Abstract
A number of the subunits within the family of K2P background K+ channels are sensitive to changes in extracellular pH in the physiological range, making them likely candidates to mediate various pH-dependent processes. Based on expression patterns within several brainstem neuronal cell groups that are believed to function in CO2/H+ regulation of breathing, three TASK subunits – TASK-1, TASK-2 and TASK-3 – were specifically hypothesized to contribute to this central respiratory chemoreflex. For the acid-sensitive TASK-1 and TASK-3 channels, despite widespread expression at multiple levels within the brainstem respiratory control system (including presumptive chemoreceptor populations), experiments in knockout mice provided no evidence for their involvement in CO2 regulation of breathing. By contrast, the alkaline-activated TASK-2 channel has a more restricted brainstem distribution and was localized to the Phox2b-expressing chemoreceptor neurons of the retrotrapezoid nucleus (RTN). Remarkably, in a Phox2b27Ala/+ mouse genetic model of congenital central hypoventilation syndrome (CCHS) that is characterized by reduced central respiratory chemosensitivity, selective ablation of Phox2b-expressing RTN neurons was accompanied by a corresponding loss of TASK-2 expression. Furthermore, genetic deletion of TASK-2 blunted RTN neuronal pH sensitivity in vitro, reduced alkaline-induced respiratory network inhibition in situ and diminished the ventilatory response to CO2/H+ in vivo. Notably, a subpopulation of RTN neurons from TASK-2−/− mice retained their pH sensitivity, at least in part due to a residual pH-sensitive background K+ current, suggesting that other mechanisms (and perhaps other K2P channels) for RTN neuronal pH sensitivity are yet to be identified.
INTRODUCTION
It has long been known that potassium selective channels contribute to the “leak” currents that establish a negative resting membrane potential in cells. With the discovery of the two-pore-domain family of background K+ channels (so-called K2P channels, of the KCNK gene family) [18,20,22,37], a molecular basis for this leak K+ current was identified. Aside from the unique two-pore-domain structure, dimeric assembly and relative voltage-independence of the K2P background K+ channel group, a remarkable feature that was quickly noted was their exquisite sensitivity to modulation by various endogenous neurochemicals, clinically-active drugs and physicochemical factors [21,35,58]. In excitable cells, like neurons, this modulation allows K2P channels to control cell membrane potential and resistance, and thereby regulate electrical excitability. Among the K2P channels, a number are sensitive to changes in extracellular proton concentrations in the physiological range [36]. Here, we specifically examine pH-sensitive K2P channels that have been implicated in regulation of breathing by CO2/H+, a major homeostatic reflex. We direct readers to a number of recent comprehensive reviews that describe the general properties of K2P channels, including the pH-sensitive K2P channels [36], and their contributions to physiological and clinical processes [2,15,16,24,27,30,40,43]. We do not address the role of K2P channels in peripheral chemoreception [6], a topic that is addressed elsewhere in this volume.
TASK channels generate pH-sensitive neuronal background K+ currents
The two-pore-domain K+ channel family includes 15 separate K2P subunits that are divided among 6 different subgroups based on sequence similarity and some shared functionality [20]. As depicted in Fig. 1A, K2P channel subunits have a predicted topology that includes 4 transmembrane spanning domains, two re-entrant pore helices with variable selectivity filter residues, intracellular N- and C-termini and an extended initial extracellular loop that was proposed to form a cap-like channel subunit interaction domain [38]. The expectation that these subunits would form channels as dimers was supported by experiments with concatenated subunits [4,39] and ultimately confirmed by the crystal structures for two of these channels, TWIK-1 (K2P1.1) and TRAAK (K2P4.1) [5,45],; these structures revealed that the channel conduction pathway was indeed generated by a pseudosymmetric arrangement of the 4 pore domains, and they allowed visualization of the previously proposed cap-like dimer interaction domain (Fig. 1B). It is likely that K2P channels are not gated at the intracellular bundle crossing, as with some other K channels [42]. Instead, it appears that ions have access to the intracellular pore vestibule in both open and closed states, and that gating occurs at the selectivity filter via a C-type mechanism [1,42,51].
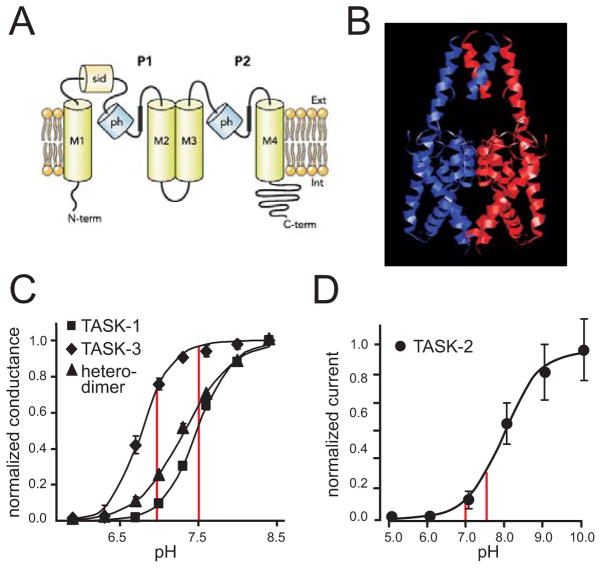
Figure 1. TASK channel K2P subunits generate pH-sensitive K+ currents.
A. Schematic of two-pore-domain (P1–P2) channel subunit, comprising 4 transmembrane domains (M1–M4) and two reentrant pore helical regions (ph), and intracellular N- and C-terminal domains, and a subunit interaction domain (sid) in the first extracellular loop (reproduced from [36]). B. Model of TASK-3 channel based on crystal structures of TWIK-1 (3UKM; [45]) and TRAAK (3UM7; [5]), with the two subunits of the dimeric channel depicted in blue and red. C. The pH sensitivity curves for recombinant homomeric TASK-1 and TASK-3, and for a concatenated heteromeric TASK-1:TASK-3 construct, expressed in HEK293 cells. Note that significant changes in conductance occur over the range from pH 7.0 to pH 7.5 (red vertical lines). (Adapted from [4]). D. The pH sensitivity curve for recombinant TASK-2 channels is displaced toward more alkaline levels, with substantial changes in current again observed through the physiological pH range. (Adapted from [55]).
Of the 15 members of this K2P subunit family, three have not yet been recorded in any heterologous or native system, and their functional properties are not known [20]. Among the remaining 12 functionally characterized K2P subunits, at least 8 (representing 4 of the subgroups) produce currents that show some significant variation with changes in extracellular pH [36]. The molecular mechanisms that underlie pH sensitivity in these different channels are distinct, accounting for the fact that they respond over widely different pH ranges, even if all encompass in part the physiological range [36]. For reasons discussed below, three TASK subunits have been considered likely mediators of an important pH-dependent homeostatic reflex – the ventilatory response to changes in CO2, the central respiratory chemoreflex.
Despite their similar moniker, these TASK subunits are members of different subgroups of the K2P channel family, with distinct molecular mechanisms of proton modulation and different pH-sensitivities. TASK-1 (K2P3.1) and TASK-3 (K2P9.1) are the functional members of one subgroup of acid-sensitive channels [36]; they can form homodimeric and heterodimeric channels which carry background K+ currents in native and heterologous systems that are regulated by protons over a relatively tight physiological pH range (see Fig. 1C; over 1–2 pH units, with pKa values of approximately 7.4, 6.7 and 7.2 for TASK-1, TASK-3 and TASK-1:TASK-3, respectively). The pH sensitivity of TASK-1 and TASK-3 is due to a common titratable histidine residue (His-98) immediately following the canonical GYG selectivity sequence in the first pore domain [46,53], with different pKa values attributed to effects of their individual M1P1 loops [10]. By contrast, TASK-2 (K2P5.1) is a member of the subgroup of alkaline-activated channels (that also includes TALK-1, K2P16.1 and TALK-2, K2P17.1) [36]. TASK-2 has a broader pH sensitivity, with activation occurring over a range from pH 7 to pH 10 and a pKa of ~8 (Fig. 1D); in the case of TASK-2, there is no corresponding pore histidine residue, and pH sensitivity has instead been attributed to an arginine residue in the final extracellular loop (Arg-224 in P2M4 of mouse TASK-2) [50]. For all three of these TASK channels, the pH sensitivity profile allows changes in pH over a physiological range to have significant effects on channel conductance (Fig. 1C, 1D), making them attractive candidates to mediate pH-dependent effects on neuronal excitability [36].
TASK-1 and TASK-3 are expressed in many respiratory-related brainstem neurons but are not required for central respiratory chemosensitivity
The recognition of TASK-1 and TASK-3 as neuronal background K+ channels that are potently inhibited by protons in the physiological range, suggested that modulation of these channels would provide a dynamic regulation of neuronal activity that could be exploited for pH-dependent behavioral reflexes. Indeed, for the case of the central respiratory chemoreflex, unidentified pH-dependent background K+ channels had already been implicated in different populations of presumptive chemoreceptor neurons [12], reviewed in [52]. Moreover, the prevailing theory for central respiratory chemoreception held that CO2/H+ chemosensation in the respiratory system was a distributed phenomenon, with pH-dependent activity of numerous constituent neurons contributing to the overall reflex response [17,49]. Pleasingly consistent with this notion, both TASK-1 and TASK-3 were found in a broad expression pattern throughout the brainstem [29,59,63]. Thus, a role for these TASK channels in mediating central respiratory chemosensitivity was hypothesized [3,66].
As will be reviewed below, initial studies that utilized molecular neuroanatomy and/or cellular electrophysiology to characterize TASK-1 and TASK-3 channels in various respiratory-related cell groups provided further evidence in favor of this hypothesis. However, subsequent single and double gene deletion experiments, combined with ventilatory measurements in conscious unrestrained mice, failed to support an obligate role for these TASK channels in the central respiratory chemoreflex.
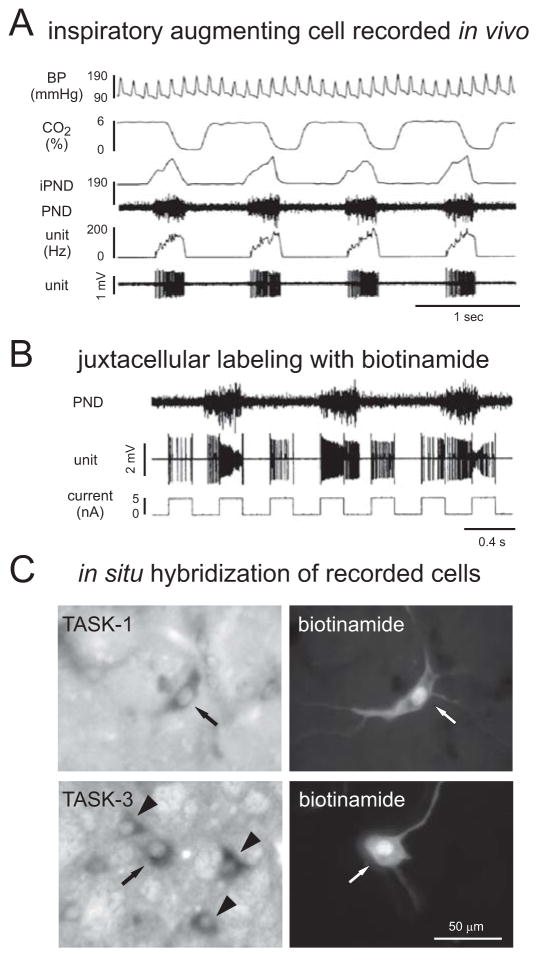
Expression of TASK-1 and TASK-3 was demonstrated in a variety of identified brainstem respiratory neurons. By in situ hybridization, high levels of these TASK transcripts were observed in motoneurons of the brainstem and spinal cord, the ultimate output neurons for the respiratory motor system [29,59,63]. In addition, a corresponding TASK-like background K+ current was identified in those cells by whole cell recording in slice preparations [4,57]; key characteristics of that motoneuronal TASK-like current were activation by inhalational anesthetics, weak outward rectification and inhibition by extracellular acidification [4,56,57]. Evidence for TASK-1 and TASK-3 expression was also obtained in respiratory rhythm-generating pre-Bötzinger neurons and cells of the rostral ventral respiratory group (rVRG) by combining in situ hybridization with immunohistochemistry for markers of those respiratory neuron populations (e.g., neurokinin 1 receptor, somatostatin) [66] and by recording pH- and anesthetic-sensitive TASK-like currents from identified pre-Bötzinger neurons in rat brain slices [31]. Direct functional confirmation that the TASK expressing cells in the rVRG were indeed bulbospinal respiratory neurons was obtained by in vivo juxtacellular labeling of extracellularly recorded neurons with an inspiratory activity pattern (Fig. 2) that were antidromically activated by electrical stimulation of the spinal cord [66]. These data provided compelling evidence that the central respiratory pattern generator (respiratory CPG) including the rhythm-generating core (pre-Bötzinger neurons), inspiratory pre-motor neurons and respiratory motor neurons, all express pH-sensitive TASK-1 and TASK-3 channels [4,31,57,66].
Figure 2. TASK-1 and TASK-3 are expressed in respiratory cells of the rat.
A. In vivo extracellular recording from an inspiratory augmenting cell located in the ventral respiratory group (VRG) of an anesthetized rat. The firing rate of the neuron (unit) increased progressively during inspiration, the period of phrenic nerve discharge (PND, and integrated iPND) when end-tidal CO2 is decreasing. The corresponding blood pressure (BP) measurements are also provided. This was a bulbospinal neuron, as determined by antidromic activation of the cell from the spinal cord (data not shown). B. The cell was juxtacellularly labeled by using current injection from the biotinamide-containing recording electrode to entrain the firing of the neuron. C. Examples of two functionally-characterized, juxtacellularly-labeled bulbospinal inspiratory augmenting neurons of the VRG that express TASK-1 or TASK-3 are indicated (see arrows; arrowheads point to other nearby TASK expressing neurons. (Adapted from [66]).
TASK-1 and TASK-3 expression was also demonstrated in putative chemoreceptor neuron populations upstream of the respiratory CPG [17,60], most notably in noradrenergic neurons of the locus coeruleus and serotonergic neurons of the raphe nuclei (Fig. 3) [56,67]. In both of these nuclei, strong expression of TASK-3 and more modest expression of TASK-1 was detected by in situ hybridization [48,56,67]. In addition, since there is a substantial non-serotonergic population of neurons within the raphe nuclei, immunohistochemistry for the transmitter-synthesizing enzyme, tryptophan hydroxylase, was combined with in situ hybridization to verify expression of TASK-1 and TASK-3 in the serotonergic neurons (Fig. 3A) [48,67]. For both cell populations, whole cell neuronal recordings in brain slices from neonatal animals uncovered a TASK-like current (i.e., a pH- and anesthetic-sensitive, weakly rectifying K+ current) [48,56,67]. The TASK-like current was observed in dorsal and caudal raphe cell groups (Fig. 3Ba, 3Bb), and a serotonergic phenotype was confirmed both by post hoc immunostaining for tryptophan hydroxylase in recorded neurons and by a characteristic 5-HT-mediated inhibition of cell firing observed in those anesthetic- and pH-sensitive cells (Fig. 3Bc) [48,67]. TASK-1 and TASK-3 were also proposed to underlie a glucose- and pH-sensitive background K+ current in hypothalamic orexinergic neurons [7,68], raising the possibility that those brainstem-projecting cells might also provide a TASK-dependent respiratory chemosensory role [7,68]. However, later work using different lines of knockout mice failed to support a role for TASK-1 and TASK-3 channels in either the glucose or pH sensitivity of those neurons [23,26]. So, aside from these negative results from orexinergic neurons, there is excellent evidence that TASK-1 and TASK-3 channels account for pH sensitivity in two presumptive respiratory chemosensory neuron populations, the locus coeruleus and the raphe [48,56,67].
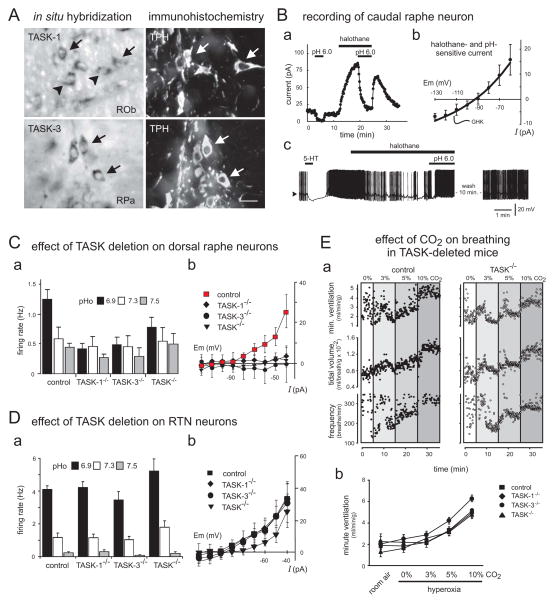
Figure 3. TASK-1 and TASK-3 are required for pH-sensitivity in neonatal raphe neurons; they are dispensable for both the pH sensitivity of RTN chemoreceptor neurons and the hypercapnic ventilatory reflex.
A. In situ hybridization for TASK subunits combined with tryptophan hydroxylase (TPH) immunohistochemistry reveals expression of TASK-1 and TASK-3 in neurons of the raphe obscurus (ROb) and the raphe pallidus (RPa). Ba. Voltage clamp recording from caudal raphe neuron showing effect of bath acidification and inhalational anesthetics on holding current; bath acidification caused a decrease in outward current that was increased in magnitude in the presence of halothane. Bb. The current-voltage relationship of the anesthetic- and pH-sensitive current in caudal raphe neurons was well-fitted by the Goldman-Hodgkin-Katz (GHK) equation and reminiscent of recombinant TASK channel currents. Bc. Under current clamp, caudal raphe neurons hyperpolarized in response to 5-HT, as expected for serotonergic cells. After wash, an exposure to halothane caused membrane hyperpolarization that was reversed by bath acidification. Ca. The effect of pH on firing rate in raphe neurons seen in wild type animals was eliminated in mice deleted for TASK-1, TASK-3 or both TASK-1 and TASK-3 (TASK−/−). Cb. Likewise, the pH- and anesthetic-sensitive TASK-like background K+ current was also eliminated in raphe neurons from all of the genetic TASK deletion models. D. In RTN neurons, there was no effect of TASK-1 or TASK-3 subunit deletion on pH-dependent firing (Da) or pH-sensitive background K+ current (Db). Ea. Example plethysmography records showing effect of increased levels of inspired CO2 (3%, 5% & 10%, balance O2) on respiratory frequency, tidal volume and minute ventilation in conscious unrestrained control and TASK−/− mice. Eb. Averaged data illustrate that TASK-1 and TASK-3 deletion had no effect on the ventilatory response to CO2. (A & B, adapted from [67]; C–E, adapted from [48])
A pH-sensitive background K+ current was also identified in a separate group of putative respiratory chemosensory neurons – the glutamatergic and Phox2b-expressing neurons of the retrotrapezoid nucleus (RTN) [25,47]. In this case, however, the initial suggestion that the pH-sensitive current might be mediated by TASK-1 or TASK-3 was not supported by other channel properties. Specifically, unlike currents mediated by these TASK channels, the pH-sensitive background K+ current in RTN neurons was distinct from the anesthetic-sensitive current [33,48]. Also, anesthetics caused inhibition rather than activation of a distinct background K+ current in RTN cells, properties opposite those of TASK channels and more reminiscent of a different K2P channel, namely THIK-1 (K2P13.1) [33].
So, these results indicated that TASK-1 and TASK-3 channels are expressed in many respiratory neuron populations, where they could account for native pH-sensitive background K+ currents that confer dynamic regulation of neuronal excitability by extracellular protons. This includes elements of the respiratory CPG and its output neurons, as well as certain populations of putative respiratory chemoreceptor neurons (noradrenergic locus coeruleus and serotonergic raphe neurons). On the other hand, TASK-1 and TASK-3 do not appear to account for those pH-sensitive currents in RTN neurons, another prominent putative respiratory chemosensory cell group. Together, these data provided a rationale for exploring a role for these TASK channels in the central respiratory chemoreflex. In addition, they also implied that genetic deletion of TASK-1 and TASK-3 channels would disrupt cellular pH sensitivity in some, but not all, of the putative respiratory chemosensory cell groups.
We used reverse genetics in mice, knocking out TASK-1 and TASK-3 subunits alone and in combination, to examine effects on TASK-like currents in different populations of respiratory-related neurons [34,48]. In respiratory-related hypoglossal motoneurons, anesthetic- and pH-sensitive TASK-like background K+ currents were reduced in amplitude following deletion of either TASK-1 or TASK-3, and eliminated in TASK-1−/−:TASK-3−/− double knockout mice [34]. A quantitative assessment of currents from the different knockout lines, specifically the fact that deletion of either subunit individually accounted for more than half of the TASK-like current, confirmed earlier suggestions that motoneurons express a combination of homomeric and heteromeric TASK channels [4,34].
In neonatal serotonergic raphe neurons studied in vitro, the TASK-like pH-sensitive background K+ current was virtually eliminated in all three mouse models – TASK-1−/−, TASK-3−/− and TASK-1−/−:TASK-3−/− double knockout mice – consistent with a major, if not exclusive, expression of heteromeric TASK channels (Fig. 3Cb) [48]. In addition, the effect of changing bath pH on firing rate was abolished in serotonergic raphe neurons from TASK knockout mice (Fig. 3Ca) [48]. These effects were seen in dorsal raphe neurons and in caudal raphe neurons (i.e., in raphe obscurus and pallidus) [48]. By contrast, pH-dependent firing and the associated pH-sensitive background K+ current were retained in chemosensitive RTN neurons from all three lines of TASK-1 and TASK-3 single and double knockout mice (Fig. 3Da, 3Db), as expected given the distinctive pharmacology of the K+ current in those cells [33,48]. In sum, pH sensitivity was eliminated from brainstem respiratory motoneurons and at least one candidate chemoreceptor neuron population (serotonergic dorsal and caudal raphe cells) in TASK-1 and TASK-3 knockout mice lines, but remained intact in the RTN group of respiratory chemoreceptor neurons.
This situation afforded an opportunity to test the role of TASK-1 and TASK-3 in the central respiratory chemoreflex, and also to examine how the chemoreflex is affected by loss of TASK-dependent pH sensitivity in serotonergic raphe neurons. Using whole body plethysmography, the ventilatory effect of raised CO2 (hypercapnia) was examined in conscious unrestrained mice from all three TASK knockout lines (TASK-1−/−, TASK-3−/− and TASK-1−/−:TASK-3−/−) [48]; experiments were performed under hyperoxic conditions, to minimize input from TASK-expressing peripheral chemoreceptors (Fig. 3E). The increases in ventilation caused by hyperoxic hypercapnia (3% CO2, 5% CO2, and 10% CO2; balance O2) were essentially identical in wild type and all three lines of TASK knockout mice (Fig. 3Eb). These data demonstrated that TASK-1 and TASK-3 are not required for the central respiratory chemoreflex; they also dissociated the cellular pH sensitivity of serotonergic raphe neurons in vitro from central respiratory chemosensitivity in vivo [48].
These data were surprising on a number of levels. First, as mentioned above, TASK-1 and TASK-3 channels are inherently pH sensitive. Therefore, it was reasonable to expect that they would confer a pH-dependent excitability on TASK-1/3-expressing respiratory-related neurons, and that this would be reflected in some measurable decrement in effects of CO2/H+ on breathing in the knockout mice. It is possible that a compensatory pH-dependent channel was expressed in some of the respiratory-related neurons that were not directly examined in the knockout mice. It is also worth noting that evidence for expression in a number respiratory-related neuron groups has relied on measures of TASK channel transcripts [66], with functional characterization of TASK-like currents restricted to a few select populations assessed in vitro. However, it is clear that TASK-1 and TASK-3 together account for pH sensitivity in both hypoglossal motoneurons [4,34,57] and serotonergic raphe neurons [48,67], with no compensatory pH-sensitive current evident in those neurons from the knockout mice [34,48]. We suggest that this implies either that the TASK-1/3-dependent pH sensitivity observed in these neurons using in vitro preparations from neonatal mice is not present in these neurons of adult animals in vivo, or that such pH sensitivity is not required for the central respiratory chemoreflex. Currently, there are no data that allow us to discriminate between these two possibilities.
A number of caveats have been raised with respect to this conclusion that merit further discussion [60]. Stated concerns regarding the pH range over which TASK-1 and TASK-3 channels are modulated fail to consider the extensive characterization of the pH sensitivity of the homomeric and heteromeric channels, with titration curves that indicate sharp changes in channel current through the physiological pH range (e.g. see Fig. 1, reviewed in [36]). Similar titration curves are obtained in vitro for neonatal motoneurons and serotonergic raphe neurons from wild type mice [4,57,67]. As noted above, TASK channel-dependent pH sensitivity in raphe serotonergic neurons has only been observed from neonatal in vitro preparations [48,67]. Despite the continued presence of TASK-1 and TASK-3 transcripts in serotonergic neurons of adult animals [29,59,63,67], it remains to be clearly established whether or not CO2/H+ sensitivity is retained in mature serotonergic neurons in vivo [61,62], but see [13,47]. It is possible that only a select subgroup of adult serotonergic neurons are CO2/H+-sensitive in vivo, as noted using an in situ preparation [28], and that those particular cells could employ a distinct mechanism for pH sensitivity. In this respect, a preliminary report has suggested that raphe neurons express a pH-sensitive, Ca2+-activated, mixed cationic channel (see Massey et al. in [60]). However, those data evidently are derived from cultured neonatal neurons and no molecular correlate has yet been identified for this unusual channel current [60]. Thus, until such an alternative ionic mechanism for H+-sensitivity in adult serotonergic raphe neurons is adequately defined, the most parsimonious conclusion from currently available data is that TASK-1 and TASK-3 channels are essential for pH regulation of serotonergic raphe neurons. On the other hand, those TASK channels do not contribute to pH sensitivity of RTN neurons and they are dispensable for central respiratory chemoreception in vivo.
TASK-2 has a restricted brainstem expression and contributes to pH sensitivity of RTN chemoreceptor neurons and the respiratory chemoreflex
In contrast to the relatively broad distribution of TASK-1 and TASK-3 channel expression throughout the brain, including at multiple levels of the respiratory system, expression of the alkaline-activated TASK-2 channel is restricted to only a few sites in the mouse brainstem (Fig. 4A–4C) [19]. This was first revealed by taking advantage of a β-galactosidase construct that was expressed from the TASK-2 locus of a gene-trap mouse (TASK-2LacZ/+ or TASK-2LacZ/LacZ). Thus, using X-gal staining as a reporter, TASK-2 expression was identified in the lateral superior olive and the parvocellular reticular nucleus pars alpha in the pons, and in the dorsal raphe nucleus and in the intermediate lateral lemniscus in the midbrain [19]. Of relevance here, TASK-2 expression in the hindbrain was localized specifically to the area of the RTN [19], where it co-localized with Phox2b in neurons along the ventral medullary surface (Fig. 4B, 4C) [64]. Remarkably, the LacZ-expressing cells in the RTN region were completely eliminated in a Phox2b27Ala/+ mouse model of CCHS (Fig. 4A) [19]. This is further consistent with the idea that TASK-2 is expressed in the presumptive RTN chemoreceptor population, since those glutamatergic and Phox2b+ chemoreceptor cells are also ablated in the same Phox2b27Ala/+ mouse model [14]. Thus, these data provided strong evidence that the pH-sensitive TASK-2 channel is expressed in the RTN group of brainstem respiratory chemoreceptor neurons.
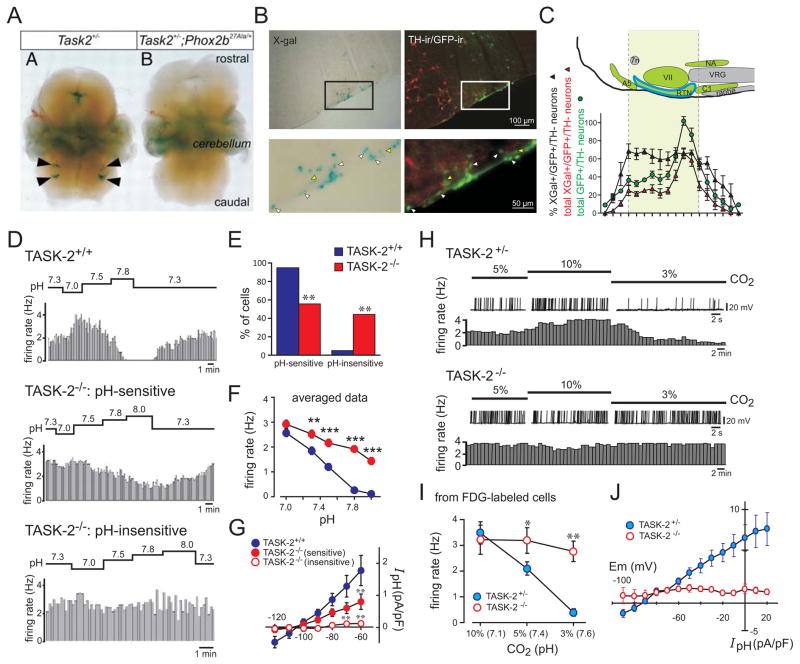
Figure 4. TASK-2 is expressed in Phox2b-expressing RTN neurons and contributes to neuronal pH sensitivity.
A. Whole mount X-gal staining of embryos from gene trap TASK-2+/− mice, on a Phox2b+/+ or a Phox2b27Ala/+ background; note that the X-gal staining in the RTN region is eliminated in the Phox2b27Ala/+ mouse model of CCHS. B. Histochemical staining for X-gal (left) and GFP and TH (right) in the RTN region from TASK-2−/− mice crossed with a Phox2b-eGFP BAC transgenic line of mice; double-positive neurons (GFP+/X-gal+; white arrowheads) and GFP+ neurons in the RTN that were not stained with X-gal (yellow arrowheads) were observed. C. Brainstem schematic (upper) and cell counts (lower) of the Phox2b-expressing chemoreceptor neuron population (GFP+/TH−) and the number/percentage of those RTN neurons that were X-gal-positive (~63% over the rostrocaudal extent of the nucleus). 7n, Facial nerve; VII, facial nucleus; VRG, ventral respiratory group; NA, nucleus ambiguus. D. Firing rate histograms from cell-attached patch recordings of GFP-targeted RTN neurons in slices obtained from TASK-2+/+ and TASK-2−/− mice during bath acidification and alkalization. E. There was a significantly greater proportion of pH-insensitive RTN neurons from TASK-2−/− mice. F. Averaged firing rate-pH curves of GFP-labeled RTN neurons from TASK-2+/+ and TASK-2−/− mice (i.e., both pH-sensitive and pH-insensitive); the aggregate pH sensitivity was significantly blunted. G. Averaged I–V relationships of pH-sensitive current density (pH 8.0 minus pH 7.0) for RTN neurons from TASK-2+/+ and TASK-2−/− mice. The current was reduced in pH-sensitive RTN neurons from TASK-2−/− mice and eliminated in pH-insensitive TASK-2−/− RTN neurons. H. Exemplar whole-cell current-clamp recordings of membrane potential (upper) and associated firing rate (lower) from RTN neurons from heterozygous TASK-2+/− and homozygous TASK-2−/− mice stained with FDG and exposed to bath solutions equilibrated with different levels of CO2 (3, 5, and 10%, corresponding to pH 7.6, pH 7.4, and pH 7.1). All FDG-labeled TASK-2−/− RTN neurons were pH-insensitive. I. Averaged firing rate-pH curves of FDG-labeled RTN neurons from TASK-2+/− and TASK-2−/− mice. J. Averaged I–V relationships of pH-sensitive current density (10% CO2 minus 3% CO2) for FDG-labeled RTN neurons from TASK-2+/− and TASK-2−/− mice (recorded in tetraethylammonium, 10 mm; 4-AP, 3 mm; and barium, 10 μm to block voltage-dependent K+ currents); the pH-sensitive current was abolished in RTN neurons from TASK-2−/− mice. (A, adapted from [19]; other panels adapted from [64]).
To examine the possibility that TASK-2 contributes to pH sensitivity of RTN neurons, we recorded from RTN neurons in slices from wild type and TASK-2 knockout mice [64]. In one set of experiments (Fig. 4D–4G), TASK-2 knockout mice were crossed with a BAC transgenic line in which the Phox2b promoter directs expression of enhanced green fluorescent protein (eGFP). From TASK-2+/+ wild type mice, essentially all of the GFP-expressing RTN neurons (n=58/61, ~95%) were pH sensitive (i.e., they showed a >30% decrease in firing rate from pH 7.0 to pH 7.8 under cell-attached recording conditions). However, only ~56% of RTN neurons from the TASK-2−/− mice were pH sensitive (n=49/88), with ~44% characterized as pH-insensitive (n=39/88) (Fig. 4D, 4E). Among the group of pH-sensitive RTN neurons from TASK-2−/− mice, the firing rate response to pH changes was less pronounced and many cells continued to discharge even at alkalized pH levels (pH 7.8) where nearly all wild type RTN neurons were silenced (Fig. 4D); in aggregate, the averaged firing response to changes in pH across all RTN neurons from TASK-2−/− mice was strongly blunted (Fig. 4F). Consistent with these differences in effects of bath pH on firing rate, we also found a prominent alkaline-activated background K+ current in wild type RTN neurons that was reduced in amplitude in the pH-sensitive RTN neurons from TASK-2−/− mice and undetectable in the group of pH-insensitive TASK-2−/− neurons (Fig. 4G).
In corresponding histochemical experiments examining the overlap in staining for X-gal (i.e., TASK-2) and GFP (i.e., Phox2b), we found that all LacZ-expressing neurons were immunopositive for GFP [64]. In addition, as shown in Fig. 4C, the fraction of X-gal- stained RTN neurons (~63%) was roughly comparable to the fraction of RTN neurons that depended on TASK-2 for their cellular pH sensitivity (~44%). By using a highly-sensitive single cell RT-PCR approach, however, TASK-2 transcripts were detected in 85% of GFP-expressing RTN neurons. These data suggested that TASK-2 is expressed in a majority of RTN neurons, but that levels of expression may be higher in a subset of those cells (i.e., in the X-gal-stained group).
To test if the X-gal-positive subset of RTN neurons represent the same group of cells that require TASK-2 expression for their chemosensitivity, an approach was developed to identify those neurons for recording based on LacZ activity (Fig. 4H–4J) [64]. In this case, brainstem slices were incubated with fluorescein di-β-D-galactopyranoside (FDG), a fluorogenic LacZ substrate, and whole cell recordings were obtained for the FDG-labeled subpopulation of RTN neurons from heterozygous control (TASK-2LacZ/+) and homozygous TASK-2 knockout mice (TASK-2LacZ/LacZ). When selected based on FDG fluorescence, all RTN neurons from control mice responded like the wild type cells described above (i.e., their discharge rate varied with acidification and alkalization, and they displayed an alkaline-activated background K+ current). By contrast, none of the FDG-labeled RTN neurons from TASK-2 knockout mice showed pH-dependent effects on firing or pH-sensitive membrane current (Fig. 4H–4J). The FDG-labeled TASK-2−/− RTN neurons had a significantly more depolarized resting membrane potential at pH 7.4, as expected from the absence of a major background K+ current in those cells. Thus, the TASK-2−/− RTN neurons selected based on β-galactosidase activity were functionally equivalent to the pH-insensitive group of Phox2b- (and GFP)-expressing neurons that require TASK-2 for cellular pH sensitivity. In aggregate, these results indicate that TASK-2 plays a critical role in the pH sensitivity of a subgroup of RTN neurons, likely those in which the channel is expressed at the highest levels; loss of the alkaline-activated TASK-2 channel in RTN neurons from knockout mice yields a more depolarized membrane potential at normal pH (pH 7.4) and progressively higher firing rates relative to control cells during extracellular alkalization from pH 7.0/7.1 to pH 7.6/7.8 [64].
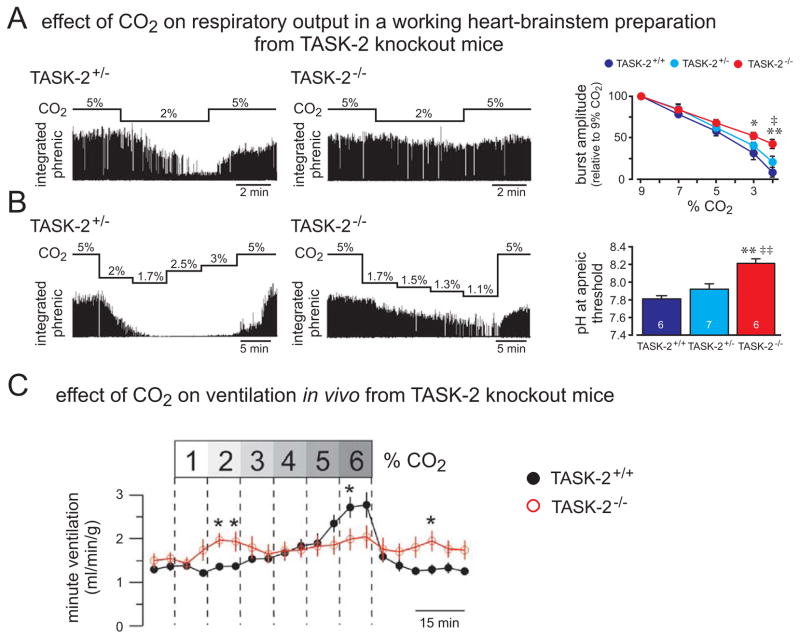
The contribution of TASK-2 to the integrated central respiratory chemoreflex has been ascertained using in situ and in vivo preparations from wild type and TASK-2 knockout mice (Fig. 5) [19,64]. In the in situ working heart-brainstem model, respiratory output was relatively maintained in TASK-2 knockout mice during progressive alkalization (Fig. 5A), and the CO2/H+ threshold for a neurophysiological equivalent of apnea (loss of phrenic nerve discharge) occurred at significantly more alkaline pH values (Fig. 5B) [64]. This is consistent with the known pH sensitivity of TASK-2 channels, where increasing activation of this background K+ channel activity by alkalization would act to lower RTN neuronal activity at raised pH; in TASK-2 knockout mice, the removal of this inhibitory mechanism would better preserve RTN activity and respiratory output under alkalized conditions (low CO2/H+), as was observed. On the other hand, there was no obvious effect of TASK-2 deletion on respiratory output under control or acidified conditions in this particular preparation [64]. Even though a more pronounced effect of TASK-2 deletion with increasingly alkalized conditions is expected given the properties of this alkaline-activated channel, the pKa of TASK-2 predicts some contribution of the channel at normal and slightly acidified physiological pH conditions (see Fig. 1). Indeed, we found that RTN neurons from TASK-2-deleted mice were more depolarized and fired faster than wild type RTN cells under those control pH conditions (Fig. 4F, 4I and [64]). Thus, we suspect that the absence of any measurable difference between wild type and TASK-2−/− mice in respiratory output from in situ preparation at neutral and acidified conditions likely reflects some peculiarity of the working heart-brainstem preparation (e.g., a less prominent contribution from RTN neurons, especially at normal to high PCO2/low pH when respiratory frequency is already maximal in that preparation), and is not due solely to TASK-2 channel pH sensitivity.
Figure 5. TASK-2 deletion leads to reduced sensitivity to hypocapnic alkalosis in a working heart-brainstem preparation and a blunted hypercapnic ventilatory response in vivo.
A. A working heart-brainstem preparation was used to examine effects of respiratory alkalosis (2% CO2) on integrated phrenic nerve activity in TASK-2+/− and TASK-2−/− mice. Right: The averaged inhibition of phrenic nerve amplitude by 2% CO2 was less pronounced in TASK-2−/− mice. B. Perfusate CO2 levels were progressively lowered in preparations from TASK-2+/− and TASK-2−/− mice in order to determine an apneic threshold (i.e., the pH when phrenic nerve activity was eliminated). Right: The averaged pH at apneic threshold was significantly higher in TASK-2−/− mice. C. Whole animal plethysmography from conscious unrestrained TASK-2+/+ and TASK-2−/− mice was used to examine the hypercapnic ventilatory reflex, under hyperoxic conditions; effects of TASK-2 deletion were manifest as a hypersensitivity to low CO2 and reduced ventilatory response to higher CO2, such that the overall reflex was blunted. (A & B, adapted from [64]; C, adapted from [19]).
Whole animal plethysmography was used to examine effects of elevated inspired CO2 and respiratory acidosis on ventilation in TASK-2 knockout mice (Fig. 5C) [19]. By comparison to wild type littermates, the TASK-2 knockouts displayed an exaggerated ventilatory response during low CO2 challenges (2%) but a diminished response at higher levels of inspired CO2 (6%), yielding an overall blunted effect of CO2 on breathing [19]. This blunted in vivo ventilatory CO2 response curve in TASK-2 knockout mice under conditions of respiratory acidosis agrees well with the overall reduction in RTN neuronal pH sensitivity, including through the pH range acidified to control (see Fig. 4F, 4I). It is also notable that the TASK-2 knockout mice display chronic metabolic acidosis, due to effects of TASK-2 deletion on the kidney [65], and thus there may have been compensatory changes in the CO2/H+ threshold for breathing in these mice. Finally, some CO2/H+ regulation of breathing is preserved even after elimination of RTN neurons in mouse genetic models of CCHS, presumably via enhanced peripheral chemoreceptor activity [54]. This is potentially relevant since the effects of hypercapnia were studied in TASK-2 knockout mice with intact peripheral chemoreceptors, even if hyperoxic conditions were employed to minimize peripheral effects [19]. So, although contributions from compensatory mechanisms likely influenced the overall respiratory response to CO2/H+ measured in TASK-2 knockout mice, they nevertheless showed a marked reduction in ventilatory response to CO2, under conditions of respiratory acidosis [19].
Summary and conclusions
The TASK channels – TASK-1, TASK-2 and TASK-3 – are pH-sensitive members of the K2P family of background K+ channels that are expressed in brainstem neurons associated with control of breathing. Their contributions to chemical regulation of breathing by CO2/H+ have been assessed by combining molecular neuroanatomy, cellular physiology and whole animal plethysmography in both wild type and TASK channel gene knockout mice.
The acid-sensitive TASK-1 and TASK-3 channels are evidently dispensable for CO2/H+-regulation of breathing despite widespread representation at multiple levels of the respiratory control system [48], including expression in respiratory motoneurons [4,34,57], rhythm-generating and pattern-modulating cells of the respiratory CPG [66], and even in some prominent groups of presumptive chemosensory neurons [48,56,67]. In particular, TASK-1 and TASK-3 channels appear to account entirely for the pH sensitivity of dorsal and caudal raphe serotonergic neurons recorded in vitro from neonatal mice animals [48,67]. As discussed above, it remains to be determined if these raphe cells (or other TASK-expressing respiratory neurons) retain their pH sensitivity in adult animals, in vivo, and if so, whether some compensatory or alternative pH sensing mechanism may contribute to any CO2/H+-regulation of breathing mediated by those neurons in wild type or TASK knockout mice. In any case, the relatively normal CO2-ventilatory curve in TASK-1−/−: TASK-3−/− double knockout mice reveals that the channels themselves are not critical for central respiratory chemosensitivity [48].
On the other hand, TASK-2 is necessary for normal regulation of breathing by CO2 [19,64]. The alkaline-activated TASK-2 channel displays a remarkably discrete brainstem localization, with expression in the medulla oblongata restricted to Phox2b-expressing RTN chemoreceptor neurons as demonstrated by the loss of Phox2b- and TASK-2-expressing neurons in the Phox2b27Ala/+ mouse line, a genetic model of CCHS [19]. Conversely, genetic deletion of TASK-2 leads to a complete loss of pH sensitivity in a subgroup of RTN neurons, with correspondingly blunted respiratory CO2 sensitivity, in situ and in vivo [19,64]. Thus, the intrinsic pH sensitivity of TASK-2 may allow the channel to function as a molecular sensor for changes in CO2/H+ in some RTN neurons, and thereby contribute to chemical regulation of breathing. A definitive test of this sensor function will require experiments using mice in which the intrinsic pH-dependence of an otherwise normal TASK-2 channel is selectively disrupted [50].
A number of outstanding questions remain. The results with TASK-2 knockout mice imply that there must be other cellular/molecular mechanisms for pH sensitivity in RTN neurons and ventilatory responses to CO2 [19,64]. What accounts for the residual pH sensitivity in the pH-sensitive RTN neurons from TASK-2 knockout mice? Evidently, that cellular pH sensitivity involves another non-TASK background K+ channel that remains to be identified [64]. Parenthetically, although not discussed here, TASK-2 channels may also contribute to a central O2 chemosensitivity since the respiratory depression induced by long term hypoxia observed in wild type mice was lost in TASK-2 knockout animals [19]. In this case, O2 sensing is probably not a direct function of the channel, but could be due to the generation of reactive O2 species that are known to activate TASK-2 currents [32].
Another unresolved issue centers on the role of TASK-1 and TASK-3 in multiple groups of respiratory neurons. For raphe neurons and motoneurons, the intrinsic pH sensitivity of those channels confers pH sensitivity on the neurons, at least in vitro [48,34]. Is the intrinsic pH sensitivity of TASK-1 and TASK-3 retained in vivo, and exploited for any physiological purpose? It is possible that these channels are utilized instead to provide a neuromodulator-regulated leak current in respiratory neurons, since TASK-1 and TASK-3 are effectors for many different neurotransmitter-receptor systems [8,11,41,44,57]. Even if that is the case, given that TASK-1 and TASK-3 channels are expressed in many neurons embedded in a homeostatic system regulated by CO2/H+, it remains surprising that their removal had no impact on breathing and its control by chemoreceptors. Of course, it is easy to imagine that some compensatory mechanisms could develop in a system as crucial as that controlling breathing, as suggested in the case of animals with outright ablation of chemosensitive RTN neurons [54]. In addition, pH changes can occur with neuronal activity in many brain regions where TASK-1 and TASK-3 are expressed [9], and it will be interesting to determine if their pH sensitivity is exploited for other physiological contexts.
So, in conclusion, TASK-2 contributes to central respiratory chemosensitivity by mediating the pH response of RTN neurons but additional molecular sensors are also involved. Those other mechanisms appear to include alternative background K+ channels as effectors (at least in RTN neurons), but neither TASK-1 nor TASK-3 are necessary for mediating this important homeostatic reflex.
Acknowledgments
This work was supported by grants from the NIH (HL108609, DAB and HL074011, PGG), and from French National Agency for Research Grants (ANR RESPITASK, JB, CG and ANR-11-LABX-0015-01, JB) and from CNRS (JB, CG).
References
- 1.Bagriantsev SN, Peyronnet R, Clark KA, Honore E, Minor DL., Jr Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci. 2008;29:566–575. doi: 10.1016/j.tips.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurons. Respir Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- 4.Berg AP, Talley EM, Manger JP, Bayliss DA. Motoneurons express heteromeric TWIK-related acid-sensitive K+ (TASK) channels containing TASK-1 (KCNK3) and TASK-3 (KCNK9) subunits. J Neurosci. 2004;24:6693–6702. doi: 10.1523/JNEUROSCI.1408-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brohawn SG, del Marmol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckler KJ. Two-pore domain K+ channels and their role in chemoreception. Adv Exp Med Biol. 2010;661:15–30. doi: 10.1007/978-1-60761-500-2_2. [DOI] [PubMed] [Google Scholar]
- 7.Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 8.Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 2003;22:5403–5411. doi: 10.1093/emboj/cdg528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 10.Clarke CE, Veale EL, Wyse K, Vandenberg JI, Mathie A. The M1P1 loop of TASK3 K2P channels apposes the selectivity filter and influences channel function. J Biol Chem. 2008;283:16985–16992. doi: 10.1074/jbc.M801368200. [DOI] [PubMed] [Google Scholar]
- 11.Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. TASK (TWIK-related acid- sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 12.Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76:656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- 13.Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C. Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci. 2009;29:14836–14846. doi: 10.1523/JNEUROSCI.2623-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 16.Es-Salah-Lamoureux Z, Steele DF, Fedida D. Research into the therapeutic roles of two-pore-domain potassium channels. Trends Pharmacol Sci. 2010;31:587–595. doi: 10.1016/j.tips.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15 (24):6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 19.Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci USA. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein SA, Bayliss DA, Kim D, Lesage F, Plant LD, Rajan S. International Union of Pharmacology. LV. Nomenclature and molecular relationships of two-P potassium channels. Pharmacol Rev. 2005;57:527–540. doi: 10.1124/pr.57.4.12. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein SA, Bockenhauer D, O’Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein SA, Price LA, Rosenthal DN, Pausch MH. ORK1, a potassium-selective leak channel with two pore domains cloned from Drosophila melanogaster by expression in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:13256–13261. doi: 10.1073/pnas.93.23.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, Burdakov D. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur J Neurosci. 2009;30:57–64. doi: 10.1111/j.1460-9568.2009.06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38:305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- 25.Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyon A, Tardy MP, Rovere C, Nahon JL, Barhanin J, Lesage F. Glucose inhibition persists in hypothalamic neurons lacking tandem-pore K+ channels. J Neurosci. 2009;29:2528–2533. doi: 10.1523/JNEUROSCI.5764-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 28.Iceman KE, Richerson GB, Harris MB. Medullary serotonin neurons are CO2 sensitive in situ. J Neurophysiol. 2013;110:2536–2544. doi: 10.1152/jn.00288.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karschin C, Wischmeyer E, Preisig-Muller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- 30.Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- 31.Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J Neurosci. 2010;30:4273–4284. doi: 10.1523/JNEUROSCI.4017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.L’Hoste S, Poet M, Duranton C, Belfodil R, e Barriere H, Rubera I, Tauc M, Poujeol C, Barhanin J, Poujeol P. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J Biol Chem. 2007;282:36692–36703. doi: 10.1074/jbc.M703933200. [DOI] [PubMed] [Google Scholar]
- 33.Lazarenko RM, Fortuna MG, Shi Y, Mulkey DK, Takakura AC, Moreira TS, Guyenet PG, Bayliss DA. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K+ current. J Neurosci. 2010;30:9324–9334. doi: 10.1523/JNEUROSCI.1956-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA. Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010;30:7691–7704. doi: 10.1523/JNEUROSCI.1655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesage F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- 36.Lesage F, Barhanin J. Molecular physiology of pH-sensitive background K2P channels. Physiology (Bethesda) 2011;26:424–437. doi: 10.1152/physiol.00029.2011. [DOI] [PubMed] [Google Scholar]
- 37.Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- 38.Lesage F, Reyes R, Fink M, Duprat F, Guillemare E, Lazdunski M. Dimerization of TWIK-1 K+ channel subunits via a disulfide bridge. EMBO J. 1996;15:6400–6407. [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes CM, Zilberberg N, Goldstein SA. Block of Kcnk3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J Biol Chem. 2001;276:24449–24452. doi: 10.1074/jbc.C100184200. [DOI] [PubMed] [Google Scholar]
- 40.Lotshaw DP. Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys. 2007;47:209–256. doi: 10.1007/s12013-007-0007-8. [DOI] [PubMed] [Google Scholar]
- 41.Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathie A, Al-Moubarak E, Veale EL. Gating of two pore domain potassium channels. J Physiol. 2010;588:3149–3156. doi: 10.1113/jphysiol.2010.192344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathie A, Veale EL. Therapeutic potential of neuronal two-pore domain potassium-channel modulators. Curr Opin Investig Drugs. 2007;8:555–562. [PubMed] [Google Scholar]
- 44.Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci USA. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–436. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- 46.Morton MJ, O’Connell AD, Sivaprasadarao A, Hunter M. Determinants of pH sensing in the two-pore domain K+ channels TASK-1 and-2. Pflugers Arch. 2003;445:577–583. doi: 10.1007/s00424-002-0901-2. [DOI] [PubMed] [Google Scholar]
- 47.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- 48.Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- 50.Niemeyer MI, Gonzalez-Nilo FD, Zuniga L, Gonzalez W, Cid LP, Sepulveda FV. Neutralization of a single arginine residue gates open a two-pore domain, alkali-activated K+ channel. Proc Natl Acad Sci USA. 2007;104:666–671. doi: 10.1073/pnas.0606173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piechotta PL, Rapedius M, Stansfeld PJ, Bollepalli MK, Ehrlich G, Andres-Enguix I, Fritzenschaft H, Decher N, Sansom MS, Tucker SJ, Baukrowitz T. The pore structure and gating mechanism of K2P channels. EMBO J. 2011;30:3607–3619. doi: 10.1038/emboj.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- 53.Rajan S, Wischmeyer E, Xin Liu G, Preisig-Muller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- 54.Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- 56.Sirois JE, Lei Q, Talley EM, Lynch C, 3rd, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 58.Talley EM, Sirois JE, Lei Q, Bayliss DA. Two-pore-Domain (KCNK) potassium channels: dynamic roles in neuronal function. Neuroscientist. 2003;9:46–56. doi: 10.1177/1073858402239590. [DOI] [PubMed] [Google Scholar]
- 59.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teran FA, Massey CA, Richerson GB. Serotonin neurons and central respiratory chemoreception: where are we now? Prog Brain Res. 2014;209:207–233. doi: 10.1016/B978-0-444-63274-6.00011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. J Neurosci. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Single-unit responses of serotonergic dorsal raphe neurons to specific motor challenges in freely moving cats. Neuroscience. 1997;79:161–169. doi: 10.1016/s0306-4522(96)00673-2. [DOI] [PubMed] [Google Scholar]
- 63.Vega-Saenz de Miera E, Lau DH, Zhadina M, Pountney D, Coetzee WA, Rudy B. KT3.2 and KT3.3, two novel human two-pore K+ channels closely related to TASK-1. J Neurophysiol. 2001;86:130–142. doi: 10.1152/jn.2001.86.1.130. [DOI] [PubMed] [Google Scholar]
- 64.Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci. 2013;33:16033–16044. doi: 10.1523/JNEUROSCI.2451-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warth R, Barriere H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Poujeol P, Barhanin J. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA. 2004;101:8215–8220. doi: 10.1073/pnas.0400081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Washburn CP, Bayliss DA, Guyenet PG. Cardiorespiratory neurons of the rat ventrolateral medulla contain TASK-1 and TASK-3 channel mRNA. Respir Physiol Neurobiol. 2003;138:19–35. doi: 10.1016/s1569-9048(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 67.Washburn CP, Sirois JE, Talley EM, Guyenet PG, Bayliss DA. Serotonergic raphe neurons express TASK channel transcripts and a TASK-like pH- and halothane-sensitive K+ conductance. J Neurosci. 2002;22:1256–1265. doi: 10.1523/JNEUROSCI.22-04-01256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104 (25):10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]