Abstract
Our objective was to determine if increased cardiovascular (CV) stiffness is associated with disability in middle-aged and older adults at risk for congestive heart failure. CV stiffness (brachial pulse pressure/left ventricular stroke volume indexed to body surface area) and total disability (the summed assessment of activities of daily living, mobility, and instrumental activities of daily living) were measured in 445 individuals. A subset of 109 randomly selected individuals also underwent physical function testing. Total disability was associated with CV stiffness (p = .01), driven by an association with mobility (p = .005), but not activities of daily living (p = .13) or instrumental activities of daily living (p = .61). After accounting for age, these correlations remained significant for men (p = .04), but not for women. CV stiffness was also associated with increased 400-m walk time (p = .02). In middle-aged and elderly men at risk for congestive heart failure, CV stiffness is associated with decreased mobility and physical function, and increased overall disability.
Key Words: Cardiovascular stiffness, Disability, Congestive heart failure.
Physical disability reduces quality of life, increases health care costs (due to increased need for support services), and is an independent predictor of mortality (1,2). Mobility and activities of daily living are necessary for maintaining basic independent functioning in middle-aged and older individuals (3). Therapy targeted to attenuate the factors that promote disability could improve quality of life and prognosis in older individuals. Such therapy could provide a mortality benefit, as it has been shown in the elderly individual that all levels of physical activity are associated with lower risk of incident acute myocardial infarction, stroke, and cardiovascular (CV) mortality (4).
Previously, it has been shown that abnormally increased cardiac and aortic stiffness is independently associated with reduced peak exercise capacity in elderly individuals with and without left ventricular (LV) systolic dysfunction and congestive heart failure (CHF) (5–10). We hypothesized that similar to exercise capacity, increased CV stiffness would be associated with increased disability in middle-aged and elderly individuals at risk for their first episode of symptomatic CHF. To test this hypothesis, we noninvasively assessed CV stiffness (using CV magnetic resonance or CMR) and disability. Additionally, in a randomly selected subgroup of individuals, we formally measured physical function.
Methods
Study Population and Design
The study was approved by the institutional review board of the Wake Forest School of Medicine, and each participant provided witnessed informed consent. A total of 445 consecutive participants from the National Institutes of Health–funded cohort study, “Pulmonary Edema and Stiffness of the Vascular System” (PREDICT) were enrolled. PREDICT was designed to identify abnormalities of the CV system that forecast the first onset of symptomatic heart failure. To accomplish this purpose, middle-aged and elderly individuals (aged 55–85 years) with risk factors for CHF were recruited from rural western North Carolina to undergo a collection of historical, physical exam, laboratory, and CMR data. Each component of the data acquisition was accomplished by personnel blinded to other components of the study. Hypertension, diabetes, or coronary artery disease (CAD), risk factors for the first onset of symptomatic heart failure were identified and recorded. For the purpose of this study, hypertension was defined according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC VII) as a systolic blood pressure of greater than or equal to 140mm Hg, a diastolic blood pressure greater than or equal to 90mm Hg, or the concurrent use of antihypertensive medications (11). Diabetes was defined, according to guidelines of the American Diabetic Association, as a random plasma glucose concentration greater than or equal to 200mg/dL (11.1 mmol/L), fasting plasma glucose level of greater than or equal to 126mg/dL (7.0 mmol/L), or concurrent receipt of antiglycemic treatment (12). CAD was defined in accordance with American College of Cardiology/American Heart Association guidelines (13).
The study excluded those not suitable for CMR testing due to any of the following: (a) pacemakers, defibrillators, functioning neural stimulator devices, or other implanted electronic devices, (b) ferromagnetic cerebral aneurysm clips, or other intraorbital and intracranial metal, (c) an allergy to gadolinium or other severe drug allergies, (d) acute myocardial infarction within 4 months, (e) moderate or severe valvular stenosis or regurgitation, (f) claustrophobia, (g) closed angle glaucoma, (h) participants unable to provide informed consent, (i) renal dialysis (subjects with eGFR <60 mL/min were eligible but did not receive gadolinium), (j) those with a LV ejection fraction less than 25%, (k) those with FEV1 less than 0.5, or (l) those with chronic obstructive pulmonary disease receiving home oxygen and/or a nebulizer. Additionally, those with antecedent diagnosis of CHF, as defined according to American College of Cardiology/American Heart Association guidelines, were excluded from this study (14).
Anthropometric measurements including weight and height were performed in loose clothing without shoes. Laboratory assessments including fasting serum electrolytes, creatinine, glucose, lipids, and C-reactive protein were acquired, and then, each participant underwent a CMR exam. During the CMR exam, images were acquired to determine LV volumes and CV stiffness. On the same day as the CMR study, participants completed the Pepper Assessment Tool for Disability (PAT-D) questionnaire (15), and a randomly selected subset of 109 participants underwent formal physical function testing.
CMR Assessment of CV Stiffness
Each patient was imaged on an Avanto 1.5T (Siemens Medical Solutions, Malvern, PA) whole-body imaging system utilizing a phased-array cardiac surface coil placed on the chest. Multislice steady-state free precession images with a temporal resolution of 40ms were acquired and used to calculate LV volumes according to Simpson’s rule formula (16). These slices were 8-mm thick with a 2-mm gap and were positioned perpendicular to the long axis of the ventricle, spanning base to apex. Image parameters were as follows: 256 × 192 matrix, a 38-cm field of view, a 35-degree flip angle, a 14-ms repetition time, and a 6.7-ms echo time. Brachial pulse pressure was acquired during image acquisition (Invivo Monitoring Systems, Gainesville, FL). According to previously published techniques (17–19), CV stiffness was calculated as brachial pulse pressure divided by the LV stroke volume indexed to body surface area (PP/LVSVi) (20).
Pulse Wave Velocity
A subset of 390 individuals underwent measurement of pulse wave velocity as another index of arterial stiffness. Pulse wave velocity was not obtained in all patients because access to the technology to measure this was not available until after the study protocol was initiated. All images were analyzed according to previously published techniques (9,21,22) and were transferred to a processing work station where the boundary of the aortic lumen was defined on the magnitude image of the reference scan by manually tracing a region of interest. To precisely trace the boundary of the flow lumen, each image was magnified by 400%–800% (21,22).
Relative Wall Thickness
Relative wall thickness (RWT) was measured for each participant using the formula septal wall thickness + posterior wall thickness divided by LV diastolic diameter. This formula is recommended when septal asymmetry (intraventricular septum thickness/posterior wall thickness [IVST/PWT] > 1.3) is present (23).
PAT-D Questionnaire
The PAT-D (15), a 19-item self-report disability questionnaire that was developed and refined at the Wake Forest University Claude D. Pepper Older Americans Independence Center, was implemented to assess difficulty with daily function in society (24–27). The PAT-D has been widely used in randomized controlled trials and observational studies in a variety of chronic health conditions (15). In addition to being a valid measure with excellent psychometric properties, in previous intervention studies, it has been shown to be sensitive to change (15). The PAT-D assesses the following three standard domains of function and disability: (a) ADL, such as eating, toileting, and bathing; (b) mobility, such as walking several blocks; and (c) instrumental activities of daily living (IADL), such as answering the telephone and paying bills. The questionnaire asks respondents how much difficulty they have had with a range of activities in the past month and if they believe any perceived difficulties were related to their health. For each item, respondents answer whether they (a) have no difficulty, (b) have a little difficulty, (c) have some difficulty, (d) have a lot of difficulty, (e) are unable to do, or a box can be checked that reads “usually did not do for other reasons.” Independent scores for ADL, mobility, and IADL were obtained by averaging the scale score of the questions pertaining particularly to those subsets. A summary score, an indication of a person’s overall perceived disability, was calculated as a scaled mean of all 19 questions.
Physical Function Testing
The following physical function tests were completed by a subset of 109 randomly selected study participants. Due to funding, roughly 25% of the participants were randomly selected to participate in the performance measures of physical function.
Gait speed: Each participant completed two, timed 4-m walk tests, and the time to complete each trial was measured to the nearest 0.01 second using a stopwatch. Gait speed was calculated by dividing 4 m by the time in seconds of the shorter of the two trials (28–30).
Isometric leg strength: Isometric strength was measured in Newton-meters (torque) for hip flexion, hip extension, knee flexion, and knee extension by using strain gauges connected to a computerized data-collecting unit (31). The highest recorded measurement during the last 3 seconds of effort was used in analyses. During measurement, the knee extension angle was 120 degrees and the knee flexion angle was 135 degrees. The hip flexion and extension angles were approximately 90 degrees. Each participant completed two trials for each leg, and the average of all four trials was used for statistical analysis.
Leg extensor power: Power was measured in watts with a Nottingham Power Rig (32). The seated participant pressed a footplate as hard and fast as possible. Seat position was adjusted so that the knee angle at the start of the push was 90 degrees. The measurement was repeated for at least five efforts for each leg until no further improvements were seen. Verbal encouragement and visual feedback were given, and the best power output for each leg was recorded. The average of all 10 trials was used for statistical analysis.
400-m walk time: Participants were asked to walk 400 m at their usual pace without any assistive devices and without overexerting themselves, as described by Roland and colleagues (33). During the walk, participants were allowed to rest if they needed by standing in one place without sitting. They were instructed to resume walking as soon as they were able to do so. If they were unable to continue after a 60-second rest stop or if they needed to sit down, the test was discontinued. There were no limits to the number of allowable rest stops, as long as the participant could complete the walk within 15 minutes without sitting. The test was discontinued after 15 minutes, a time that corresponds to a slow walking speed (0.45 m/s), and that translates into a walking capacity that has little utility in daily life (34).
We chose the previously mentioned physical function tests because they are descriptors of mobility and quantify physical performance and decline over time. Gait speed is one of three tests that make up the Short Physical Performance Battery, which is a well-studied universal standard for measuring physical function (26). The 400-m usual-pace walk test was implemented because, compared with other commonly used walk tests such as the 6-minute walk, the 400-m usual-pace walk test assesses disability as opposed to exercise tolerance (33). Leg extensor power has been shown to strongly mediate the variance in gait speed in nursing home residents, and in community-dwelling elderly individuals, it is a strong independent predictor of performance-based measures of physical function (34–38). Finally, reductions in muscle strength have long been identified as important factors leading to limitations in mobility and are associated with reduced gait speed (39–41). For this reason, isometric leg strength was measured. Additionally, it has been shown that isometric and isokinetic measurements of muscle strength strongly correlate in various patient populations (42,43).
Statistical Analysis
Categorized data were summarized by percentages. Comparison of proportions between groups was tested for significance using the Fisher exact test. Continuous data were presented as mean ± standard deviation, and intergroup comparisons were performed using Student’s t test. Measurements from the LV volumes were used to determine CV stiffness (PP/LVSVi) as described previously. In all patients, associations between PP/LVSVi or pulse wave velocity (PWV) and overall PAT-D score, ADL score, mobility score, and IADL score were estimated and tested using Spearman’s rank correlations. The estimate of group effect was made after controlling for age, then stratifying by gender. In the subset of 109 randomly selected individuals who underwent physical function testing, associations between PP/LVSVi and physical function test score for gait speed, isometric leg strength, and leg extensor power were estimated and tested using Spearman’s rank correlations. Again, the estimate of group effect was made after controlling for age, then stratifying by gender. Mediation analysis was conducted using linear regression. Additionally, PAT-D data were stratified by tertile of CV stiffness to show trends, and a p-value was calculated testing the continuous linear trend with CV stiffness as well as ordinal trend test comparing the means in the first and third tertile. Physical performance testing (PPT) data were also stratified by tertile of CV stiffness to show trends, and a p-value was calculated using the continuous correlation between the continuous measure of CV stiffness and the continuous measure of disability. The statistical comparisons were two tailed, and p values less than .05 were considered statistically significant.
Results
Demographic data of the study participants are shown in Table 1. Men and women exhibited similar age, body mass index, and medication use, and had similar prevalence of diabetes mellitus, hypertension, and dyslipidemia. More women were black than men (p = .001). CAD and smoking were more prevalent among the men (p < .001 and p = .02, respectively). Mean resting systolic blood pressure was similar in men and women; however, mean resting diastolic blood pressure was higher in men (p = .001), and mean heart rate was higher in women (p = .003).
Table 1.
Baseline Characteristics of PREDICT Participants
| All (n = 445) | Men (n = 213) | Women (n = 232) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) | 69±8 | 69±8 | 69±8 | 1.00 |
| 55–64 | 129 (36%) | 77 (36%) | 75 (32%) | .42 |
| 65–74 | 126 (35%) | 68 (32%) | 93 (40%) | .08 |
| 75+ | 106 (30%) | 68 (32%) | 64 (36%) | .35 |
| Race | ||||
| Caucasian | 293 (79%) | 182 (85%) | 171 (74%) | .002 |
| Black | 70 (19%) | 25 (12%) | 56 (24%) | .001 |
| Hispanic | 5 (1%) | 2 (0.9%) | 4 (2%) | .69 |
| Other | 4 (1%) | 4 (1.9%) | 1 (0.4%) | .20 |
| Body mass index (kg/m2) | 30±6 | 30±5 | 31±7 | 1.00 |
| Historical data | ||||
| Coronary artery disease | 121 (27%) | 83 (39%) | 38 (16%) | <.001 |
| Diabetes mellitus | 172 (39%) | 86 (40%) | 86 (37%) | .50 |
| Hypertension | 408 (92%) | 191 (90%) | 217 (94%) | .17 |
| Dyslipidemia | 230 (52%) | 107 (50%) | 123 (53%) | .57 |
| Smoker | 196 (44%) | 106 (50%) | 90 (39%) | .02 |
| Selected medications | ||||
| β-Blocker | 182 (41%) | 87 (41%) | 95 (41%) | 1.00 |
| Calcium-channel blocker | 125 (28%) | 62 (29%) | 63 (27%) | .67 |
| Nitrate | 24 (5%) | 11 (5%) | 13 (6%) | 1.00 |
| ACE inhibitor/ARB | 318 (71%) | 150 (70%) | 168 (72%) | .67 |
| Statin | 339 (76%) | 164 (77%) | 175 (75%) | .74 |
| Resting SBP, mm Hg | 141±18 | 141±16 | 142±19 | .55 |
| Resting DBP, mm Hg | 79±13 | 81±12 | 77±13 | .001 |
| Resting heart rate, bpm | 65±11 | 63±10 | 66±11 | .003 |
Note: Values are expressed as mean ± standard deviation or percent (%). ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; bpm = beats per minute; DBP = diastolic blood pressure; PREDICT = Pulmonary Edema and Stiffness of the Vascular System; SBP = systolic blood pressure.
LV and CV stiffness data are shown in Table 2. LV volumes and mass were higher in men, except for resting LV stroke volume index, which was similar in men and women. Women had a higher LV ejection fraction (p < .001), resting pulse pressure (p = .003), and CV stiffness (p = .01).
Table 2.
Cardiac Magnetic Resonance, Pepper Assessment Tool for Disability, and Physical Function Testing Data
| All Subjects (n = 445) | Men (n = 213) | Women (n = 232) | p Value (men vs women) | |
|---|---|---|---|---|
| Resting EF (%) | 63±8 | 61±8 | 66±7 | <.001 |
| Resting pulse pressure (mm Hg) | 62±17 | 59±14 | 64±18 | .003 |
| LV mass | 133±35 | 152±39 | 115±24 | <.001 |
| Resting EDV | 120±35 | 131±31 | 110±35 | <.001 |
| Resting ESV | 45±19 | 52±19 | 37±15 | <.001 |
| Resting SV | 75±19 | 80±20 | 71±18 | <.001 |
| LV mass index | 67±13 | 73±14 | 62±11 | <.001 |
| Resting EDV index | 61±15 | 63±14 | 59±16 | .01 |
| Resting ESV index | 23±9 | 25±10 | 20±8 | <.001 |
| Resting SV index | 38±9 | 38±9 | 38±9 | .00 |
| Resting PP/SV index | 1.69±0.55 | 1.62±0.51 | 1.75±0.58 | .01 |
| Overall PAT-D | 1.53±0.59 | 1.44±0.57 | 1.61±0.60 | .001 |
| ADL | 1.35±0.50 | 1.31±0.51 | 1.39±0.49 | .09 |
| Mobility | 1.85±0.91 | 1.63±0.79 | 2.05±0.96 | <.001 |
| IADL | 1.41±0.66 | 1.39±0.67 | 1.43±0.64 | .52 |
| Subgroup Analysis (n = 109) | Men (n = 48) | Women (n = 61) | ||
| Leg extensor power (watts) | 76±42 | 98±43 | 59±32 | <.001 |
| Leg isometric strength (Newton-meter) | 76±30 | 96±28 | 60±21 | <.001 |
| Gait speed (m/s) | 1.1±0.2 | 1.1±0.2 | 1.0±0.2 | .02 |
| 400-m walk time (s) | 388±81 | 366±85 | 403±83 | .02 |
Note: Values are expressed as mean ± standard deviation. ADL = activities of daily living; EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; IADL = instrumental activities of daily living; LV = left ventricular; PAT-D = Pepper Assessment Tool for Disability; SV = stroke volume.
Mean overall PAT-D score and mean score for ADL, mobility, and IADL are also shown in Table 2. Women exhibited a higher mean overall score reflective of greater disability relative to men (p = .002). Women also possessed higher mobility scores relative to men (p < .001). There was no difference between men and women with regard to their ADL or IADL scores. Table 2 also displays mean PPT scores in men and women. Consistent with the disability questionnaire-derived data, women were generally more disabled than men. Men had greater leg extensor power and isometric strength (both p < .001), a faster walk speed (p = .02), and a shorter walk time (p = .04) than women.
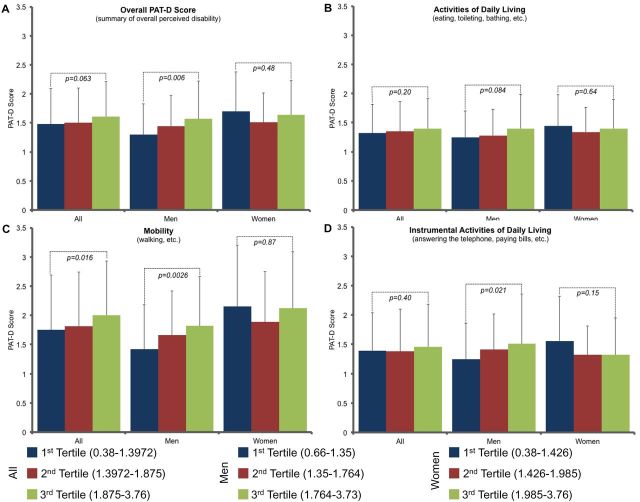
Figure 1 illustrates overall PAT-D score, ADL score, mobility score, and IADL score divided by tertile of CV stiffness in all subjects, and then separately for men and women. Pairwise comparisons between the tertiles were calculated. In all subjects, there was a nonsignificant trend between increased CV stiffness and overall PAT-D score (p = .063; Figure 1A). When subdivided by domain, this relationship was observed with mobility (p = .016; Figure 1C), but not ADL or IADL. Figure 1A also illustrates a significant increase in overall PAT-D score in men with increasing CV stiffness (p = .006), but not in women. A similar trend was found in men with regard to mobility score and increasing CV stiffness (p = .0026; Figure 1C), but not in women. There were no significant trends between ADL score and CV stiffness in men or women (Figure 1B). There was no significant trend between IADL score and CV stiffness in women, but there was a trend in men (p = .021; Figure 1D).
Figure 1.
Pepper Assessment Tool for Disability (PAT-D) data by tertile of cardiovascular (CV) stiffness in all subjects, men, and women. (A) CV stiffness vs overall PAT-D score. A trend is seen in all subjects (p = .063), and a significant trend is seen in men (p = .006). (B) CV stiffness vs ADL score. No significant trends were identified. (C) CV stiffness vs mobility score. A significant trend between increasing CV stiffness and mobility score was seen in all subjects (p = .016) and in men (p = .0026), but not in women. (D) CV stiffness vs IADL. A significant trend was seen in men (p = .021), but not in women.
Similar analysis was completed for the 109 randomly selected individuals who underwent physical function testing and is shown in Table 3. p-Values illustrate a trend between continuous CV stiffness and the disability measure. There was an increasing trend between CV stiffness and gait speed, and 400-m walk time in all individuals (p = .011 and p = .002, respectively). When stratified by gender, this relationship remained significant in men (p = .044 and p = .002, respectively), but not in women (p = .28 and p = .30, respectively).
Table 3.
Pepper Assessment Tool for Disability Data and Physical Function Testing Data by Tertile of Cardiovascular Stiffness (all = 109, men = 48, and women = 61)
| First Tertile | Second Tertile | Third Tertile | p Value* | ||
|---|---|---|---|---|---|
| Leg extensor power (watts) | All | 90±51 | 76±36 | 69±36 | .27 |
| Men | 112±38 | 99±38 | 92±45 | .12 | |
| Women | 55±39 | 59±31 | 59±25 | .65 | |
| Leg isometric strength (Newton-meter) | All | 85±28 | 76±31 | 70±31 | .15 |
| Men | 95±27 | 95±27 | 98±31 | .71 | |
| Women | 67±25 | 57±17 | 56±20 | .080 | |
| Gait speed (m/s) | All | 1.12±0.18 | 1.06±0.18 | 1.03±0.18 | .011 |
| Men | 1.16±0.18 | 1.14±0.17 | 1.06±0.21 | .044 | |
| Women | 1.05±0.19 | 1.03±0.18 | 1.00±0.14 | .28 | |
| 400-m walk time (s) | All | 356±70 | 337±88 | 403±88 | .002 |
| Men | 339±58 | 370±149 | 399±99 | .002 | |
| Women | 386±74 | 396±84 | 406±76 | .30 |
Notes: Values are expressed as mean ± standard deviation.
*Trend test between continuous cardiovascular stiffness and disability measure.
Further adjusted analysis is shown in Table 4. After accounting for age, there was a trend toward a correlation between CV stiffness and total overall PAT-D score (p = .10), whereas the correlation with the PAT-D mobility score remained significant (p = .04). After stratifying by gender, the correlation between CV stiffness and overall PAT-D score was significant in men (rho = 0.138, p = .04), but not in women (rho = −0.037, p = .58; Figure 1A). The correlation between CV stiffness and mobility score was also significant for men (rho = 0.134, p = .05), but not for women (rho = −0.004, p = .95; Table 4). There was no significant correlation between CV stiffness and either ADL or IADL score in either men or women, even after accounting for age. After accounting for CAD, dyslipidemia, diabetes mellitus, and smoking history, a strong trend between CV stiffness and mobility score remained (p = .06–.10), and the percent mediation for these three variables combined was only 26%. After accounting for age, CV stiffness trended toward an association with walk speed (p = .08) and was associated with the 400-m walk time (p = .02; Table 3). After stratifying for gender, this correlation was present in men (p = .03), but not in women (p = .53).
Table 4.
Correlations and Partial Correlations Between Cardiac Stiffness and Self-reported and Measured Physical Function: the PREDICT Study
| PP/SVi | |||
|---|---|---|---|
| Adjusted for age | All Subjects (n = 445) | Men (n = 213) | Women (n = 232) |
| Total overall | 0.078 | 0.138 | −0.037 |
| p = .10 | p = .04 | p = .58 | |
| Total ADL | 0.032 | 0.059 | −0.034 |
| p = .50 | p = .39 | p = .61 | |
| Total mobility | 0.096 | 0.134 | −0.004 |
| p = .04 | p = .05 | p = .95 | |
| Total IADL | −0.001 | 0.064 | −0.107 |
| p = .86 | p = .35 | p = .10 | |
| Adjusted for age | Subgroup Analysis (n = 109) | Men (n = 48) | Women (n = 61) |
| Leg extensor power (watts) | −0.083 | −0.071 | 0.059 |
| p = .39 | p = .63 | p = .65 | |
| Isometric leg strength (Newton-meter) | −0.121 | 0.120 | −0.152 |
| p = .21 | p = .29 | p = .24 | |
| 4-m gait speed (m/s) | −0.173 | −0.157 | −0.085 |
| p = .08 | p = .29 | p = .53 | |
| 400-m walk time (s) | 0.227 | 0.322 | 0.094 |
| p = .02 | p = .03 | p = .53 | |
Note: Correlations are Spearman’s nonparametric rank correlations. ADL = activities of daily living; IADL = instrumental activities of daily living; PAT-D = Pepper Assessment Tool for Disability; PREDICT = Pulmonary Edema and Stiffness of the Vascular System.
In the subset of 390 patients undergoing PWV measures, there were nonsignificant trends between PWV and overall PAT-D score (rho = 0.096, p = .058) and between PWV and mobility score (rho = 0.084, p = .097).
The mean RWT for the entire population was 0.43±0.22 cm. The mean RWT for men was 0.45±0.16 cm, and the mean RWT for women was 0.41±0.26 cm. The mean LV mass index (g/m2) for the entire population was 66±13. The mean LV mass index (g/m2) was 72±14 for the men and 61±10 for the women. Using Spearman’s rank correlation, there was no significant association between RWT and any of the disability measures (p = .25–.88) in either men or women. From this data, it does not appear that concentric remodeling contributes to disability in either men or women.
Discussion
The results of this study indicate that in middle-aged and elderly patients at risk for but yet to experience symptomatic heart failure: (a) resting CV stiffness is higher in women than men; (b) CV stiffness is associated with increased overall disability as measured by PAT-D score (this association persists after accounting for age in men but not in women, and is driven by an association with decreased mobility as opposed to disability associated with ADL or IADL); (c) CV stiffness is associated with increased walk time (this association persists after accounting for age in men but not in women); and (d) in men, the relationship between CV stiffness and disability is only partially accounted for by traditional risk factors.
Several demographic features of the study participants including body mass index, medical history, and CV medication use were similar to those found in other studies evaluating middle-aged and older adults both at risk for or with a diagnosis of compensated CHF (5,44–48). There were, however, some differences in our population of 445 individuals relative to these other previously studied populations. Our study included a higher percentage of women aged 65 years and older, with more of these women being of black race. In addition, the majority of our study population (92%) was hypertensive.
The resting CV stiffness and mobility scores identified in the participants in this study are similar to those reported previously in other populations of elderly individuals (44,45). With regard to cardiac metrics, women exhibited a higher LV ejection fraction (p < .001) and CV stiffness (p = .01). Similar to other studies (33,48), men had lower overall and mobility PAT-D scores (p = .001, p < .001) and higher physical function testing scores (range: p = .02 to p < .001), indicative of less disability and increased strength, compared with women.
Many authors have shown that both CV and aortic stiffness increase with age (34,49–51), and several studies have shown that independent of this age-related change, increased vascular stiffness is associated with reduced exercise capacity (5–9). An association between CV stiffness and gait speed has been shown in individuals with peripheral arterial disease (52). Additionally, an association has been shown between pulse pressure and long-distance gait speed in individuals with known poor functional performance and sedentary lifestyle (53). We hypothesized that a similar relationship could exist between CV stiffness and disability in elderly individuals at risk for but yet to develop heart failure. We found a positive relationship between increasing CV stiffness and overall PAT-D score. This association was driven largely by an inverse association between CV stiffness and mobility, as there was no association of CV stiffness with ADL or IADL score.
After adjusting for age and stratifying by gender, a significant correlation was found between increasing CV stiffness and mobility score in men (p = .05), but not in women. Similar findings were observed in the randomly selected subset of individuals who underwent physical function testing. In all subjects independent of age, walk speed decreased (p = .08) and walk time increased (p = .02) as CV stiffness increased. These data suggest that CV stiffness may play a role in the pathogenesis of disability in men.
In our regression model, we included other variables that previously have been shown to contribute to CV stiffness. In these analyses, only 25% of the relationship between disability and CV stiffness could be accounted for by the combined effects of CAD, dyslipidemia, diabetes mellitus, and smoking history. These results suggest that other nontraditional variables, which affect CV stiffness, may play a larger role in the relationship between CV stiffness and disability in men.
In this study, we wanted to explore the relationship between CV stiffness and physical function, or functional limitation, rather than exercise capacity. Because of this we utilized validated measures of disability, such as the Short Physical Performance Battery, isometric leg strength, leg extensor power, and 400-m usual walk pace. We did not intend to make an association between CV stiffness and either aerobic or anaerobic exercise capacity. We found the association between CV stiffness and disability was related to mobility function, not ADL or IADL. The nature of our study does not prove a causal relationship or allow for delineation of the mechanism by which an increase in CV stiffness results in increased disability. Further studies are required to address this issue.
The resting average CV stiffness was higher in women (p = .03), and there are several factors that could account for this. First, a smaller body height has been associated with early return of reflected waves to the central aorta in systole rather than diastole (54), which causes a decrease in pulse wave amplification, exemplified by an increase in pulse pressure (55). Further attenuation in pulse pressure amplification has also been shown in postmenopausal women (12,56). This again would increase our measured brachial pulse pressure, and thus impact our measure of CV stiffness. Despite this increase in resting CV stiffness in women, we found no significant correlation between CV stiffness and disability in women. This suggests factors other than CV stiffness, such as alteration in musculoskeletal function, may contribute to disability in women, and further studies are needed to determine what these may be.
Persons aged 65 years and older represent the second fastest growing segment of the U.S. population (57). Limitations in mobility affect almost 25% of individuals aged 65 years or older and 75% of those living in nursing homes (58,59). Physical disability reduces quality of life, increases health care costs and is an independent predictor of mortality (1,2). Additionally, fall, and injuries due to falls, often leads to fear and consequent limitation of activity, which can start a vicious cycle of inactivity-related disability (60). Mobility is necessary for maintaining basic independent functioning in middle-aged and older individuals (3). Our analysis suggests CV stiffness is associated with poor mobility in elderly men at risk for CHF, and increased CV stiffness in the young old may be a risk factor for incident disability. Identifying potentially treatable predictors of functional decline is important to assist in designing preventive strategies to help maintain function in late life, as preventing disability and maintaining independence throughout later life is an important public health goal (61,62).
There were limitations to our study. First, this analysis was cross-sectional, and therefore, we could not assess causality. Second, the subjective nature of questionnaire-derived data may exhibit reduced precision. To minimize this, we chose to use the PAT-D questionnaire, which was shown to have strong internal consistency as well as test-retest reliability, and correlates with objective physical function testing (15). Third, only a subset of randomly selected individuals underwent formal physical function testing. Despite this, we were able to discern a correlation between increased CV stiffness and some of the physical function measures. Finally, VO2 during max exercise was not measured, as our focus was on disability. However, collecting this measure would provide further insight into peripheral versus central limitations.
In conclusion, in a group of middle-aged and elderly subjects at high risk for but yet to experience symptomatic heart failure, we found that CV stiffness correlated with functional status in men independent of age. Further studies are needed to understand the mechanism by which increases in CV stiffness are associated with or promote disability in men. In women, resting CV stiffness is elevated relative to men of similar age, but this elevation was not associated with disability, suggesting other factors promote the pathogenesis of disability in women.
Funding
This research is supported in part by National Institutes of Health (R01 HL076438-02 and P30 AG21332).
References
- 1. Branch LG, Jette AM. A prospective study of long-term care institutionalization among the aged. Am J Public Health. 1982;72:1373–1379. :10.2105/AJPH.72.12.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. J Am Med Assoc. 2006;295:2018–2026. :10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 3. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. J Am Med Assoc. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 4. Patel K, Sui X, Zhang Y, et al. Prevention of heart failure in older adults may require higher levels of physical activity than needed for other cardiovascular events. Int J Cardiol. 2013;168:1905–1909. 10.1016/j.ijcard.2012.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. :10.1161/ 01.CIR.88.4.1456 [DOI] [PubMed] [Google Scholar]
- 6. Lakatta EG. Cardiovascular aging research: the next horizons. J Am Geriatr Soc. 1999;47:613–625. [DOI] [PubMed] [Google Scholar]
- 7. Chen CH, Nakayama M, Talbot M, et al. Verapamil acutely reduces ventricular-vascular stiffening and improves aerobic exercise performance in elderly individuals. J Am Coll Cardiol. 1999;33:1602–1609. :10.1016/S0735-1097(99)00052-2 [DOI] [PubMed] [Google Scholar]
- 8. Rerkpattanapipat P, Hundley WG, Link KM, et al. Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002;90:1221–1225. :10.1016/S0002-9149(02)02838-2 [DOI] [PubMed] [Google Scholar]
- 9. Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. :10.1016/S0735-1097(01)01447-4 [DOI] [PubMed] [Google Scholar]
- 10. Gottdiener JS, McClelland RL, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. 10.7326/0003-4819-137-8-200210150-00006 [DOI] [PubMed] [Google Scholar]
- 11. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. :10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 12. Genuth SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. :10.1161/01.CIR.96.1.308 [DOI] [PubMed] [Google Scholar]
- 13. Fraker TD, Jr, Fihn SD, Gibbons RJ, et al. 2007 chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 Guidelines for the management of patients with chronic stable angina. Circulation. 2007;116:2762–2772. :10.1016/j.jacc.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. :10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 15. Rejeski WJ, Ip EH, Marsh AP, Miller ME, Farmer DF. Measuring disability in older adults: the International Classification System of Functioning, Disability and Health (ICF) framework. Geriatr Gerontol Int. 2008;8:48–54. :10.1111/j.1447-0594.2008.00446.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hundley WG, Li HF, Willard JE, et al. Magnetic resonance imaging assessment of the severity of mitral regurgitation. Comparison with invasive techniques. Circulation. 1995;92:1151–1158. :10.1161/ 01.CIR.92.5.1151 [DOI] [PubMed] [Google Scholar]
- 17. Hundley WG, Morgan TM, Neagle CM, Hamilton CA, Rerkpattanapipat P, Link KM. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–2333. :10.1161/01.CIR.0000036017.46437.02 [DOI] [PubMed] [Google Scholar]
- 18. Lawson MA, Blackwell GG, Davis ND, Roney M, Dell’Italia LJ, Pohost GM. Accuracy of biplane long-axis left ventricular volume determined by cine magnetic resonance imaging in patients with regional and global dysfunction. Am J Cardiol. 1996;77:1098–1104. :10.1016/S0002-9149(96)00140-3 [DOI] [PubMed] [Google Scholar]
- 19. Cranney GB, Lotan CS, Dean L, Baxley W, Bouchard A, Pohost GM. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation. 1990;82:154–163. :10.1161/01.CIR.82.1.154 [DOI] [PubMed] [Google Scholar]
- 20. Palmieri V, Bella JN, Roman MJ, et al. Pulse pressure/stroke index and left ventricular geometry and function: the LIFE Study. J Hypertens. 2003;21:781–787. :10.1097/01.hjh.0000052491.18130.dc [DOI] [PubMed] [Google Scholar]
- 21. Groenink M, de Roos A, Mulder BJ, Spaan JA, van der Wall EE. Changes in aortic distensibility and pulse wave velocity assessed with magnetic resonance imaging following beta-blocker therapy in the Marfan syndrome. Am J Cardiol. 1998;82:203–208. :10.1016/S0002-9149(98)00315-4 [DOI] [PubMed] [Google Scholar]
- 22. Malayeri AA, Natori S, Bahrami H, et al. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2008;102:491–496. :10.1016/j.amjcard.2008.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savage DD, Garrison RJ, Kannel WB, et al. The spectrum of left ventricular hypertrophy in a general population sample: the Framingham Study. Circulation. 1987;75:I26–I33. [PubMed] [Google Scholar]
- 24. Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Jr, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23:60–68. :10.1097/00008483-200301000-00011 [DOI] [PubMed] [Google Scholar]
- 25. Mangani I, Cesari M, Kritchevsky SB, et al. Physical exercise and comorbidity. Results from the Fitness and Arthritis in Seniors Trial (FAST). Aging Clin Exp Res. 2006;18:374–380. :10.1007/BF03324833 [DOI] [PubMed] [Google Scholar]
- 26. Rejeski WJ, Ettinger WH, Jr, Schumaker S, James P, Burns R, Elam JT. Assessing performance-related disability in patients with knee osteoarthritis. Osteoarthritis Cartilage. 1995;3:157–167. :10.1016/S1063-4584(05)80050-0 [DOI] [PubMed] [Google Scholar]
- 27. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. :10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 28. Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. :10.1093/gerona/55.11.M691 [DOI] [PubMed] [Google Scholar]
- 29. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. :10.1093/gerona/55.4.M221 [DOI] [PubMed] [Google Scholar]
- 30. Cornoni-Huntley J, Brock DB, Ostfield A, et al., eds. Established Populations for Epidemiologic Studies of the Elderly, Resource Data Book. Washington, DC: NIH Publication Number 86–2443. 1986. [Google Scholar]
- 31. Herman SD, Liu K, Tian L, et al. Baseline lower extremity strength and subsequent decline in functional performance at 6-year follow-up in persons with lower extremity peripheral arterial disease. J Am Geriatr Soc. 2009;57:2246–2252. :10.1111/j.1532-5415.2009.02562.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–390. :10.1007/BF00713504 [DOI] [PubMed] [Google Scholar]
- 33. Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–976. :10.1111/j.1532-5415.2004.52267.x [DOI] [PubMed] [Google Scholar]
- 34. Hoxie RE, Rubenstein LZ. Are older pedestrians allowed enough time to cross intersections safely? J Am Geriatr Soc. 1994;42:241–244. [DOI] [PubMed] [Google Scholar]
- 35. Whipple RH, Wolfson LI, Amerman PM. The relationship of knee and ankle weakness to falls in nursing home residents: an isokinetic study. J Am Geriatr Soc. 1987;35:13–20. [DOI] [PubMed] [Google Scholar]
- 36. Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond). 1992;82:321–327. [DOI] [PubMed] [Google Scholar]
- 37. Suzuki T, Bean JF, Fielding RA. Muscle strength and power of the ankle plantar and dorsi flexors predict functional performance in community dwelling older women. J Am Geriatr Soc. 2001;49:1161–1167. [DOI] [PubMed] [Google Scholar]
- 38. Foldavari M, Clark M, Laviolette MJA, et al. Association of muscle power with functional status in community dwelling elderly women. Med Sci Sports Exerc. 1999;31:S378. :10.1093/gerona/55.4.M192 [DOI] [PubMed] [Google Scholar]
- 39. Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. :10.1046/j.1532-5415.2002.50111.x [DOI] [PubMed] [Google Scholar]
- 40. Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23:132–137. :10.1093/ageing/23.2.132 [DOI] [PubMed] [Google Scholar]
- 41. Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50:55–59. [DOI] [PubMed] [Google Scholar]
- 42. Quittan M, Wiesinger GF, Crevenna R, et al. Isokinetic strength testing in patients with chronic heart failure–a reliability study. Int J Sports Med. 2001;22:40–44. :10.1055/s-2001-11360 [DOI] [PubMed] [Google Scholar]
- 43. Reinking MF, Bockrath-Pugliese K, Worrell T, Kegerreis RL, Miller-Sayers K, Farr J. Assessment of quadriceps muscle performance by hand-held, isometric, and isokinetic dynamometry in patients with knee dysfunction. J Orthop Sports Phys Ther. 1996;24:154–159. :10.2519/jospt.1996.24.3.154 [DOI] [PubMed] [Google Scholar]
- 44. Charoenpanichkit C, Little WC, Mandapaka S, et al. Impaired left ventricular stroke volume reserve during clinical dobutamine stress predicts future episodes of pulmonary edema. J Am Coll Cardiol. 2011;57:839–848. :10.1016/j.jacc.2010.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chahal H, McClelland RL, Tandri H, et al. Obesity and right ventricular structure and function: the MESA-Right Ventricle Study. Chest. 2012;141:388–395. :10.1378/chest.11-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jeevanantham V, Chughtai H, Little WC, et al. Aging reduces left atrial performance during adrenergic stress in middle aged and older patients. Cardiol J. 2012;19:45–52. :10.5603/CJ.2012.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. :10.1046/j.1532-5415.2003.51104.x [DOI] [PubMed] [Google Scholar]
- 48. Ettinger WH, Jr, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). J Am Med Assoc. 1997;277:25–31. :10.1001/jama.277.1.25 [PubMed] [Google Scholar]
- 49. Greig CA, Young A, Skelton DA, Pippet E, Butler FM, Mahmud SM. Exercise studies with elderly volunteers. Age Ageing. 1994;23:185–189. :10.1093/ageing/23.3.185 [DOI] [PubMed] [Google Scholar]
- 50. Kelly R, Hayward C, Avolio A, O’Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80:1652–1659. :10.1161/01.CIR.80.6.1652 [DOI] [PubMed] [Google Scholar]
- 51. Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. :10.1161/01.CIR.68.1.50 [DOI] [PubMed] [Google Scholar]
- 52. Watson NL, Sutton-Tyrrell K, Youk AO, et al. Arterial stiffness and gait speed in older adults with and without peripheral arterial disease. Am J Hypertens. 2011;24:90–95. :10.1038/ajh.2010.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heffernan KS, Manini TM, Hsu FC, et al. Relation of pulse pressure to long-distance gait speed in community-dwelling older adults: findings from the LIFE-P study. PLoS One. 2012;7:e49544. :10.1371/journal.pone.0049544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nichols WW, O’Rourke MF, Avolio AP, et al. Effects of age on ventricular-vascular coupling. Am J Cardiol. 1985;55:1179–1184. :10.1016/0002-9149(85)90659-9 [DOI] [PubMed] [Google Scholar]
- 55. Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. :10.1016/S0735-1097(98)00374-X [DOI] [PubMed] [Google Scholar]
- 56. Smulyan H, Marchais SJ, Pannier B, Guerin AP, Safar ME, London GM. Influence of body height on pulsatile arterial hemodynamic data. J Am Coll Cardiol. 1998;31:1103–1109. :10.1016/S0735-1097(98)00056-4 [DOI] [PubMed] [Google Scholar]
- 57. U.S. Census Bureau. Age and sex composition: 2010 http://www.census.gov/population/age/. Retrieved April 14, 2014.
- 58. Zimmerman SI, Fox K, Magaziner J. Demography and epidemiology of disabilities in the aged. In: Felsenthal G, Garrison SJ, Steinberg FU, eds. Rehabilitation of the Aging and Elderly Patient. Baltimore, MD: William and Wilkins; 1994:11–22. [Google Scholar]
- 59. Jette AM, Branch LG. The Framingham Disability Study: II. Physical disability among the aging. Am J Public Health. 1981;71:1211–1216. :10.2105/AJPH.71.11.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Simey P, Pennington B. Physical Activity and the Prevention and Management of Falls and Accidents Among Older People. London: Health Education Authority; 1999. :10.1056/NEJM198311173092005 [Google Scholar]
- 61. Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. N Engl J Med. 1983;309:1218–1224. [DOI] [PubMed] [Google Scholar]
- 62. Branch LG, Guralnik JM, Foley DJ, et al. Active life expectancy for 10,000 Caucasian men and women in three communities. J Gerontol. 1991;46:M145–M150. :10.1093/geronj/46.4.M145 [DOI] [PubMed] [Google Scholar]