Abstract
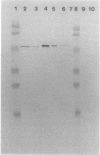
Cigarette smoking is a leading risk factor for atherosclerosis. Endothelial injury may be the initial event in this process. The carcinogenic metabolites of the polycyclic aromatic hydrocarbons found in cigarette smoke tars could cause this injury. We tested this model by examining the effect of 3-methylcholanthrene administration on aortic polycyclic aromatic hydrocarbon metabolism. Immunoblotting with a monoclonal antibody (mAb 1-7-1) specific for cytochromes CYPIA1 and CYPIA2 showed that aortic microsomes from treated, but not from control, animals contained CYPIA1; the CYPIA1 was primarily in the endothelium. Aortic microsomes from induced animals metabolized benzo[a]pyrene (BaP) to the 7R,8S,9,10-tetrahydrotetrol-, 7,8-dihydrodiol-, 1,6 quinone-, 3,6 quinone-, 6,12 quinone-, 3-hydroxy-, and 9-hydroxy-BaP. mAb 1-7-1 inhibited the formation of the tetrahydrotetrol, the dihydrodiol-BaP, and the 3-hydroxy-BaP but did not inhibit the quinones or the 9-hydroxy-BaP. Arachidonic acid did not affect metabolism. These data suggest that the aortas of induced animals metabolize the BaP in cigarette smoke to carcinogenic and toxic products and that this metabolism may initiate vessel injury and lead to the accelerated atherosclerosis seen in cigarette smokers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham N. G., Pinto A., Mullane K. M., Levere R. D., Spokas E. Presence of cytochrome P-450-dependent monooxygenase in intimal cells of the hog aorta. Hypertension. 1985 Nov-Dec;7(6 Pt 1):899–904. doi: 10.1161/01.hyp.7.6.899. [DOI] [PubMed] [Google Scholar]
- Adeagbo A. S., Breen C. A., Cutz E., Lees J. G., Olley P. M., Coceani F. Lamb ductus venosus: evidence of a cytochrome P-450 mechanism in its contractile tension. J Pharmacol Exp Ther. 1990 Feb;252(2):875–879. [PubMed] [Google Scholar]
- Alvares A. P., Schilling G., Garbut A., Kuntzman R. Studies on the hydroxylation of 3,4-benzpyrene by hepatic microsomes. Effect of albumin on the rate of hydroxylation of 3,4-benzpyrene. Biochem Pharmacol. 1970 Apr;19(4):1449–1455. doi: 10.1016/0006-2952(70)90060-2. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988 Nov 17;319(20):1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- Bond J. A., Yang H. Y., Majesky M. W., Benditt E. P., Juchau M. R. Metabolism of benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in chicken aortas: monooxygenation, bioactivation to mutagens, and covalent binding to DNA in vitro. Toxicol Appl Pharmacol. 1980 Feb;52(2):323–335. doi: 10.1016/0041-008x(80)90119-2. [DOI] [PubMed] [Google Scholar]
- Coceani F., Breen C. A., Lees J. G., Falck J. R., Olley P. M. Further evidence implicating a cytochrome P-450-mediated reaction in the contractile tension of the lamb ductus arteriosus. Circ Res. 1988 Mar;62(3):471–477. doi: 10.1161/01.res.62.3.471. [DOI] [PubMed] [Google Scholar]
- Debons A. F., Fani K., Jimenez F. A., Maayan M. L. Inhibition of cholesterol-induced atherosclerosis in rabbits by dimethyl sulfoxide. J Pharmacol Exp Ther. 1987 Nov;243(2):745–757. [PubMed] [Google Scholar]
- Dees J. H., Masters B. S., Muller-Eberhard U., Johnson E. F. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin and phenobarbital on the occurrence and distribution of four cytochrome P-450 isozymes in rabbit kidney, lung, and liver. Cancer Res. 1982 Apr;42(4):1423–1432. [PubMed] [Google Scholar]
- Eling T., Boyd J., Reed G., Mason R., Sivarajah K. Xenobiotic metabolism by prostaglandin endoperoxide synthetase. Drug Metab Rev. 1983;14(5):1023–1053. doi: 10.3109/03602538308991420. [DOI] [PubMed] [Google Scholar]
- Flowers-Geary L., Harvey R. G., Penning T. M. Cytotoxicity of polycyclic aromatic hydrocarbon o-quinones in rat and human hepatoma cells. Chem Res Toxicol. 1993 May-Jun;6(3):252–260. doi: 10.1021/tx00033a002. [DOI] [PubMed] [Google Scholar]
- Forkert P. G., Vessey M. L., Park S. S., Gelboin H. V., Cole S. P. Cytochromes P-450 in murine lung. An immunohistochemical study with monoclonal antibodies. Drug Metab Dispos. 1989 Sep-Oct;17(5):551–555. [PubMed] [Google Scholar]
- Giachelli C. M., Majesky M. W., Schwartz S. M. Developmentally regulated cytochrome P-450IA1 expression in cultured rat vascular smooth muscle cells. J Biol Chem. 1991 Feb 25;266(6):3981–3986. [PubMed] [Google Scholar]
- Jeffrey A. M., Fu P. P. Isotopic separations of tritium-substituted compounds from protium analogues by high-pressure liquid chromatography. Anal Biochem. 1977 Jan;77(1):298–302. doi: 10.1016/0003-2697(77)90318-9. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Jennette K. W., Blobstein S. H., Weinstein I. B., Beland F. A., Harvey R. G., Kasal H., Miura I., Nakanishi K. Letter: Benzo[a]pyrene-nucleic acid derivative found in vivo: structure of a benzo[a]pyrenetetrahydrodiol epoxide-guanosine adduct. J Am Chem Soc. 1976 Sep 1;98(18):5714–5715. doi: 10.1021/ja00434a060. [DOI] [PubMed] [Google Scholar]
- Juchau M. R., Bond J. A., Benditt E. P. Aryl 4-monooxygenase and cytochrome P-450 in the aorta: possible role in atherosclerosis. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3723–3725. doi: 10.1073/pnas.73.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin W., Wood A., Chang R., Ryan D., Thomas P., Yagi H., Thakker D., Vyas K., Boyd C., Chu S. Y. Oxidative metabolism of polycyclic aromatic hydrocarbons to ultimate carcinogens. Drug Metab Rev. 1982;13(4):555–580. doi: 10.3109/03602538209011087. [DOI] [PubMed] [Google Scholar]
- Liu G., Gelboin H. V., Myers M. J. Role of cytochrome P450 IA2 in acetanilide 4-hydroxylation as determined with cDNA expression and monoclonal antibodies. Arch Biochem Biophys. 1991 Feb 1;284(2):400–406. doi: 10.1016/0003-9861(91)90315-a. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Benditt E. P., Juchau M. R. Focal smooth muscle proliferation in the aortic intima produced by an initiation-promotion sequence. Proc Natl Acad Sci U S A. 1985 May;82(10):3450–3454. doi: 10.1073/pnas.82.10.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Yang H. Y., Benditt E. P., Juchau M. R. Carcinogenesis and atherogenesis: differences in monooxygenase inducibility and bioactivation of benzo[a]pyrene in aortic and hepatic tissues of atherosclerosis-susceptible versus resistant pigeons. Carcinogenesis. 1983;4(6):647–652. doi: 10.1093/carcin/4.6.647. [DOI] [PubMed] [Google Scholar]
- Meehan T., Straub K., Calvin M. Benzo[alpha]pyrene diol epoxide covalently binds to deoxyguanosine and deoxyadenosine in DNA. Nature. 1977 Oct 20;269(5630):725–727. doi: 10.1038/269725a0. [DOI] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Murphy S. E., Hecht S. S. Dual-label high-performance liquid chromatographic assay for femtomole levels of benzo[a]pyrene metabolites. Anal Biochem. 1985 May 1;146(2):442–447. doi: 10.1016/0003-2697(85)90567-6. [DOI] [PubMed] [Google Scholar]
- Nam S. C., Lee W. M., Jarmolych J., Lee K. T., Thomas W. A. Rapid production of advanced atherosclerosis in swine by a combination of endothelial injury and cholesterol feeding. Exp Mol Pathol. 1973 Jun;18(3):369–379. doi: 10.1016/0014-4800(73)90032-4. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Goujon F. M., Gielen J. E. Aryl hydrocarbon hydroxylase induction by polycyclic hydrocarbons: simple autosomal dominant trait in the mouse. Nat New Biol. 1972 Mar 29;236(65):107–110. doi: 10.1038/newbio236107a0. [DOI] [PubMed] [Google Scholar]
- Paigen B., Holmes P. A., Morrow A., Mitchell D. Effect of 3-methylcholanthrene on atherosclerosis in two congenic strains of mice with different susceptibilities to methylcholanthrene-induced tumors. Cancer Res. 1986 Jul;46(7):3321–3324. [PubMed] [Google Scholar]
- Park S. S., Cha S. J., Miller H., Persson A. V., Coon M. J., Gelboin H. V. Monoclonal antibodies to rabbit liver cytochrome P-450LM2 and cytochrome P-450LM4. Mol Pharmacol. 1982 Jan;21(1):248–258. [PubMed] [Google Scholar]
- Park S. S., Fujino T., West D., Guengerich F. P., Gelboin H. V. Monoclonal antibodies that inhibit enzyme activity of 3-methylcholanthrene-induced cytochrome P-450. Cancer Res. 1982 May;42(5):1798–1808. [PubMed] [Google Scholar]
- Pearson T. A., Wang B. A., Solez K., Heptinstall R. H. Clonal characteristics of fibrous plaques and fatty streaks from human aortas. Am J Pathol. 1975 Nov;81(2):379–387. [PMC free article] [PubMed] [Google Scholar]
- Penn A., Snyder C. Arteriosclerotic plaque development is 'promoted' by polynuclear aromatic hydrocarbons. Carcinogenesis. 1988 Dec;9(12):2185–2189. doi: 10.1093/carcin/9.12.2185. [DOI] [PubMed] [Google Scholar]
- Pinto A., Abraham N. G., Mullane K. M. Arachidonic acid-induced endothelial-dependent relaxations of canine coronary arteries: contribution of a cytochrome P-450-dependent pathway. J Pharmacol Exp Ther. 1987 Mar;240(3):856–863. [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Selkirk J. K., Croy R. G., Roller P. P., Gelboin H. V. High-pressure liquid chromatographic analysis of benzo(alpha)pyrene metabolism and covalent binding and the mechanism of action of 7,8-benzoflavone and 1,2-epoxy-3,3,3-trichloropropane. Cancer Res. 1974 Dec;34(12):3474–3480. [PubMed] [Google Scholar]
- Serabjit-Singh C. J., Nishio S. J., Philpot R. M., Plopper C. G. The distribution of cytochrome P-450 monooxygenase in cells of the rabbit lung: an ultrastructural immunocytochemical characterization. Mol Pharmacol. 1988 Mar;33(3):279–289. [PubMed] [Google Scholar]
- Singer H. A., Saye J. A., Peach M. J. Effects of cytochrome P-450 inhibitors on endothelium-dependent relaxation in rabbit aorta. Blood Vessels. 1984;21(5):223–230. doi: 10.1159/000158515. [DOI] [PubMed] [Google Scholar]
- Soucek P., Gut I. Cytochromes P-450 in rats: structures, functions, properties and relevant human forms. Xenobiotica. 1992 Jan;22(1):83–103. doi: 10.3109/00498259209053106. [DOI] [PubMed] [Google Scholar]
- Srivastava S. P., Chen N. Q., Liu Y. X., Holtzman J. L. Purification and characterization of a new isozyme of thiol:protein-disulfide oxidoreductase from rat hepatic microsomes. Relationship of this isozyme to cytosolic phosphatidylinositol-specific phospholipase C form 1A. J Biol Chem. 1991 Oct 25;266(30):20337–20344. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Straub K. M., Meehan T., Burlingame A. L., Calvin M. Identification of the major adducts formed by reaction of benzo(a)pyrene diol epoxide with DNA in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5285–5289. doi: 10.1073/pnas.74.12.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Reik L. M., Ryan D. E., Levin W. Induction of two immunochemically related rat liver cytochrome P-450 isozymes, cytochromes P-450c and P-450d, by structurally diverse xenobiotics. J Biol Chem. 1983 Apr 10;258(7):4590–4598. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W. C., Green A., Stampfer M. J., Speizer F. E., Colditz G. A., Rosner B., Monson R. R., Stason W., Hennekens C. H. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987 Nov 19;317(21):1303–1309. doi: 10.1056/NEJM198711193172102. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Miller H., Gelboin H. V., Friedman F. K. Variation in inducibility of cytochrome P-450c and aryl hydrocarbon hydroxylase in rat liver, lung, kidney, pancreas and nasopharynx. Pharmacology. 1990;41(5):256–262. doi: 10.1159/000138729. [DOI] [PubMed] [Google Scholar]