Summary
The immune system recognizes and is poised to eliminate cancer, but is held in check by inhibitory receptors and ligands. These immune checkpoint pathways, which normally maintain self-tolerance and limit collateral tissue damage during anti-microbial immune responses, can be co-opted by cancer to evade immune destruction. Drugs interrupting immune checkpoints, such as anti-CTLA-4, anti-PD-1, anti-PD-L1, and others in early development can unleash anti-tumor immunity and mediate durable cancer regressions. The complex biology of immune checkpoint pathways still contains many mysteries, and the full activity spectrum of checkpoint-blocking drugs, used alone or in combination, is currently the subject of intense study.
INTRODUCTION
In the current era in oncology emphasizing personalized therapy, immune checkpoint blockade is distinguished by its “common denominator” approach. While the vast somatic mutational diversity found in most human cancers creates challenges for therapies targeting individual mutations, it exposes a panoply of new antigens for potential immune recognition. However, cells of the adaptive and innate immune systems that recognize and are poised to attack cancer are held in check by molecular pathways that suppress their activation and effector functions. The seminal observation that blocking the prototypical immune checkpoint receptor Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) could mediate tumor regression in murine models [Leach et al., 1996] led to the clinical development and approval of anti-CTLA-4 as a treatment for patients with advanced melanoma [Hodi et al., 2010]. Subsequently, drugs blocking the distinct checkpoints Programmed Death 1 (PD-1) and its major ligand PD-L1 have shown great promise in treating many diverse cancer types, fueling the intensive examination of a growing cohort of unique checkpoint molecules as potential therapeutic targets. This has revealed new treatment options for patients and has revolutionized our approach to cancer therapy.
BIOLOGY OF IMMUNE CHECKPOINTS: THE BASICS
The rapid-fire clinical successes from blocking CTLA-4 and PD-1, the first checkpoint receptors to be discovered, have opened prospects for extending the potential of cancer immunotherapy by inhibiting more recently discovered checkpoint ligands and receptors. It is clear that despite some commonalities, CTLA-4 and PD-1 have distinct patterns of expression, signaling pathways, and mechanisms of action. Although discovered over 20 years ago, there are still many unanswered questions about their biology, particularly in the context of cancer.
The CD28/CTLA-4 system of immune modulation
The conventional wisdom underlying our vision of how CTLA-4 blockade mediates tumor regression is that it systemically activates T cells that are encountering antigen. CTLA-4 represents the paradigm for regulatory feedback inhibition; its engagement down-modulates the amplitude of T cell responses, largely by inhibiting co-stimulation by CD28, with which it shares the ligands CD80 (B7.1) and CD86 (B7.2) (Figure 1) [Lenshow et al., 1996]. As a “master T cell co-stimulator”, CD28 engagement amplifies TCR signaling when the T cell receptor (TCR) is also engaged by cognate peptide-MHC [Schwartz, 1992]. However, CTLA-4 has a much higher affinity for both CD80 and CD86 compared to CD28 [Linsley et al., 1994], so its expression on activated T cells dampens CD28 co-stimulation by out-competing CD28 binding and possibly also via depletion of CD80 and CD86 via “trans-endocytosis” [Querishi et al., 2011]. Because CD80 and CD86 are expressed on antigen presenting cells (APCs, e.g., dendritic cells, monocytes) but not on non-hematologic tumor cells, CTLA-4’s suppression of anti-tumor immunity has been viewed to reside primarily in secondary lymphoid organs where T cell activation occurs, rather than within the tumor microenvironment (TME). Furthermore, CTLA-4 is predominantly expressed on CD4+ “helper” and not CD8+ “killer” T cells, thus heightened CD8 responses in anti-CTLA-4 treated patients likely occur indirectly through increased activation of CD4+ cells. Of note, a few studies suggest that CTLA-4 can act as a direct inhibitory receptor of CD8 T cells [Fallarino et al., 1998; Chambers et al., 1998], although this role in down-modulating anti-tumor CD8 T cell responses remains to be directly demonstrated.
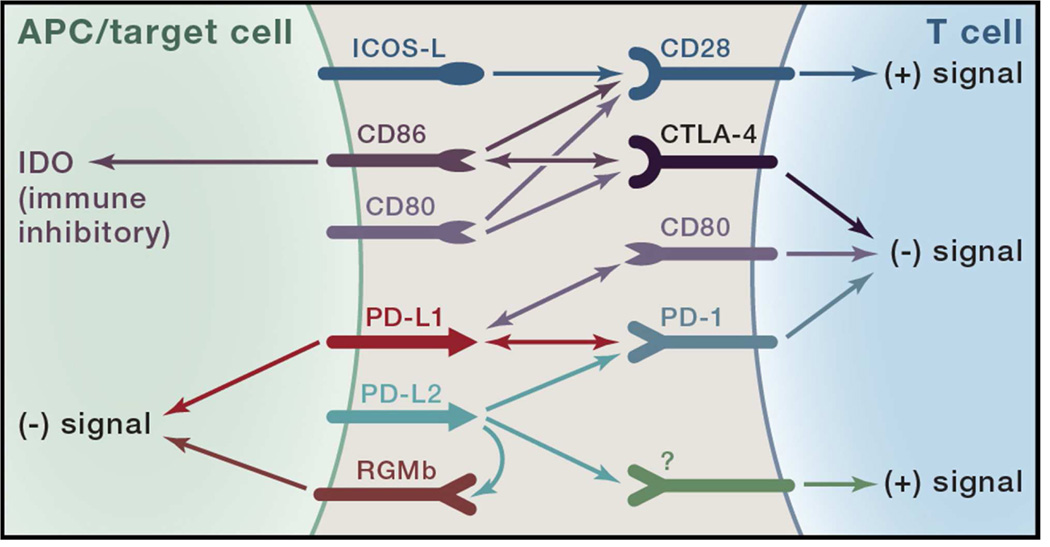
Figure 1. Complex interactions between the CTLA-4/CD28 and PD-1 families of receptors and ligands.
Shown are the defined interactions between the co-inhibitory (checkpoint) receptors, CTLA-4 and PD-1, and their ligands and related receptors. The two known ligands for CTLA-4 are CD80 (B7.1) and CD86 (B7.2). CD86 can “backwards signal” into antigen presenting cells (APCs) when engaged by CTLA-4, inducing the immune inhibitory enzyme indolamine 2’3’ dioxygenase (IDO). CD80 and CD86 also bind the co-stimulatory receptor CD28 on T cells. Recently, another B7 family member, ICOS-L, which was discovered as the ligand for the co-stimulatory receptor ICOS (not shown), was reported to bind to CD28 leading to co-stimulation independent of CD80 or CD86. The two defined ligands for PD-1, namely PD-L1 (B7-H1) and PD-L2 (B7-DC), bind to additional molecules. PD-L1 binds CD80 molecules expressed on activated T cells, mediating inhibition. Additionally, PD-L1 on APCs appears to provide inhibitory signals (“backwards signaling”) when it is engaged by PD-1. PD-L2 binds another molecule, repulsive guidance molecule b (RGMb), which is expressed on macrophages and some epithelial cell types and appears to deliver an inhibitory immune signal through an as yet undefined mechanism. Though not identified, genetic evidence from PD-1 knockout T cells and knockout mice suggests the existence of another receptor for PD-L2 that is co-stimulatory.
The specific signaling pathways by which CTLA-4 inhibits T cell activation are still under investigation, although activation of the phosphatases SHP2 and PP2A appears to be important in counteracting both tyrosine and serine/thronine kinase signals induced by TCR and CD28 [Rudd et al., 2009]. CTLA-4 engagement also interferes with the “TCR stop signal”, which maintains the immunological synapse long enough for extended or serial interactions between TCR and its peptide-MHC ligand [Schneider et al., 2006]. Naïve and resting memory T cells express CD28, but not CTLA-4, on the cell surface, allowing co-stimulation to dominate upon antigen recognition. However, CTLA-4 is rapidly mobilized to the cell surface from intracellular protein stores, allowing feedback inhibition to occur within an hour after antigen engagement. The central role of CTLA-4 in maintaining immune tolerance is dramatically demonstrated by the rapidly lethal systemic immune hyperactivation phenotype of Ctla-4 knockout mice [Tivol et al., 1995; Waterhouse et al., 1995]. In humans, anti-CTLA-4 treatment induces expression of activation markers on circulating T cells [Maker et al., 2005] and a high rate of inflammatory side effects [Phan et al., 2003]. However, because melanoma patients appear to possess an unusually high proportion of tumor-reactive T cells, anti-tumor responses balance autoimmune toxicity and provide clinical benefit to roughly 20% of patients with this disease (see below).
PD-1: similarities to and differences from CTLA-4
The PD-1 system of immune modulation bears similarities to CTLA-4 as well as key distinctions [Parry et al., 2005]. Similar to CTLA-4, PD-1 is absent on resting naïve and memory T cells and is expressed upon TCR engagement. However, in contrast to CTLA-4, PD-1 expression on the surface of activated T cells requires transcriptional activation, and thus is delayed (6–12 hr). Also in contrast to CTLA-4, PD-1 contains a conventional immunoreceptor tyrosine inhibitory motif (ITIM) as well as an immunoreceptor tyrosine switch motif (ITSM). PD-1’s ITIM and ITSM bind the inhibitory phosphatase SHP-2. PD-1 engagement can also activate the inhibitory phosphatase PP2A. PD-1 engagement directly inhibits TCR-mediated effector functions and increases T cell migration within tissues, thereby limiting the time that a T cell has to survey the surface of interacting cells for the presence of cognate peptide-MHC complexes; thus, T cells may “pass over” target cells expressing lower levels of peptide-MHC complexes [Honda et al., 2014].
In contrast to CTLA-4, PD-1 blockade is viewed to work predominantly within the TME where its ligands are commonly overexpressed by tumor cells as well as infiltrating leukocytes [Keir et al., 2008]. This mechanism is thought to reflect its important physiologic role in restraining collateral tissue damage during T cell responses to infection. In addition, tumor infiltrating lymphocytes (TILs) commonly express heightened levels of PD-1 and are thought to be “exhausted” due to chronic stimulation by tumor antigens, analogous to the exhausted phenotype seen in murine models of chronic viral infection which is partially reversible by PD-1 pathway blockade [Barber et al., 2006].
Importantly, the phenotypes of murine knockouts of PD-1 and its two known ligands are very mild, consisting of late-onset organ-specific inflammation, particularly when crossed to autoimmune-prone mouse strains [Nishimura et al., 1999; Nishimura et al., 2001]. This contrasts sharply to the Ctla-4 knockout phenotype and highlights the importance of the PD-1 pathway in restricting peripheral tissue inflammation. Further, it is consistent with clinical observations that autoimmune side effects of anti-PD-1 drugs are generally milder and less frequent than with anti-CTLA-4.
Despite the conventional wisdom that CTLA-4 acts early in T cell activation in secondary lymphoid tissues whereas PD-1 inhibits execution of effector T cell responses in tissue and tumors, this distinction is not absolute. Beyond its role in dampening activation of effector T cells, CTLA-4 plays a major role in driving the suppressive function of T regulatory (Treg) cells [Wing et al., 2008; Peggs et al., 2009]. Tregs, which broadly inhibit effector T cell responses, are typically concentrated in tumor tissues and are thought to locally inhibit anti-tumor immunity. Thus, CTLA-4 blockade may affect intratumoral immune responses by inactivating tumor-infiltrating Tregs. Recent evidence demonstrates anti-tumor effects from CTLA-4 blockade even when S1P inhibitors block lymphocyte egress from lymph nodes [Spranger et al., 2014], indicating that this checkpoint exerts at least some effects directly in the TME as opposed to secondary lymphoid tissues. Conversely, PD-1 has been shown to play a role in early fate decisions of T cells recognizing antigens presented in the lymph node. In particular, PD-1 engagement limits the initial “burst size” of T cells upon antigen exposure and can partially convert T cell tolerance induction to effector differentiation [Goldberg et al., 2007].
Complex receptor-ligand interactions in the PD-1 pathway: links and analogies to the CD28/CTLA-4 system
The receptor-ligand interactions of the PD-1 system appear even more complex than the CD28/CTLA-4 system (Figure 1). The two ligands for PD-1 are PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273), which share 37% sequence homology and arose via gene duplication [Dong et al., 1999, Latchman et al., 2001; Tseng et al., 2001]. However, their regulation is highly divergent. PD-L1 is induced on activated hematopoietic cells and on epithelial cells by the inflammatory cytokine IFN-gamma (IFN-g), which is produced by some activated T and NK cells. PD-L2 has much more selective expression on activated dendritic cells and some macrophages. It is induced to a much greater extent by IL-4 than by IFN-g, further emphasizing differences in regulation of expression of PD-L1 and PD-L2.
Beyond their role as ligands for PD-1, PD-L1 and PD–L2 appear to have additional partners, indicating additional layers of immune modulation. An unexpected molecular interaction between PD-L1 and CD80 was discovered [Butte et al., 2007; Park et al., 2010], whereby CD80 expressed on activated T cells (and possibly APCs) can function as a receptor rather than a ligand, delivering inhibitory signals when engaged by PD-L1 – the relevance of this interaction in tumor immune resistance has not yet been determined. Recently, PD-L2 was shown to bind to repulsive guidance molecule b (RGMb), which itself binds at least three other molecules in cis (neogenin, and BMP receptors type I and II) [Xiao et al., 2014]. This interaction appears to be inhibitory, independent of PD-1, as demonstrated in a pulmonary tolerance model. Finally, evidence from murine models suggests that PD-L2, and possibly PD-L1, may bind to a co-stimulatory T cell receptor [Shin et al., 2003; Shin et al., 2005], an arrangement reminiscent of the CD80/CD86 ligand pair for the co-stimulatory CD28 and co-inhibitory CTLA-4 receptors. Understanding the roles of these various interactions in cancer is highly relevant for the development of immunomodulatory drugs and the discovery of biomarkers predictive of therapeutic response.
Mechanisms of PD-1 ligand induction: implications for cancer immunotherapy
A key finding that encouraged the development of drugs blocking the PD-1 pathway for cancer immunotherapy was that PD-1 ligands are up-regulated in many human cancers [Dong et al., 2002] while PD-1 is highly expressed on tumor infiltrating lymphocytes [Ahmadzedeh et al., 2009; Sfanos et al., 2009]. Indeed, PD-L1 appears to be the major ligand expressed in solid tumors, while PD-L2 (together with PD-L1) is highly expressed in certain subsets of B cell lymphomas [Ansell et al., 2014]. Exploration of this phenomenon as a central process by which cancers resist elimination by endogenous tumor-specific T cells revealed two mechanisms for PD-1 ligand up-regulation in cancer, known as intrinsic and adaptive immune resistance (Figure 2). These mechanisms are not mutually exclusive and may co-exist in the same TME. Intrinsic resistance refers to the constitutive expression of PD-L1 by tumor cells due to genetic alterations or activation of certain signaling pathways, such as the AKT pathway and STAT3 which are commonly activated in many cancers [Parsa et al., 2007; Marzec et al., 2008]. While PD-L1 induction by AKT and STAT3 signaling has been demonstrated in some tumor cell lines, the importance of this intrinsic pathway in PD-L1 expression by tumors in vivo remains to be determined. Genetic alterations in B cell lymphoma subtypes can drive expression of either or both PD-L1 and PD-L2. Primary mediastinal lymphomas commonly display gene fusions between MHC class II transactivator CIITA and PD-L1 or PD-L2, placing PD-1 ligands under the transcriptional control of the CIITA promoter which is highly active in B cell lymphomas [Steidl et al., 2011]. A significant subset of Hodgkin’s lymphoma has amplification of chromosome 9p23-24, where PD-L1 and PD-L2 reside, resulting in over-expression of both ligands. Other cancers, such as a subset of EBV-induced gastric cancers, also display gene amplification with consequent induction of PD-L1 and PD-L2.
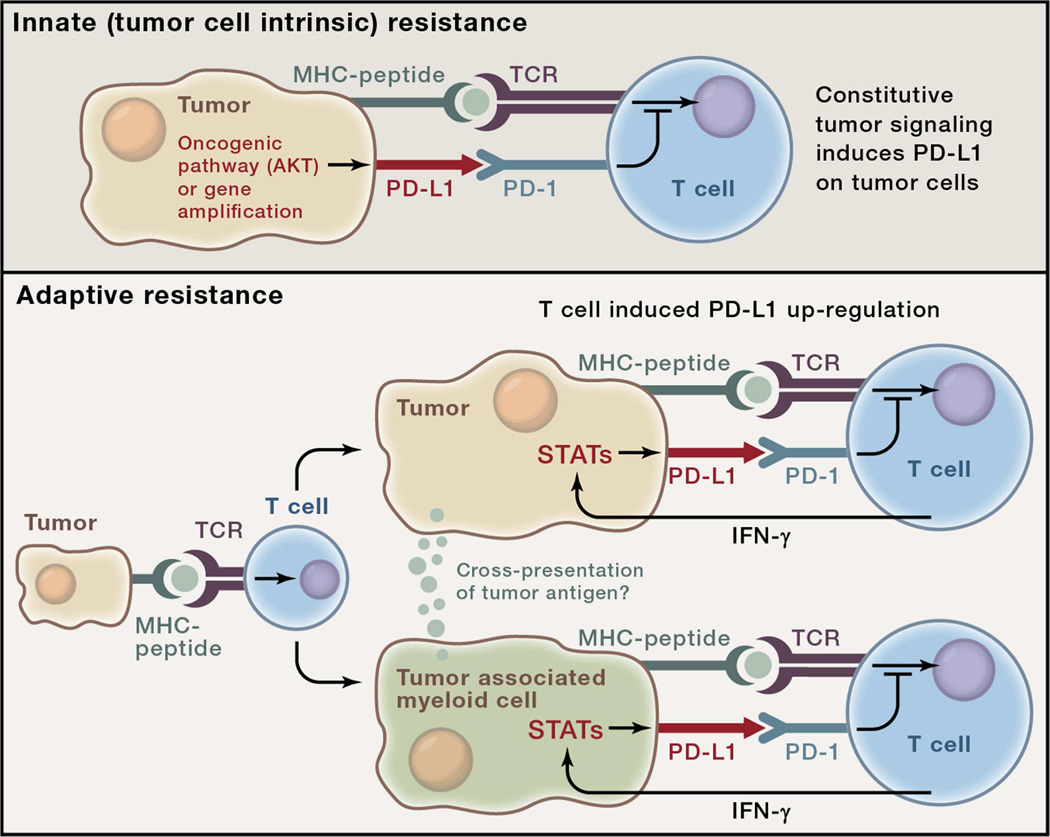
Figure 2. Two general mechanisms for expression of checkpoint ligands in the tumor microenvironment (TME).
The examples in this figure use the PD-1 ligand, PD-L1 for illustrative purposes although the concept likely applies to multiple checkpoint ligands. Top: Innate immune resistance. In some tumors, constitutive oncogenic signaling, such as through activation of the AKT pathway or gene amplification, can up-regulate PD-L1 expression on tumor cells independently of inflammatory signals in the TME. Bottom: Adaptive immune resistance refers to PD-L1 induction in tumors as an adaptation to sensing of immune attack. In adaptive resistance, PD-L1 is not constitutively expressed but rather, is induced by inflammatory signals such as IFN-g produced by T cells attempting to execute an active anti-tumor response. Expression of PD-L1 in a non-uniform distribution associated with lymphocyte infiltrates suggests adaptive induction in response to immune reactivity within the TME. Adaptive resistance can be generated by cytokine-induced PD-L1 expression on either tumor cells themselves or on leukocytes (macrophages, myeloid suppressor cells, dendritic cells or even lymphocytes) in the TME. Inhibition of tumor specific T cells by PD-L1- (or PD-L2)-expressing leukocytes may involve cross-presentation of tumor antigens such that PD-1-dependent inhibition is in cis. Adaptive resistance may be a common mechanism for the intratumoral expression of multiple immune checkpoint molecules.
The second mechanism, adaptive resistance, refers to the induction of PD-L1 expression on tumor cells in response to specific cytokines, in particular IFN-g. As IFN-g is only produced by activated Th1-type helper CD4 cells, activated CD8 cells and NK cells, this mechanism represents an adaptation of tumor cells upon “sensing” an inflammatory immune microenvironment that “threatens” the tumor. Indeed, human tumors show significant correlations between PD-L1 expression, levels of T cell infiltration, and IFN-g in the TME [Taube et al., 2012; Spranger et al., 2013]. Other inhibitory molecules in the TME, such as indoleamine 2’3’ dioxygenase (IDO), which inhibits immunity locally via conversion of tryptophan to kynurenines, are also induced by IFN-g and coordinately up-regulated with PD-L1. The concept of adaptive resistance does not solely apply to induction of PD-L1 on tumor cells. Early studies demonstrated that PD-L1 expression on myeloid cells, including dendritic cells, can significantly impair activation of tumor-specific T cells. Inhibition of T cell responses can be mediated by PD-L1+ suppressive myeloid cells or DC in the TME as well as in tumor draining lymph nodes [Curiel et al., 2003]. In some tumors, such as MSI colon cancer, myeloid rather than tumor cells are the major cell type expressing PD-L1 [Llosa et al., 2014]. A recent report suggests that PD-L1 expression by infiltrating myeloid cells rather than tumor cells is more predictive of response to PD-1 pathway blockade [Herbst et al., 2014]. The relative importance of PD-L1 expression on leukocytes in the TME, which would provide “third party” inhibition, vs. direct expression by the tumor cells, remains to be determined.
Implications of adaptive immune resistance
The adaptive resistance mechanism of intratumoral PD-L1 induction, together with the broad therapeutic activity of PD-1 pathway blockade in human cancer, validates one of the most important tenets underlying cancer immunology and immunotherapy, namely, that many cancer patients contain a significant repertoire of tumor-specific T cells capable of killing their tumor save for the adaptive induction of immune checkpoints in the TME. It also implies that PD-L1 expression in the tumor represents a measure of the potential for a patient’s immune system to recognize their tumor. One of the major unanswered questions is: what are the dominant antigenic targets that T cells recognize when checkpoints are blocked? Circumstantial evidence supports the notion that neoantigens created by the multiple somatic mutations in cancers provide such targets. Indeed, a recent report demonstrated that melanomas with higher mutational loads were more responsive to anti-CTLA-4 therapy [Snyder et al., 2014]. Also, the tumor types that have been shown to respond to anti-PD-1/PD-L1 therapy tend to be those with higher median mutational loads (i.e., carcinogen-induced cancers such as melanoma, lung, bladder, and head and neck cancers). However, there is much evidence over the past 20 years that shared self-antigens up-regulated in tumors by epigenetic mechanisms (e.g., cancer-testis antigens) are also able to provide selective tumor targeting. The relative importance of mutation-dependent, tumor-specific neoantigens vs. tumor associated self-antigens as T cell targets remains to be determined.
Finally, the adaptive resistance mechanism has profound implications for developing synergistic combinatorial cancer immunotherapies. One of the most promising general approaches to immunotherapy utilizes positive drivers of anti-tumor immune responses such as vaccines, intratumoral injection of immune activators, and co-stimulatory receptor agonists. These modalities with the potential to enhance anti-tumor responses would also be expected to enhance the adaptive induction of checkpoints like PD-1 ligands. This has in fact been demonstrated in animal models of vaccination [Fu et al., 2014]. Thus, positive drivers of anti-tumor immunity may be synergistic with PD-1 pathway inhibitors. Such approaches are just beginning to enter the clinic.
CLINICAL IMPACT OF DRUGS BLOCKING CTLA-4 AND PD-1
Anti-CTLA-4
The anti-CTLA-4 monoclonal antibodies (mAbs) ipilimumab, a fully human IgG1 (Bristol-Myers Squibb), and tremelimumab, a fully human IgG2 (Pfizer, MedImmune), were the first immune checkpoint blocking drugs to enter clinical testing in oncology. Although designed as CTLA-4 blocking mAbs, these drugs have recently been postulated to have unique functions endowed by their specific isotypes, with evidence suggesting that ipilimumab may deplete Tregs over-expressing CTLA-4 [Selby et al., 2013]. In 2011, ipilimumab was approved in the US and Europe as first-line therapy for advanced unresectable melanoma, based on results from two phase III trials showing significant extensions in overall survival (OS) [Hodi et al., 2010; Robert et al., 2011]. Long-term follow-up in a pooled meta-analysis of 1861 melanoma patients receiving ipilimumab on phase II or III trials revealed durable survival in approximately 20%, in some cases extending to 10 years [Schadendorf et al., 2015]. Interestingly, this survival rate is approximately double the observed rate of tumor regressions measured by standard oncologic criteria (~10% complete and partial responses, CR+PR). Factors contributing to this phenomenon may include prolonged disease stabilization, unconventional “immune-related” response patterns, or a heightened responsiveness of ipilimumab-refractory patients to subsequent therapies. While tremelimumab, a distinct CTLA-4 blocking mAb, showed promise in early-phase melanoma trials, it did not meet its designated endpoint when randomized against standard chemotherapy in a first-line phase III melanoma trial [Ribas et al., 2013].
Ipilimumab has so far shown only modest anti-tumor effects in non-melanoma cancers, and tremelimumab is still in early testing for these indications [reviewed in Weber 2014]. Kidney, lung and prostate cancer have been the most intensively studied. In a phase II study of metastatic renal cell carcinoma (RCC, N=61), a partial response rate of 10% was observed with ipilimumab monotherapy [Yang et al., 2007]. In lung cancer, treatment-naïve patients with non-small-cell (NSCLC, n =204) or extensive-disease small-cell lung cancer (ED-SCLC, n=130) received standard chemotherapy alone, or combined with ipilimumab during initial (“concurrent”) or later (“phased”) chemotherapy cycles in a phase III trial [Lynch et al., 2012; Reck et al., 2013]. For both diseases, a brief but statistically significant 1-month extension of progression free survival measured by “immune-related” criteria (irPFS) was observed in patients receiving phased ipilimumab plus chemotherapy, compared to chemotherapy alone. In NSCLC, there was also a significant 1-month extension of PFS measured by standard criteria, in the phased ipilimumab arm. Although ipilimumab did not have a significant impact on OS in either NSCLC or ED-SCLC, a subset analysis appeared to show improved activity in patients with squamous NSCLC, providing the basis for an ongoing phase III trial of ipilimumab plus chemotherapy in this histology. Similarly, trials of ipilimumab in metastatic castration-resistant prostate cancer (mCRPC) have yielded weak but positive signals of activity. In phase I/II trials in which patients received ipilimumab alone or combined with systemic GM-CSF or focal radiotherapy, PSA reductions of ≥50% were observed in some patients and isolated examples of measurable tumor regression were reported [Fong et al., 2009; Slovin et al., 2013], supporting further study. In a phase III trial of ipilimumab vs. placebo after bone-directed radiotherapy in 799 patients with docetaxel-refractory mCRPC, median OS was 11.2 vs. 10.0 months, respectively (p=0.053), failing to meet the trial’s primary endpoint [Kwon et al., 2014]. However, there was a statistically significant 1-month improvement in PFS, and a suggestion that OS was prolonged in a subgroup of patients with favorable prognostic features. A separate phase III trial of ipilimumab in chemotherapy-naïve patients with aymptomatic or minimally symptomatic mCRPC without visceral metastases has recently completed accrual.
Valuable clinical experience gained from studies of anti-CTLA-4 mAbs paved a path for accelerated development other drugs-in-class by providing a framework for treatment strategy, toxicity management and efficacy evaluation. New principles emerged that distinguished immune checkpoint blockade from traditional cancer therapies. First, a new category of side-effects, so-called “immune-related adverse events” (irAEs), was recognized and characterized, leading to algorithm development for early detection and management. Drug-related irAEs were severe in 15–30% of patients receiving anti-CTLA-4, sometimes resulting in fatalities. These irAEs were associated with inflammation in normal tissues such as the gut, skin, and endocrine glands, and resembled phenotypes observed in human CTLA-4 heterozygotes with reduced CTLA-4 expression [Topalian and Sharpe, 2014]; their occurrence in individuals with no prior history of autoimmunity validates the mechanism of action of anti-CTLA-4 in “releasing the brakes” on immune responses and underscores the precarious balance that normally exists between self-tolerance and autoimmunity. Secondly, a new category of clinical response termed “immune-related response” was recognized, in which major and durable tumor regressions could occur after apparent initial disease progression on treatment [Wolchok et al., 2009]. Tumor enlargement measured by conventional radiologic scans may result from drug-induced inflammation at tumor sites, or could reflect actual tumor growth followed by delayed regression. Such phenomena pose challenges for the appropriate management of individual patients and the selection of informative endpoints for trials of immune checkpoint-blocking drugs.
Drugs blocking the PD-1 pathway
Information garnered from trials of anti-CTLA-4 agents fast-forwarded the development of drugs blocking PD-1 or its major ligand, PD-L1. As predicted by murine models, these drugs have heightened tumor selectivity and reduced toxicity compared to anti-CTLA-4, supporting their administration in an outpatient setting. Furthermore, while they are effective against advanced treatment-refractory melanoma, with recent regulatory approvals for two anti-PD-1 drugs in this setting, they also appear to have a much broader spectrum of anti-tumor activity than anti-CTLA-4. Reproducible and durable regressions of epithelial cancers (lung, head and neck, and bladder cancers, among others) have catapulted the launching of hundreds of ongoing clinical trials in diverse disease indications. Although several different anti-PD-1/PD-L1 blocking mAbs are currently in clinical testing (Table 1), the fact that anti-tumor activity has been observed with all of them highlights the PD-1 pathway as a dominant intratumoral immunosuppressive pathway and a key target in cancer therapy.
Table 1.
Drugs in clinical development that block PD-1 or PD-L1
| Target | Drug name | Other names | Source | Isotype and characteristics |
Clinical testing phase |
|---|---|---|---|---|---|
| PD-1 | MEDI0680 | AMP-514 | MedImmune/ AstraZeneca | Information not available | Phase I |
| Nivolumab | Opdivo; BMS-936558; MDX-1106; ONO-4538 | Bristol-Myers Squibb; Ono Pharmaceuticals | Fully human IgG4a | Approved, treatment-refractory unresectable melanoma (Japan, US) and squamous NSCLC (US) | |
| Pembrolizumab | Keytruda; MK-3475; lambrolizumab | Merck | Humanized IgG4 | Approved, treatment-refractory unresectable melanoma (US) | |
| Pidilizumab | CT-011 | CureTech | Humanized IgG1 | Phase I–II | |
| PD-L1 | BMS-936559 | MDX-1105 | Bristol-Myers Squibb | Fully human IgG4a | Phase I |
| MEDI4736 | none | MedImmune/AstraZeneca | Fc-modified human IgG1b | Phase I–III | |
| MPDL3280A | RG7446 | Genentech/ Roche | Fc-modified human IgG1b | Phase I–III | |
| MSB0010718C | none | EMD Serono | Fully human IgG1a | Phase I–II |
Fully human mAbs were produced in genetically engineered mice.
Fc-modified mAbs were engineered to abrogate antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
The first-in-human trial of nivolumab anti-PD-1 provided seminal evidence that this treatment approach could potentially impact diverse cancer types including common epithelial cancers, with objective responses reported in patients with melanoma, kidney and colorectal cancer [Brahmer et al., 2010]. A transient tumor regression in one patient with NSCLC provided the impetus for investigating a larger NSCLC cohort in a follow-up multi-dose trial of nivolumab in multiple cancer types [Topalian et al., 2012]. Results from this trial showed notable objective response rates in patients with advanced treatment-refractory NSCLC (17%, n=129), RCC (27%, n=34), and melanoma (31%, n=107). Importantly, responses were quite durable with many persisting even after drug discontinuation, and long-term follow-up revealed OS of 9.9, 22.4, and 16.8 months, respectively [Topalian et al., 2014; McDermott et al., 2015]. These non-randomized data compared favorably to historical response rates in similar patient populations, spurring phase III testing of nivolumab in all three cancers. A recent phase III report showed the superiority of first-line nivolumab vs. standard chemotherapy in patients with advanced melanoma [Robert et al., 2014]. These findings have incentivized the aggressive clinical development of PD-1 pathway blocking drugs by multiple pharmaceutical and biotechnology companies (Table 1), and the clinical activity of these drugs in melanoma, RCC and NSCLC has been confirmed [Brahmer et al., 2012; Hamid et al., 2013; Herbst et al., 2014; Motzer et al., 2014]. However, the full activity spectrum of PD-1 pathway blocking drugs is not yet known, with recent evidence of efficacy in advanced chemotherapy-refractory bladder cancer [Powles et al., 2014], Hodgkin’s lymphoma [Ansell et al., 2014], head and neck, gastric, triple negative breast, and ovarian cancers.
Combination therapies based on PD-1 pathway blockade
Despite these promising results, the majority of patients treated with anti-PD-1/PD-L1 monotherapies do not achieve objective responses, and most tumor regressions are partial rather than complete. Animal models suggest that treatment combinations based on PD-1 pathway blockade may be synergistic, including anti-CTLA-4 or other checkpoint inhibitors, chemotherapy, tyrosine kinase inhibitors, focal irradiation, cancer vaccines, or immune agonist mAbs. Appropriate preclinical models are valuable in providing a basis for prioritizing clinical translation. A wide variety of treatment combinations are now under clinical development in diverse cancer types. Early and substantial tumor regressions observed with a combination of anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab) in advanced melanoma have garnered attention, although associated irAEs were also amplified [Wolchok et al., 2013]. Results from ongoing prospectively randomized trials will be needed to define the role of this treatment combination in melanoma and other cancers.
Biomarkers of response
As mentioned earlier, studies of peripheral blood have yielded pharmacodynamic evidence of global T cell activation in patients receiving anti-CTLA-4 [Maker et al., 2006], although these changes do not appear to correlate with clinical outcomes. Peripheral T cell activation does not occur to the same degree in patients receiving anti-PD-1/PD-L1 [Brahmer et al., 2010], as might be anticipated since the TME is thought to be the main site of activity of this pathway. Accordingly, tumor tissue has become the focal point for exploring potential biomarkers of response to anti-PD-1 drugs. Early studies revealed a correlation between pretreatment tumor cell expression of the ligand PD-L1 by immunohistochemistry (IHC), and the likelihood of response to anti-PD-1 [Brahmer et al, 2010; Topalian et al., 2012]. With the advent of several new automated PD-L1 IHC tests and interrogation of hundreds of patients with a variety of cancer types, a significant but not absolute relationship between PD-L1 expression in the TME and responsiveness to PD-1 pathway blockade has been confirmed. The potential importance of PD-L1 expression by infiltrating immune cells [Herbst et al., 2014], the presence and location of CD8+ tumor infiltrating lymphocytes [Tumeh et al., 2014], and other factors [Taube et al., 2014] are currently under intense study individually and in combination to discern more sensitive and specific predictors of clinical outcomes [reviewed in Lipson et al., 2015].
ON THE HORIZON: TARGETING NOVEL CHECKPOINTS
While antibody blockers of CTLA-4 and PD-1 are the focus of the clinical attention at this time, it is likely that blockade of additional checkpoints will result in even further clinical activity. This is because multiple checkpoints appear to be co-expressed with PD-L1 and PD-1 in tumors. We review here some of the most actively studied “next generation” checkpoint molecules for which antibody blockers are already in the clinic or soon to be tested in clinical trials, many in combination with anti-PD-1 or anti-PD-L1.
Lymphocyte activation gene 3 (LAG-3)
LAG-3 (CD223) is an immune checkpoint molecule expressed on activated T cells [Huard et al., 1994], NK cells [Triebel et al., 1990], B cells [Kisielow et al., 2005] and plasmacytoid dendritic cells [Workman et al., 2009]. Structurally, LAG-3 is highly homologous to the CD4 T cell co-receptor and lies proximal to the CD4 gene on human chromosome 12, but at the amino acid level it is less than 20% homologous to CD4, indicating that the two genes likely diverged early in evolution [Dijkstra et al., 2006]. The only known ligand for LAG-3 is MHC II [Huard et al., 1997], although its structural interactions with MHC II are more limited than those of CD4 [Fleury et al., 1991; Moebius et al., 1993]. Early studies showed that LAG-3 was selectively up-regulated on CD4+ Tregs [Huang et al., 2004]. Here, a LAG-3 blocking antibody mitigated Treg activity in vivo, and transfection of antigen-specific CD4 T cells with full length, but not truncated, LAG-3 could confer in vitro Treg function. More recent studies also suggest that LAG-3 blockade (or genetic knockout) affects the ability of conventional T cells (Tconv) to be suppressed by Tregs [Sega et al., 2014; Durham et al., 2014]. Additionally, LAG-3 has a CD8 T-cell-intrinsic role as LAG-3 blocking antibodies were found to augment CD8 T cell function in vivo in the absence of CD4 T cells [Grosso et al., 2007]. As described above, “exhausted” or dysfunctional T cells can express multiple immune checkpoint molecules, and LAG-3 and PD-1 are commonly co-expressed in models of chronic infection [Blackburn et al., 2009] as well as models of self-antigen recognition [Grosso et al., 2009]. These studies have been extended to human tumors in that a significant fraction of antigen-specific CD8 T cells in patients with ovarian cancer and melanoma co-express LAG-3 and PD-1 [Matsuzaki et al., 2010; Baitsch et al., 2012]. Evidence for synergistic immunosuppression mediated by LAG-3 and PD-1 comes from studies in which double-knockout mice were generated; although neither LAG-3 nor PD-1 single knockout animals succumb to autoimmunity, combined knockout results in multi-organ lymphocytic infiltration and early death [Woo et al., 2012]. Nearly identical results were obtained in models of autoimmunity [Okazaki et al., 2011], reinforcing the notion that LAG-3 and PD-1 are potentially synergistic in regulating T cell function. A role for dual blockade of LAG-3 and PD-1 in tumor immunity is suggested by studies in which most tumors implanted in PD-1/LAG-3 double knockout mice were rejected, while PD-1 single knockout mice showed delayed tumor growth. Similarly, combined antibody-mediated blockade of LAG-3 and PD-1 resulted in tumor rejection in several models, without any short-term evidence of autoimmune side effects. An anti-LAG-3 blocking mAb has recently entered clinical testing in cancer (NCT01968109), in a phase I trial that includes cohorts receiving anti-LAG-3 monotherapy or combination therapy with anti-PD-1.
Killer inhibitory receptors (KIRs)
Natural killer (NK) cells are a population of innate immune cells with well-documented roles in infectious and tumor immunity [Marcus et al., 2014]. Like activated CD8 T cells, NK cells mediate target cell apoptosis via secretion of preformed granules containing perforin and granzymes. However, unlike CD8 T cells, NK cells do not recognize unique peptides in the context of classical MHC I molecules. Instead, NK function is controlled by the complex interplay of a series of activating receptors and killer inhibitory receptors (KIRs) and their ligands. In humans, KIR molecules are polymorphic and bind to certain MHC I alleles, and not all KIR/ligand pairs are equally capable of inhibiting NK cell function. Indeed, bone marrow transplants in which donor NK cells lack the ability to be inhibited by host KIR ligands have been shown to result in lower relapse rates and improved OS, supporting the importance of this cell type in cancer immunity [Benson, Jr. and Caligiuri, 2014]. The relative importance of NK cells in murine models of cancer immunotherapy has been documented by multiple studies, but is especially highlighted by studies in which NK cell activation via IL-15 can eradicate fairly advanced tumors in the absence of CD8 T cells [Liu et al., 2012]. So, in a sense, KIRs can be thought of as immune checkpoint molecules, and blocking KIRs on NK cells could be exploited to augment anti-tumor immunity. To that end, a fully human anti-KIR mAb has entered clinical testing. This antibody (initially IPH-2101, Innate Pharma; now lirilumab, Bristol-Myers Squibb) binds to the human KIR molecules KIR2DL-1, KIR2DL-2, and KIR2DL-3 as well as to KIR2DS-1 and KIR2DA-2, preventing their binding to HLA-C MHC I molecules [Romagne et al., 2009]. A phase I trial of anti-KIR in acute myelogenous leukemia has been completed. Several studies in hematologic and solid cancers are ongoing, but of particular interest are trials in which lirilumab is being combined with anti-PD-1 (nivolumab; NCT01714739) or with anti-CTLA-4 (ipilimumab; NCT01750580). These trials are important in that each seeks to combine innate immune activation via anti-KIR with activation of the adaptive immune system, thus offering the potential for additive or synergistic anti-tumor efficacy.
B7-H3
B7-H3 (CD276) was initially identified using a bioinformatics approach in which human genome databases were queried for sequences with homology to previously identified B7 family members [Chapoval et al., 2001]. It is a type I transmembrane protein with single variable and constant immunoglobulin domains. B7-H3 mRNA is widely expressed in normal tissues [Sun et al., 2002], but protein expression is more restricted and is controlled by post-transcriptional mechanisms. The understanding of B7-H3 biology is complicated by the fact that it can be expressed on both immune and non-immune cells. On immune cells, B7-H3 appears to exert a stimulatory role: down-regulation of B7-H3 expression using anti-sense oligonucleotides inhibits T cell production of IFN-g [Chapoval et al., 2001]. Thus, B7-H3 might be considered not as a classical immune checkpoint molecule, but rather a co-stimulatory receptor more analogous to CD28. While this model is supported by numerous studies [Yi and Chen, 2009], several studies suggest an alternative model, in which B7-H3 down-modulates T cell activation. These studies include the finding that B7-H3 blocking antibodies exacerbate disease in the experimental autoimmune encephalomyelitis (EAE) murine model, as well as in several other models [Suh et al., 2003]. In terms of cancer immunity, there is a similar lack of clarity, in that the induction of expression of B7-H3 in tumor cell lines increases their immunogenicity and leads to more rapid rejection [Luo et al., 2004]. But in many human tumors, expression of B7-H3 in situ has been associated with poor outcome; this is especially notable in RCC and prostate cancer, where expression correlates with an increased risk of death [Crispen et al., 2008; Chavin et al., 2009]. Based on the notion that B7-H3 protein is over-expressed in multiple tumor types, a mAb with enhanced ADCC function has been developed [Loo et al., 2012] and has entered clinical trials (NCT01391143). This agent is not being deployed as an immune checkpoint blocking antibody, rather it is being tested as a traditional tumor-targeting antibody similar in concept to rituximab or trastuzumab.
T cell immunoglobulin and mucin-3 (TIM-3)
TIM-3 is an immune checkpoint molecule expressed on activated human T cells, NK cells and monocytes. TIM-3 knockout mice, similar to LAG-3 knockouts, do not develop overt autoimmunity [Sanchez-Fueyo et al., 2003], suggesting that TIM-3 and LAG-3 may have similarly subtle effects in modulating immune cell function. Consistent with this hypothesis, TIM-3 blockade accelerates the disease phenotype in murine models prone to developing autoimmunity, including non-obese diabetic (NOD) [Sanchez-Fueyo et al., 2003] and EAE models [Monney et al., 2002]. Functionally, TIM-3 binds to galectin-9 (as well as several other ligands), as supported by data showing that administration of galectin-9 in vitro causes cell death of Th1 cells in a TIM-3 dependent manner [Zhu et al., 2005]. Recent studies showed that TIM-3 is co-expressed with and binds to CECAM1, and that this interaction is important in TIM-3’s regulatory function [Huang et al., 2015]. In other work, the role of the TIM-3 immune checkpoint was studied in several murine cancer models [Sakuishi et al., 2010], including the CT26 colon carcinoma, 4T1 mammary carcinoma, and B16 melanoma. Interestingly, TIM-3 was nearly universally co-expressed with PD-1 on the majority of TILs. Co-expression of both checkpoint molecules reflected a more exhausted phenotype, functionally defined by a T cell’s reduced ability to proliferate and secrete IFN-g, IL-2 and TNF-a. Combined blockade was more effective in controlling tumour growth than blocking either checkpoint alone, confirming the notion that combined immune checkpoint blockade offers a potential treatment strategy for a wide variety of cancers, and that, besides CTLA-4 and LAG-3, other checkpoints might synergize with PD-1 to down-modulate T cell responses to tumours. Anti-human TIM-3 blocking antibodies have not yet entered the clinic but are under development.
V-domain Ig-containing Suppressor of T-cell Activation (VISTA)
VISTA is a relatively recently described negative regulator of T cell function [Wang et al., 2011]. Unlike PD-1 and CTLA-4, VISTA is predominantly expressed on myeloid and granulocytic cells, with only weak T cell expression in mice and humans [Lines et al., 2014; Wang et al., 2011]. Functionally, VISTA blockade attenuates tumour outgrowth, especially when combined with a cancer vaccine [Wang et al., 2011]. In terms of human cancers, VISTA expression has been described in colorectal tumors – here expression appears to be confined to CD11b+ cells, while expression on CD8 T cells was not detected [Lines et al., 2014]. These early studies are relatively limited in scope, and a more comprehensive analysis of VISTA expression in various human tumour types is warranted. In addition, the relative efficacy of VISTA blockade as compared to PD-1 or CTLA-4 blockade awaits the development of suitable reagents, but as is the case for the other checkpoint molecules discussed above, the notion that VISTA expression thus far appears to be selective for the myeloid compartment of tumors suggests the possibility of clinical effects distinct from those mediated by currently available checkpoint blocking antibodies, as well as the potential for additive or synergistic benefit.
T cell ImmunoGlobulin and ImmunoTyrosine inhibitory motif (ITIM) domain (TIGIT)
Like B7-H3, TIGIT was initially identified through a genomic search for structures shared among regulatory receptors, including a conserved ITIM motif [Yu et al., 2009]. Initial studies suggested that TIGIT functions by transmitting a negative signal to DCs, decreasing IL-12 secretion while simultaneously enhancing IL-10 levels. A more recent study, however, shows that TIGIT functions as an immune checkpoint, down-regulating proliferation of both murine and human T cells [Johnston et al., 2014]. The ligand for TIGIT is the poliovirus receptor (PVR), but PVR also binds to the T cell surface molecule CD226. In this way, TIGIT biology is perhaps reminiscent of the interaction between B7 molecules and CTLA-4/CD28: binding of PVR to TIGIT mediates an inhibitory signal, while binding of PVR to CD226 transmits a positive co-stimulatory signal to T cells. Blocking TIGIT with a specific mAb showed efficacy in both viral and tumor models – including an additive anti-tumor effect when both PD-L1 and TIGIT were blocked simultaneously. The relevance of these data to human cancer awaits future clinical development, but it is worth noting that genomic profiling studies showed that CD8a expression correlates closely with TIGIT expression in tissue from lung cancer patients [Johnston et al., 2014].
Indoleamine 2,3-dioxygenase (IDO)
Although not an immune checkpoint in the classical sense, several inhibitory pathways mediated by overexpression of IDO in various tumor types play an important role in down-regulating anti-tumor immunity [Prendergast et al., 2014]. As briefly mentioned above, IDO catabolizes the breakdown of tryptophan to kynurenine (and other metabolites). T cells require adequate tryptophan levels for survival and effector function, and thus IDO-mediated tryptophan deficiency results in T cell tolerance and lack of effector function, and promotes the differentiation of naïve CD4 T cells into Treg [Fallarino et al., 2006]. In addition, IDO expression in a relatively small population of tumor-associated DC allows the suppression of effector T cell responses [Mellor and Munn, 2004]. Both the IDO pathway inhibitor D-1MT and small molecule enzymatic inhibitors of IDO1 (INCB024360, NLG919) have entered clinical trials, and phase I data from a trial combining D-1MT (indoximod) with chemotherapy were recently published, demonstrating tolerability for the combination as well as evidence of antitumor activity [Solimon et al., 2014].
CONCLUSIONS
Recent years have seen a rapid expansion of our knowledge of immune regulation. Basic principles established in laboratory models of infection, autoimmunity and transplantation have proved to be transportable to human cancer, supporting the development of drugs modulating anti-tumor immunity. The successful application of the immune checkpoint blockers anti-CTLA-4 in melanoma, and anti-PD-1/PD-L1 in multiple cancer types, has established immunotherapy as a viable treatment option for patients with advanced cancers and has opened the doors to developing a new generation of immune modulators which may be most effective if employed in treatment combinations. Armed with new understanding and unprecedented opportunities, the field of immunotherapy is now standing on the threshold of great advances in the war against cancer.
ACKNOWLEDGEMENTS
The authors thank Dr. Julie Brahmer (Johns Hopkins University) for helpful discussions. This work was supported by research funding from Bristol-Myers Squibb (to S.L.T., C.G.D., and D.M.P.), the Melanoma Research Alliance (to S.L.T., C.G.D., and D.M.P.), the National Cancer Institute/NIH (R01 CA142779 to S.L.T. and D.M.P.; R01 CA154555 to C.G.D.), the Barney Family Foundation (to S.L.T.), the Laverna Hahn Charitable Trust (to S.L.T.), the Commonwealth Foundation (to D.M.P.), and Moving for Melanoma of Delaware (to S.L.T. and D.M.P.). S.L.T. and D.M.P. were also supported by a Stand Up To Cancer—Cancer Research Institute Cancer Immunology Translational Cancer Research Grant (SU2C-AACR-DT1012). Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
The authors have declared the following financial relationships. S.L.T.: research grants from Bristol-Myers Squibb and consulting for Five Prime Therapeutics, GlaxoSmithKline, and Jounce Therapeutics; C.G.D.: research grants from Bristol-Myers Squibb and Janssen; consulting for Bristol-Myers Squibb, Compugen, Janssen, Novartis, and Roche/Genentech; stock options in Compugen, ImmuneXcite, NexImmune, and Potenza Therapeutics; and patent royalties through his institution, Bristol-Myers Squibb and Potenza. D.M.P.: research grants from Bristol-Myers Squibb; consulting for Five Prime Therapeutics, GlaxoSmithKline, Jounce Therapeutics, MediImmune, Pfizer, Potenza Therapeutics, and Sanofi; stock options in Jounce and Potenza; and patent royalties through his institution, Bristol-Myers Squibb and Potenza.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS: All authors contributed to the design and writing of this manuscript.
REFERNCES
- Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J. Med. 2014 doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C, Bouzourene H, Rimoldi D, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7:e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Benson DM, Jr, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res. 2014;2:99–104. doi: 10.1158/2326-6066.CIR-13-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly J, Picus J, Sharfman W, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti22 programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQM, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Hauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CA, Sullivan TJ, Truong T, Allison JP. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur J Immunol. 1998;28:3137–3143. doi: 10.1002/(SICI)1521-4141(199810)28:10<3137::AID-IMMU3137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- Chavin G, Sheinin Y, Crispen PL, Boorjian SA, Roth TJ, Rangel L, Blute ML, Sebo TJ, Tindall DJ, Kwon ED, et al. Expression of immunosuppresive B7-H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res. 2009;15:2174–2180. doi: 10.1158/1078-0432.CCR-08-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich BC, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14:5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- Dijkstra JM, Somamoto T, Moore L, Hordvik I, Ototake M, Fischer U. Identification and characterization of a second CD4-like gene in teleost fish. Mol Immunol. 2006;43:410–419. doi: 10.1016/j.molimm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Durham NM, Nirschl CJ, Jackson CM, Elias J, Kochel CM, Anders RA, Drake CG. Lymphocyte Activation Gene 3 (LAG-3) Modulates the Ability of CD4 T-cells to Be Suppressed In Vivo. PLoS One. 2014;9:e109080. doi: 10.1371/journal.pone.0109080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J Exp Med. 1998;188:205–210. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- Fleury S, Lamarre D, Meloche S, Ryu SE, Cantin C, Hendrickson WA, Sekaly RP. Mutational analysis of the interaction between CD4 and class II MHC: class II antigens contact CD4 on a surface opposite the gp120-binding site. Cell. 1991;66:1037–1049. doi: 10.1016/0092-8674(91)90447-7. [DOI] [PubMed] [Google Scholar]
- Fong L, Kwek SS, O’Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- Fu J, Malm IJ, Kadayakkara DK, Levitsky H, Pardoll DM, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74:4042–4052. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso JF, Goldberg MV, Getnet D, Bruno TC, Yen HR, Pyle KJ, Hipkiss E, Vignali DA, Pardoll DM, Drake CG. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182:6659–6669. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De MA, Anders R, Netto G, Getnet D, Bruno TC, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Egen JG, Lämmermann T, Kastenmüller W, Torabi-Parizi P, Germain RN. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014;40:235–247. doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard B, Gaulard P, Faure F, Hercend T, Triebel F. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics. 1994;39:213–217. doi: 10.1007/BF00241263. [DOI] [PubMed] [Google Scholar]
- Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, Maigret B, Dreano M, Triebel F. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci U S A. 1997;94:5744–5749. doi: 10.1073/pnas.94.11.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielow M, Kisielow J, Capoferri-Sollami G, Karjalainen K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur J Immunol. 2005;35:2081–2088. doi: 10.1002/eji.200526090. [DOI] [PubMed] [Google Scholar]
- Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, Krainer M, Houede N, Santos R, Mahammedi H, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Broan JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, Ceeraz S, Suriawinata AA, Yan S, Ernstoff MS, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014;74:1924–1932. doi: 10.1158/0008-5472.CAN-13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- Lipson EJ, Forde PM, Hammers HG, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015 doi: 10.1053/j.seminoncol.2015.05.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RB, Engels B, Arina A, Schreiber K, Hyjek E, Schietinger A, Binder DC, Butz E, Krausz T, Rowley DA, et al. Densely granulated murine NK cells eradicate large solid tumors. Cancer Res. 2012;72:1964–1974. doi: 10.1158/0008-5472.CAN-11-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The Vigorous Immune Microenvironment of Microsatellite Instable Colon Cancer Is Balanced by Multiple Counter-Inhibitory Checkpoints. Cancer Discov. 2014 doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, Burke S, Ciccarone V, Li H, Yang Y, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res. 2012;18:3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, Lau JS, Dong H, Tamada K, Flies AS, et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol. 2004;173:5445–5450. doi: 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Neal J, Lu H, Cuillerot JM, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Hughes M, Yellin MJ, Haworth LR, Levy C, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29:455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, Wang L, Shifrin N, Raulet DH. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DM, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, Brahmer JR, Carvajal RD, Hammers HJ, Puzanov I, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.1041. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Moebius U, Pallai P, Harrison SC, Reinherz EL. Delineation of an extended surface contact area on human CD4 involved in class II major histocompatibility complex binding. Proc Natl Acad Sci U S A. 1993;90:8259–8263. doi: 10.1073/pnas.90.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoquchi A, Hiai H, Minato N, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol. Immunother. 2014;63:721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, Sebastian M, Lu H, Cuillerot JM, Lynch TJ. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- Ribas A, Kefford R, Marshall MA, Punt CJA, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2014 doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- Romagne F, Andre P, Spee P, Zahn S, Anfossi N, Gauthier L, Capanni M, Ruggeri L, Benson DM, Jr, Blaser BW, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analyysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metatatic melanoma. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.56.2736. pii: JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- Sega EI, Leveson-Gower DB, Florek M, Schneidawind D, Luong RH, Negrin RS. Role of lymphocyte activation gene-3 (Lag-3) in conventional and regulatory T cell function in allogeneic transplantation. PLoS One. 2014;9:e86551. doi: 10.1371/journal.pone.0086551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chaen T, Srinivasan M, Korman AJ. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, Yagita H, Chen L, Powell J, Pardoll DM, et al. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198:31–38. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Yoshimura K, Shin T, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, Scher HI, Chin K, Gagnier P, McHenry MB, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman HH, Jackson E, Neuger T, Dees EC, Harvey RD, Han H, Ismail-Khan R, Minton S, Vahanian NN, Link C, et al. A first in man phase I trial of the oral immunomodulator, indoximod, combined with docetaxel in patients with metastatic solid tumors. Oncotarget. 2014;5:8136–8146. doi: 10.18632/oncotarget.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1 IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014 doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, Johnson NA, Zhao Y, Telenius A, Neriah SB, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471:377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4:899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Sharpe AH. Balance and imbalance in the immune system: life on the edge. Immunity. 2014;41:682–684. doi: 10.1016/j.immuni.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, Lu LF, Gondek D, Wang Y, Fava RA, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Weber JS. Current perspectives on immunotherapy. Semin Oncol. 2014;41(Suppl 5):S14–S29. doi: 10.1053/j.seminoncol.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe’ C, Maio M, Binder M, Bohnsack O, Nichol G, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman CJ, Wang Y, El Kasmi KC, Pardoll DM, Murray PJ, Drake CG, Vignali DA. LAG-3 regulates plasmacytoid dendritic cell homeostasis. J Immunol. 2009;182:1885–1891. doi: 10.4049/jimmunol.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Yu S, Zhu B, Bedoret D, Bu X, Francisco LM, Hua P, Duke-Cohan JS, Umetsu DT, Sharpe AH, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211:943–959. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, et al. Ipilimumab (anti-CTLA-4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev. 2009;229:145–151. doi: 10.1111/j.1600-065X.2009.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]