-score) was used to compare the immunohistochemical staining intensities. The H-score was calculated using the following equation: H-score = Σ Pi (i + 1), where i = intensity of staining with a value of 1, 2 or 3 (weak, moderate or strong, respectively) and Pi is the percentage of stained cells for each intensity, varying from 0 to 100% (Ishibashi et al., 2003). For immunofluorescence, the sections were incubated 2 h at RT with the following secondary antibodies: Alexa Fluor 488-conjugated anti-mouse IgG (Invitrogen) and Alexa Fluor 594-conjugated anti-rabbit IgG (Invitrogen). Nuclei of the cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories).
Statistical analysis
Statistical analyses were performed using Student's t-test, Mann–Whitney U-test, or repeated measures ANOVA or one way ANOVA followed by Tukey's post hoc multiple range test using the Instat package from GraphPad (San Diego, CA, USA). The Spearman correlation coefficient was used to assess correlations between the levels of pSTAT3 and HIF1A in control and endometriosis. All data are presented as means ± SEM. P < 0.05 was considered statistically significant.
Results
Aberrant activation of STAT3 in eutopic endometrial tissue from women with endometriosis
To determine whether STAT3 signaling is dysregulated in endometriosis, we first examined the expression of total STAT3 and phosphorylated STAT3 (pSTAT3) in endometrium from patients with and without endometriosis using western blot (Fig. 1 A and B). The expression levels of total STAT3 did not differ in the presence of endometriosis. However, levels of pSTAT3 were significantly higher in the endometrium derived from women with endometriosis (the mean of relative band intensity ± SEM: 2.28 ± 0.38) when compared with controls (1.00 ± 0.21) (Student's t-test, P = 0.0158, Fig. 1A and B). To examine the cell-specific expression of STAT3 and pSTAT3, we next performed immunohistochemical analysis of endometrium from women with and without endometriosis. STAT3 expression was consistently detected in both the stromal and epithelial compartments of endometrium in both the control (the mean of H-score ± SEM: 252.25 ± 14.45) and endometriosis groups (274.35 ± 9.47) (Mann–Whitney U-test, P = 0.25, Fig. 1 C and D). In control women pSTAT3 protein was not detected in endometrial cells except decidual cells of late secretory phase (Supplementary Fig. S1). Interestingly, the level of pSTAT3 protein was significantly increased in epithelial cells of endometriosis patients (255.65 ± 9.15) when compared with control patients (14.75 ± 5.39) (Mann–Whitney U-test, P < 0.0001, Fig. 1E and F). These results suggest that dysregulation of STAT3 signaling molecules may play an important role in the pathogenesis of endometriosis.
Figure 1.
Aberrant activation of phospho-signal transducer and activator of transcription-3 (pSTAT3) in eutopic endometrial tissue from women with endometriosis. (A) Western blot analysis of STAT3 and pSTAT3 proteins in human endometrium of control and endometriosis. Actin was used as sample-loading control. (B) Quantification of pSTAT3/STAT3 western blot data obtained by densitometric analysis. Representative photomicrograph of immunohistochemical staining of STAT3 (C) and pSTAT3 (E) proteins in human endometrium of control and endometriosis. The immunohistochemical histological score (H-score) of STAT3 (D) and pSTAT3 (F) proteins. The results represent the mean ± standard error of the mean (SEM). *P < 0.05; ***P < 0.001.
HIF1A expression in endometrium from women with endometriosis
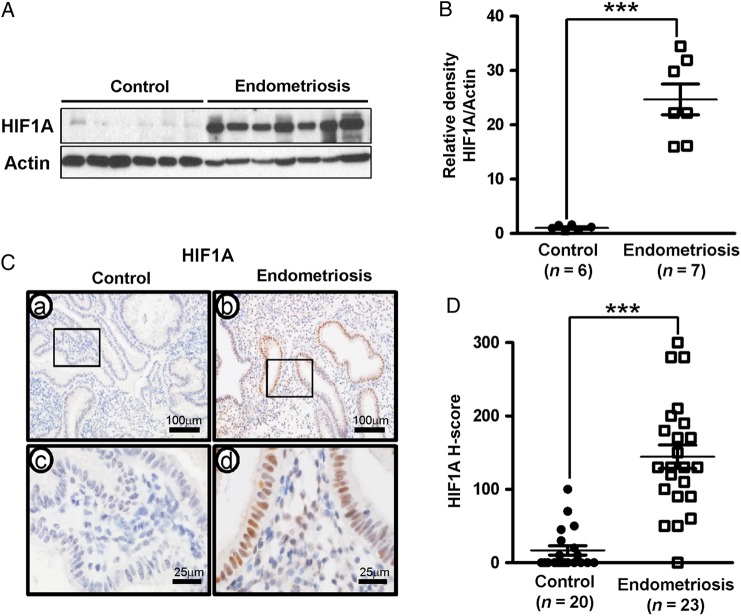
HIF1A is a known target of pSTAT3 and a key mediator of angiogenesis, inflammation, proliferation, self-renewal, and extracellular invasion (Henze and Acker, 2010). The western blot analysis of HIF1A levels show that levels of HIF1A were markedly increased in the endometrium of patients with endometriosis (the mean of relative band intensity ± SEM: 24.68 ± 2.83) when compared with controls (1.00 ± 0.24) (Student's t-test, P = 0.0002, Fig. 2A and B). Additionally, subsequent immunohistochemistry analysis of uterine paraffin wax sections confirm that HIF1A protein levels are significantly higher in the endometrial epithelium of patients with endometriosis (the mean of H-score ± SEM: 144.35 ± 15.99) when compared with the patients without endometriosis (16.75 ± 6.35) (Mann–Whitney U-test, P < 0.0001, Fig. 2C and D).
Figure 2.
Aberrant expression of hypoxia-inducible factor 1-alpha (HIF1A) in eutopic endometrial tissue from women with endometriosis. (A) Western blot analysis of HIF1A proteins in human endometrium of control and endometriosis. Actin was used as sample-loading control. (B) Quantification of HIF1A western blot data obtained by densitometric analysis. (C) Representative photomicrograph of immunohistochemical staining of HIF1A proteins in human endometrium of control and endometriosis. (D) The immunohistochemical histological score (H-score) of HIF1A proteins. The results represent the mean ± SEM. ***P < 0.001.
Correlation between pSTAT3 and HIF1A in endometriosis
Figure 3A shows the correlation between pSTAT3 and HIF1A proteins in both women without and with endometriosis. There was a significant positive correlation between pSTAT3 and HIF1A in the endometrial epithelial cells (Spearman correlation coefficient r = 0.7943, P < 0.0001). To determine whether pSTAT3 colocalized with HIF1A proteins with respect to endometriosis, we performed double immunofluorescence for pSTAT3 and HIF1A (Fig. 3B). Our immunostaining results show that pSTAT3 and HIF1A proteins were colocalized in 13% of epithelial cells from endometriosis patients. In contrast, 1% of epithelial cells were colocalized in patients without endometriosis. These results suggest a strong correlation exists between STAT3 activity and HIF1A expression in the endometrium which may play an important role in the development and progression of endometriosis.
Figure 3.

Correlation and colocalization analysis of phospho-STAT3 and HIF1A in eutopic endometrial tissue from women with endometriosis. (A) Correlation of between pSTAT3 and HIF1A H-score in control (n = 20) and endometriosis (n = 23) (P < 0.001, r = 0.7943). (B) Colocalization of pSTAT3 and HIF1A in the endometrium between women with and without endometriosis by immunofluorescence analysis.
pSTAT3 and HIF1A expression during progression of endometriosis in a baboon model
A baboon model has previously been developed to study the pathophysiology of endometriosis (Fazleabas et al., 2002; Braundmeier and Fazleabas, 2009; D'Hooghe et al., 2009). Intraperitoneal inoculation with autologous menstrual tissue under laparoscopic guidance results in the formation of endometriotic lesions with histological and morphological characteristics similar to those seen in women (Fazleabas et al., 2002). To determine if aberrant activation of STAT3 signaling was also evident in this non-human primate model, we examined pSTAT3 and HIF1A expression in the eutopic endometrium of baboons following experimental induction of the disease. pSTAT3 was strongly detected in both the stroma and epithelium of baboon endometrium following induction of the disease (Fig. 4), but levels of STAT3 protein were unchanged (Supplementary Fig. S2). pSTAT3 and HIF1A protein were weakly detected in the endometrium of pre-inoculation (control) animals (the mean of H-score ± SEM: 40.00 ± 11.40 and 12.00 ± 5.83, respectively). The levels of pSTAT3 proteins were significantly increased at 6 months post-inoculation (124.00 ± 21.59, repeated measures ANOVA, P < 0.01) and 9 months post-inoculation (184 ± 26.94, P < 0.001) during endometriosis progression (Fig. 4A). Likewise, the levels for HIF1A proteins were significantly increased in the epithelial compartment at 6 (148.00 ± 28.71) and 9 (222 ± 25.98) months post-inoculation (repeated measures ANOVA, P < 0.01 and P < 0.001, respectively) (Fig. 4B). These results suggest that aberrant activation of STAT3 may induce HIF1A proteins during the pathogenesis of endometriosis.
Figure 4.
Aberrant activation of pSTAT3 and HIF1A in induced endometriosis of baboon. Immunohistochemical analysis of pSTAT3 (A and B) and HIF1A (C and D) in endometriosis baboon model induced by intraperitoneal inoculation of menstrual endometrium during progression of endometriosis. pSTAT3 and HIF1A proteins were examined in the endometrium of pre-inoculation (a and e) and 3 (b and f), 6 (c and g) and 9 (d and h) months post-inoculation during endometriosis progression. The immunohistochemical histological score (H-score) of pSTAT3 (B) and HIF1A (D). The results represent the mean ± SEM. **P < 0.01; ***P < 0.001.
IL-6 contributes to the elevated levels of pSTAT3 and HIF1A expression
Previous studies have shown that IL-6 is over-expressed within the endometrium of women with endometriosis and pSTAT3 is regulated by inflammatory cytokines such as IL-6 (Kroon et al., 2013; Karalok et al., 2014). To determine whether IL-6 can induce phosphorylation of STAT3 and HIF1A expression in endometrial epithelial cells, we treated Ishikawa cells with IL-6 for 12 h and subsequently used western blot analysis to examine the expression levels of STAT3, pSTAT3 and HIF1A. Our results showed that IL6 treatment significantly increased pSTAT3 (4.23 ± 0.31) and HIF1A (3.45 ± 0.68) expression compared with the control (1.00 ± 0.07 and 1.00 ± 0.08, respectively) but STAT3 levels were unchanged (Fig. 5). These results indicate that this induction of pSTAT3 is not transcriptional regulation of STAT3. Estradiol (E2) + Medroxyprogesterone (MPA) are known moderators of uterine STAT3 function (Lee et al., 2013). Furthermore, E2 + MPA and IL-6 treatment significantly increased pSTAT3 levels (12.17 ± 0.50) when compared with E2 + MPA (1.26 ± 0.08, P < 0.001) and IL-6 alone (4.23 ± 0.31, P < 0.001). These results suggest that IL-6 induces phosphorylation of STAT3 and then HIF1A expression.
Figure 5.
Effects of IL-6 on STAT3 phosphorylation and HIF1A expression. Ishikawa cells were treated with interleukin 6 (IL-6) and/or Estradiol (E2) plus medroxyprogesterone acetate (MPA) for 12 h. (A) Western blot analysis of STAT3, phospho-STAT3 and HIF1A in Ishikawa cells treated with IL-6 and/or E2 plus MPA Actin was used as sample-loading control. (B and C) Quantification of pSTAT3 (B) and HIF1A (C). The results represent the mean ± SEM. **P < 0.01; ***P < 0.001.
Activated STAT3 is required for HIF1A expression
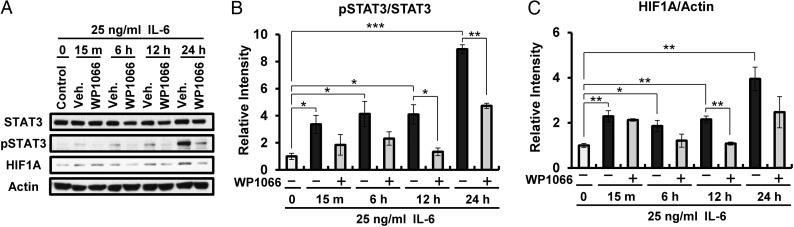
We next examined whether HIF1A expression could be interrupted by inhibition of STAT3 phosphorylation. To examine whether HIF1A expression is downstream of STAT3 activation in uterine epithelial cells, we treated Ishikawa cells with IL-6 in the presence or absence of WP1066, a STAT3 inhibitor, and examined HIF1A, pSTAT3 and STAT3 protein levels using western blot analysis. Our results show a remarkable time-dependent induction of pSTAT3 by IL-6 which could be prevented by pretreatment with WP1066. In addition, WP1066 treatment remarkably reduced HIF1A expression after 6 h of IL-6 treatment (Fig. 6). These results suggest that the HIF1A induction seen in the endometrial epithelial cells is down stream of pSTAT3 induction.
Figure 6.
Effects of STAT3 inhibitor (WP1066) in Ishikawa cells treated with IL-6. (A) Western blot analysis of STAT3, pSTAT3 and HIF1A in Ishikawa cells treated with IL-6 and/or WP1066 for 0, 15 min, 6, 12 and 24 h. Actin was used as sample-loading control. (B and C) Quantification of pSTAT3 (B) and HIF1A (C). The results represent the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Aberrant activation of STAT3 has been identified as both abnormal and oncogenic (He and Karin, 2011; Fagard et al., 2013). STAT3 signaling participates in oncogenesis by stimulating cell proliferation, promoting angiogenesis, mediating immune evasion and conferring resistance to apoptosis. Our results herein show that levels of pSTAT3 are significantly higher in the eutopic endometrium from women with endometriosis when compared with controls (Fig. 1). This suggests that tight regulation of STAT3 activity is important for normal uterine function. The relationship between STAT3 and endometriosis in humans is not well known. Given its relevance to the inflammatory process, surprisingly little is known about endometrial STAT3 and its regulation in health and disease. Examining of molecular mechanisms of uterine STAT3 signaling may suggest effective therapies for endometriosis and other uterine disorders.
This study is the first to build on the hypothesis that aberrant activation of STAT3 and HIF1A signaling within the eutopic endometrium is relevant to endometriosis due to dysregulation of cell proliferation, inflammation and steroid hormone signaling. To better understand the correlation of these two transcription factors in endometriosis, we examined correlation and colocalization of pSTAT3 and HIF1A (Fig. 3). Furthermore, this study shows that inhibition of STAT3 can prevent HIF1A expression in epithelial cells (Fig. 6). HIF1A has previously been shown to up-regulate many of the aberrant proteins and factors associated with endometriosis, including increased cellular proliferation (Semenza, 2000a,b), aberrant ESR2 expression (Juhasz-Boss et al., 2011; Wu et al., 2012), and angiogenic factors VEGF (Maybin et al., 2011) and Cyr61 (Gashaw et al., 2008). Leptin is also a known target of HIF1A and can induce endometrial cell proliferation (Wu et al., 2007; Oh et al., 2013). Silencing HIF1A (siRNA) suppresses hypoxia-induced VEGF (Spinella et al., 2007). Our results suggest that elevated pSTAT3 induces and stabilizes HIF1A within the eutopic endometrium of women with endometriosis, subsequently leading to aberrantly increased angiogenesis, MMPs, and proliferation within the eutopic endometrial compartment (Gilabert-Estelles et al., 2007; Aznaurova et al., 2014; Lu et al., 2014).
We describe herein a correlation between pSTAT3 and HIF1A expression levels, which is disease-dependent (Fig. 3). Previous studies using the non-human primate model suggest that the presence of ectopic endometrium (endometriotic lesions) alters the eutopic endometrial gene expression profile (Li et al., 2013; Meola et al., 2013). As in cancer, hypoxia is likely an important effector of the microenvironment of the ectopic endometriotic implants. Indeed, HIF1A has been shown to regulate expressions of ESR1 and ESR2 expression (Wu et al., 2012). These results suggest that dysregulation of STAT3 and HIF1A signaling may play an important role in the pathogenesis of endometriosis. Our western blot analysis showed an increase in both pSTAT3 and HIF1A expression in Ishikawa cells following IL-6 treatment (Fig. 5). Additionally, we observed more colocalization of HIF1A and pSTAT3 proteins in endometrial epithelial cells from women with endometriosis (Fig. 3B). These results suggest that cytokines and disease-specific regulation of pSTAT3 may represent a sentinel difference within the eutopic endometrium from women with and without this disease.
Previous studies have described use of a non-human primate model for the study of endometriosis (D'Hooghe et al., 1994; Fazleabas, 2006). Intraperitoneal inoculation with autologous menstrual tissue results in the formation of endometriotic lesions with histological and morphological characteristics similar to those seen in women (Fazleabas et al., 2002). This baboon model also shows similar markers of progesterone resistance as previously described in humans (Braundmeier and Fazleabas, 2009). Sequential analysis of eutopic endometrium from these baboons throughout the progression of this disease reveal increased pSTAT3 and HIF1A expression (Fig. 4). Angiogenesis plays an important role in endometriosis. The angiogenic potential of both the endometrium and the peritoneal environment influences lesion establishment, and endometriotic lesions require an adequate blood supply to survive in their ectopic sites (McLaren, 2000; Taylor et al., 2002). Therefore, these results suggest that aberrant activation of STAT3 may induce HIF1A proteins during endometriosis progression. We confirmed HIF1A induction of pSTAT3 in Ishikawa endometrial epithelial cancer cell lines (Fig. 6).
Inflammatory cytokines are responsible for the IL-6 elevation found at higher concentrations within the peritoneal fluid (Buyalos et al., 1992) and serum of women with endometriosis (Pellicer et al., 1998). In animal models of endometriosis, IL-6 is also increased in the serum of rats with surgically induced endometriosis (Boutten et al., 1992). Our results show that phosphorylation of STAT3 can be remarkably increased by IL-6 (Fig. 5) and that inhibition of STAT3 phosphorylation can reduce HIF1A expression (Fig. 6).
JAKs and STATs are critical components of many cytokine receptor systems, regulating growth, survival, differentiation, and pathogen resistance. JAK1 and JAK2 account for STAT3 phosphorylation upon docking with IL-11/gp130 receptor complex (Kamimura et al., 2003). WP1066 inhibits STAT3 activity by inhibiting its upstream transcription factor JAK (Ferrajoli et al., 2007). WP1066 shows a decrease of HIF1A levels in Ishikawa cells, suggesting that STAT3 can regulate angiogenesis through HIF1A. Additionally, S3I-201 inhibits STAT3 activity by inhibiting STAT3 dimerization (Fletcher et al., 2009), whereas cryptotanshinone inhibits STAT3 activity by inhibiting its phosphorylation (Lu et al., 2013). Furthermore, Ishikawa cells are human endometrial epithelial cancer cell lines. Ishikawa cell line, which is one of the few uterine cell lines that expresses functional ESR and PGR (Croxtall et al., 1990). This cancer cell line is modestly responsive to estrogen, but has lost its inhibitory response to progesterone, despite expression of receptors for both hormones. We have observed activation of STAT3 in Ishikawa cells treated with IL-6 (Fig. 5). Therefore, additional studies are necessary to fully understand the action of STAT3 in other endometrial epithelial cell lines with other STAT3 inhibitors.
In summary, we report for the first time that phosphorylated STAT3 and HIF1A are highly expressed and activated in the endometrium of patients with endometriosis. Our studies provides a better understanding for the aberrant gene expression previously described by many and attributed to progesterone resistance (Young and Lessey, 2010). Progress in our understanding of the etiology and pathophysiology of endometriosis and potential therapeutic interventions by targeted pharmacological agents has been hampered due, in part, to the lack of defined molecular mechanisms. These findings will provide new etiological insight into the development of this disease as well as a molecular framework for the design of new therapeutic strategies.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
B.G.K., J.-Y.Y., T.H.K. and J.F.L. performed experiments, analyzed the data and wrote the manuscript. J.-H.S. provided helpful discussion and critical analysis of data. S.D.F., A.T.F., and S.L.Y. contributed to sample collection and critical revision of the manuscript. B.A.L and J.-W.J. contributed to the design of the study, interpretation of the data and wrote the manuscript.
Funding
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Development, NIH R01 HD067721 (to S.L.Y and B.A.L) and NIH R01 HD057873 and American Cancer Society Research Grant RSG-12-084-01-TBG (to J.-W.J.).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We would like to thank the Human Female Reproductive Tract Biorepository and the Spectrum Health Medical Group, Department of Obstetrics and Gynecology in Grand Rapids, Michigan (especially Elizabeth Leary, M.D., Calvin Leazenby, M.D., Diana Bitner, M.D. and Christine Heisler, M.D.) and Meighan L. McAuliffe for their help in obtaining human samples. We would also like to thank Mark Olson and Angela Houwing for sample preparation and Amanda Sterling for manuscript preparation.
References
- Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod 2013;88:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznaurova YB, Zhumataev MB, Roberts TK, Aliper AM, Zhavoronkov AA. Molecular aspects of development and regulation of endometriosis. Reprod Biol Endocrinol 2014;12:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutten A, Dehoux M, Edelman P, Seta N, Menard A, Madelenat P, Durand G. IL6 and acute phase plasma proteins in peritoneal fluid of women with endometriosis. Clin Chim Acta 1992;210:187–195. [DOI] [PubMed] [Google Scholar]
- Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod 2009;15:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE. Endometriosis. N Engl J Med 2009;360:268–279. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Zeitoun KM, Kilic G. Expression of dioxin-related transactivating factors and target genes in human eutopic endometrial and endometriotic tissues. Am J Obstet Gynecol 2000;182:767–775. [DOI] [PubMed] [Google Scholar]
- Buyalos RP, Funari VA, Azziz R, Watson JM, Martinez-Maza O. Elevated interleukin-6 levels in peritoneal fluid of patients with pelvic pathology. Fertil Steril 1992;58:302–306. [PubMed] [Google Scholar]
- Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature 2010;463:318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto JP, Daly L, Almeida A, Knauf JA, Fagin JA, Sobrinho-Simoes M, Lima J, Maximo V, Soares P, Lyden D, et al. STAT3 negatively regulates thyroid tumorigenesis. Proc Natl Acad Sci USA 2012;109:E2361–E2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD, Elder MG, White JO. Hormonal control of proliferation in the Ishikawa endometrial adenocarcinoma cell line. J Steroid Biochem 1990;35:665–669. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science 1997;277:1630–1635. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Suleman MA, Dunselman GA, Evers HL, Koninckx PR. Development of a model of retrograde menstruation in baboons (Papio anubis). Fertil Steril 1994;62:635–638. [PubMed] [Google Scholar]
- D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, Mwenda JM. Nonhuman primate models for translational research in endometriosis. Reprod Sci 2009;16:152–161. [DOI] [PubMed] [Google Scholar]
- Fagard R, Metelev V, Souissi I, Baran-Marszak F. STAT3 inhibitors for cancer therapy: have all roads been explored? JAKSTAT 2013;2:e22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazleabas AT. A baboon model for inducing endometriosis. Methods Mol Med 2006;121:95–99. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann N Y Acad Sci 2002;955:308–317; discussion 340–342, 396–406. [DOI] [PubMed] [Google Scholar]
- Ferrajoli A, Faderl S, Van Q, Koch P, Harris D, Liu Z, Hazan-Halevy I, Wang Y, Kantarjian HM, Priebe W, et al. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res 2007;67:11291–11299. [DOI] [PubMed] [Google Scholar]
- Fletcher S, Singh J, Zhang X, Yue P, Page BD, Sharmeen S, Shahani VM, Zhao W, Schimmer AD, Turkson J, et al. Disruption of transcriptionally active Stat3 dimers with non-phosphorylated, salicylic acid-based small molecules: potent in vitro and tumor cell activities. Chembiochem 2009;10:1959–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashaw I, Stiller S, Boing C, Kimmig R, Winterhager E. Premenstrual regulation of the pro-angiogenic factor CYR61 in human endometrium. Endocrinology 2008;149:2261–2269. [DOI] [PubMed] [Google Scholar]
- Gilabert-Estelles J, Ramon LA, Espana F, Gilabert J, Vila V, Reganon E, Castello R, Chirivella M, Estelles A. Expression of angiogenic factors in endometriosis: relationship to fibrinolytic and metalloproteinase systems. Hum Reprod 2007;22:2120–2127. [DOI] [PubMed] [Google Scholar]
- He G, Karin M. NF-kappaB and STAT3—key players in liver inflammation and cancer. Cell Res 2011;21:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J 2003;374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze AT, Acker T. Feedback regulators of hypoxia-inducible factors and their role in cancer biology. Cell Cycle 2010;9:2749–2763. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Suzuki T, Suzuki S, Moriya T, Kaneko C, Takizawa T, Sunamori M, Handa M, Kondo T, Sasano H. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab 2003;88:2309–2317. [DOI] [PubMed] [Google Scholar]
- Juhasz-Boss I, Fischer C, Lattrich C, Skrzypczak M, Malik E, Ortmann O, Treeck O. Endometrial expression of estrogen receptor beta and its splice variants in patients with and without endometriosis. Arch Gynecol Obstet 2011;284:885–891. [DOI] [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol 2003;149:1–38. [DOI] [PubMed] [Google Scholar]
- Karalok HM, Karalok E, Saglam O, Torun A, Guzeloglu-Kayisli O, Lalioti MD, Kristansson H, Duke CM, Choe G, Flannery C, et al. mRNA-binding protein TIA-1 reduces cytokine expression in human endometrial stromal cells and is down-regulated in ectopic endometrium. J Clin Endocrinol Metab 2014;jc20133488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon P, Berry PA, Stower MJ, Rodrigues G, Mann VM, Simms M, Bhasin D, Chettiar S, Li C, Li PK, et al. JAK-STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem-like cells. Cancer Res 2013;73:5288–5298. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ, Lydon JP, Jeong JW. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J 2013;27:2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MQ, Shao J, Meng YH, Mei J, Wang Y, Li H, Zhang L, Chang KK, Wang XQ, Zhu XY, et al. NME1 suppression promotes growth, adhesion and implantation of endometrial stromal cells via Akt and MAPK/Erk1/2 signal pathways in the endometriotic milieu. Hum Reprod 2013;28:2822–2831. [DOI] [PubMed] [Google Scholar]
- Lu L, Li C, Li D, Wang Y, Zhou C, Shao W, Peng J, You Y, Zhang X, Shen X. Cryptotanshinone inhibits human glioma cell proliferation by suppressing STAT3 signaling. Mol Cell Biochem 2013;381:273–282. [DOI] [PubMed] [Google Scholar]
- Lu Z, Zhang W, Jiang S, Zou J, Li Y. Effect of oxygen tensions on the proliferation and angiogenesis of endometriosis heterograft in severe combined immunodeficiency mice. Fertil Steril 2014;101:568–576. [DOI] [PubMed] [Google Scholar]
- May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update 2011;17:637–653. [DOI] [PubMed] [Google Scholar]
- Maybin JA, Hirani N, Brown P, Jabbour HN, Critchley HO. The regulation of vascular endothelial growth factor by hypoxia and prostaglandin F(2)alpha during human endometrial repair. J Clin Endocrinol Metab 2011;96:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren J. Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update 2000;6:45–55. [DOI] [PubMed] [Google Scholar]
- Meola J, Hidalgo Gdos S, Silva JC, Silva LE, Paz CC, Ferriani RA. Caldesmon: new insights for diagnosing endometriosis. Biol Reprod 2013;88:122. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kimura T, Koyama S, Ogita K, Tsutsui T, Shimoya K, Taniguchi T, Koyama M, Kaneda Y, Murata Y. Mouse model of human infertility: transient and local inhibition of endometrial STAT-3 activation results in implantation failure. FEBS Lett 2006;580:2717–2722. [DOI] [PubMed] [Google Scholar]
- O'Donnell JL, Joyce MR, Shannon AM, Harmey J, Geraghty J, Bouchier-Hayes D. Oncological implications of hypoxia inducible factor-1alpha (HIF-1alpha) expression. Cancer Treat Rev 2006;32:407–416. [DOI] [PubMed] [Google Scholar]
- Oh HK, Choi YS, Yang YI, Kim JH, Leung PC, Choi JH. Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. Mol Hum Reprod 2013;19:160–168. [DOI] [PubMed] [Google Scholar]
- Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohi J, Simon C. The follicular and endocrine environment in women with endometriosis: local and systemic cytokine production. Fertil Steril 1998;70:425–431. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev 2000a;14:1983–1991. [PubMed] [Google Scholar]
- Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol 2000b;35:71–103. [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, Di Castro V, Decandia S, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 and endothelin-3 promote invasive behavior via hypoxia-inducible factor-1alpha in human melanoma cells. Cancer Res 2007;67:1725–1734. [DOI] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA 1997;94:3801–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci 2002;955:89–100; discussion 118, 396–406. [DOI] [PubMed] [Google Scholar]
- Tsudo T, Harada T, Iwabe T, Tanikawa M, Nagano Y, Ito M, Taniguchi F, Terakawa N. Altered gene expression and secretion of interleukin-6 in stromal cells derived from endometriotic tissues. Fertil Steril 2000;73:205–211. [DOI] [PubMed] [Google Scholar]
- Wang Y, van Boxel-Dezaire AH, Cheon H, Yang J, Stark GR. STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc Natl Acad Sci USA 2013;110:16975–16980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Chen KF, Lin SC, Lgu CW, Tsai SJ. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. Am J Pathol 2007;170:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Lu CW, Chang FM, Tsai SJ. Estrogen receptor expression affected by hypoxia inducible factor-1alpha in stromal cells from patients with endometriosis. Taiwan J Obstet Gynecol 2012;51:50–54. [DOI] [PubMed] [Google Scholar]
- Xiong J, Zeng P, Cheng X, Miao S, Wu L, Zhou S, Wu P, Ye D. Lipoxin A4 blocks embryo implantation by controlling estrogen receptor alpha activity. Reproduction 2013;145:411–420. [DOI] [PubMed] [Google Scholar]
- Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 2005;24:5552–5560. [DOI] [PubMed] [Google Scholar]
- Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med 2010;28:5–16. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994;264:95–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.