Abstract
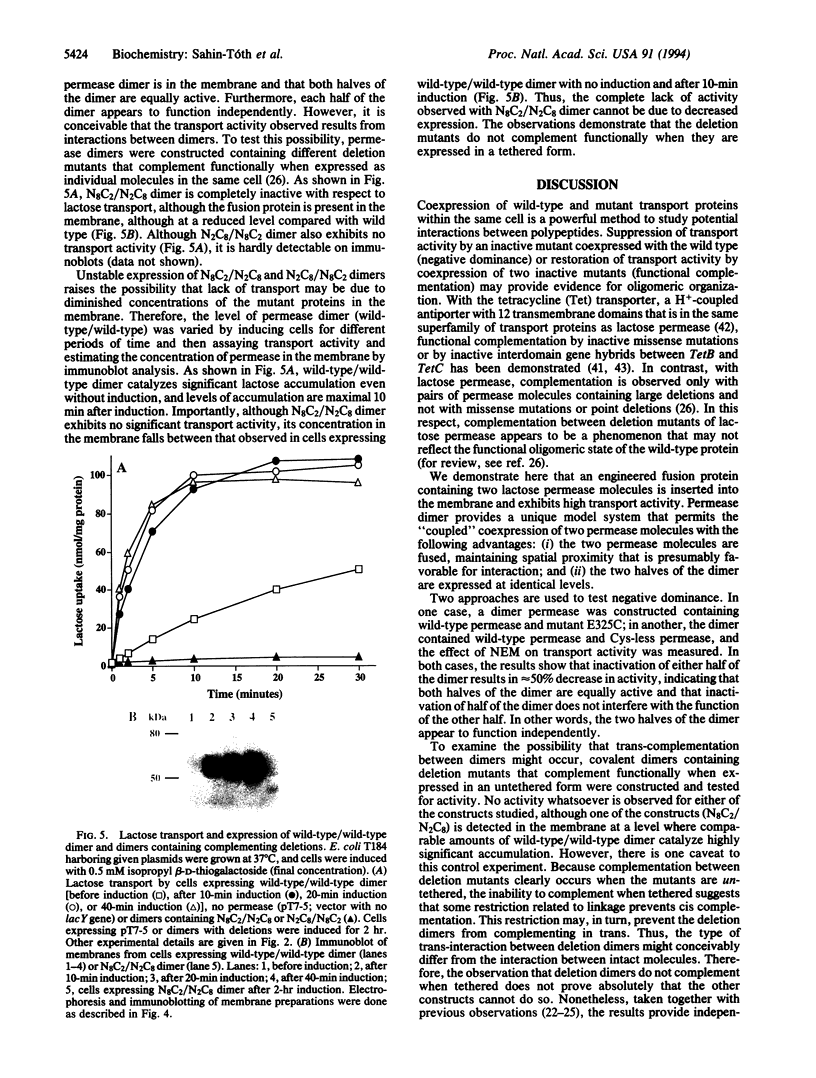
An engineered fusion protein containing two tandem lactose permease molecules (permease dimer) exhibits high transport activity and is used to test the phenomenon of negative dominance. Introduction of the mutation Glu-325-->Cys into either the first or the second half of the dimer results in a 50% decrease in activity, whereas introduction of the mutation into both halves of the dimer abolishes transport. Lactose transport by permease dimer is completely inactivated by N-ethylmaleimide; however, 40-45% activity is retained after N-ethylmaleimide treatment when either the first or the second half of the dimer is replaced with a mutant devoid of cysteine residues. The observations demonstrate that both halves of the fusion protein are equally active and suggest that each half may function independently. To test the possibility that oligomerization between dimers might account for the findings, a permease dimer was constructed that contains two different deletion mutants that complement functionally when expressed as untethered molecules. Because this construct does not catalyze lactose transport to any extent whatsoever, it is unlikely that the two halves of the dimer interact or that there is an oligomeric interaction between dimers. The approach is consistent with the contention that the functional unit of lactose permease is a monomer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibi E., Kaback H. R. Functional complementation of internal deletion mutants in the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1524–1528. doi: 10.1073/pnas.89.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi E., Kaback H. R. In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4325–4329. doi: 10.1073/pnas.87.11.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi E., Stearns S. M., Kaback H. R. The N-terminal 22 amino acid residues in the lactose permease of Escherichia coli are not obligatory for membrane insertion or transport activity. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3180–3184. doi: 10.1073/pnas.89.8.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi E., Verner G., Chang C. Y., Kaback H. R. Organization and stability of a polytopic membrane protein: deletion analysis of the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7271–7275. doi: 10.1073/pnas.88.16.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Calamia J., Manoil C. lac permease of Escherichia coli: topology and sequence elements promoting membrane insertion. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4937–4941. doi: 10.1073/pnas.87.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Herzlinger D., Mitchell R., DeChiara S., Danho W., Gabriel T. F., Kaback H. R. Intramolecular dislocation of the COOH terminus of the lac carrier protein in reconstituted proteoliposomes. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4672–4676. doi: 10.1073/pnas.81.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Tahara S. M., Patel L., Goldkorn T., Kaback H. R. Preparation, characterization, and properties of monoclonal antibodies against the lac carrier protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6894–6898. doi: 10.1073/pnas.79.22.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Viitanen P., Herzlinger D., Kaback H. R. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 1. Functional studies. Biochemistry. 1984 Jul 31;23(16):3681–3687. doi: 10.1021/bi00311a017. [DOI] [PubMed] [Google Scholar]
- Consler T. G., Tsolas O., Kaback H. R. Role of proline residues in the structure and function of a membrane transport protein. Biochemistry. 1991 Feb 5;30(5):1291–1298. doi: 10.1021/bi00219a019. [DOI] [PubMed] [Google Scholar]
- Costello M. J., Escaig J., Matsushita K., Viitanen P. V., Menick D. R., Kaback H. R. Purified lac permease and cytochrome o oxidase are functional as monomers. J Biol Chem. 1987 Dec 15;262(35):17072–17082. [PubMed] [Google Scholar]
- Curiale M. S., Levy S. B. Two complementation groups mediate tetracycline resistance determined by Tn10. J Bacteriol. 1982 Jul;151(1):209–215. doi: 10.1128/jb.151.1.209-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornmair K., Corin A. F., Wright J. K., Jähnig F. The size of the lactose permease derived from rotational diffusion measurements. EMBO J. 1985 Dec 16;4(13A):3633–3638. doi: 10.1002/j.1460-2075.1985.tb04127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kaback H. R. Topology of the lac carrier protein in the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3322–3326. doi: 10.1073/pnas.80.11.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kempner E. S., Kaback H. R. Functional molecular weight of the lac carrier protein from Escherichia coli as studied by radiation inactivation analysis. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1021–1025. doi: 10.1073/pnas.81.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Herzlinger D., Carrasco N., Kaback H. R. Functional and immunochemical characterization of a mutant of Escherichia coli energy uncoupled for lactose transport. Biochemistry. 1985 Jan 1;24(1):221–229. doi: 10.1021/bi00322a032. [DOI] [PubMed] [Google Scholar]
- Herzlinger D., Viitanen P., Carrasco N., Kaback H. R. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 2. Binding studies with membrane vesicles and proteoliposomes reconstituted with purified lac carrier protein. Biochemistry. 1984 Jul 31;23(16):3688–3693. doi: 10.1021/bi00311a018. [DOI] [PubMed] [Google Scholar]
- Houssin C., le Maire M., Aggerbeck L. P., Shechter E. The lactose permease of Escherichia coli: evidence in favor of a dimer. Arch Biochem Biophys. 1985 Aug 1;240(2):593–606. doi: 10.1016/0003-9861(85)90066-9. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Bibi E., Roepe P. D. Beta-galactoside transport in E. coli: a functional dissection of lac permease. Trends Biochem Sci. 1990 Aug;15(8):309–314. doi: 10.1016/0968-0004(90)90020-c. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. In and out and up and down with lac permease. Int Rev Cytol. 1992;137:97–125. doi: 10.1016/s0074-7696(08)62674-1. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Molecular biology of active transport: from membrane to molecule to mechanism. Harvey Lect. 1987;83:77–105. [PubMed] [Google Scholar]
- Konings W. N., Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of beta-galactosides and amino acids. J Biol Chem. 1971 Oct 10;246(19):5857–5861. [PubMed] [Google Scholar]
- König B., Sandermann H., Jr Beta-D-Galactoside transport in Escherichia coli: Mr determination of the transport protein in organic solvent. FEBS Lett. 1982 Oct 4;147(1):31–34. doi: 10.1016/0014-5793(82)81005-3. [DOI] [PubMed] [Google Scholar]
- Marger M. D., Saier M. H., Jr A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci. 1993 Jan;18(1):13–20. doi: 10.1016/0968-0004(93)90081-w. [DOI] [PubMed] [Google Scholar]
- McKenna E., Hardy D., Kaback H. R. Insertional mutagenesis of hydrophilic domains in the lactose permease of Escherichia coli. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11954–11958. doi: 10.1073/pnas.89.24.11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Page M. G., Rosenbusch J. P. Topography of lactose permease from Escherichia coli. J Biol Chem. 1988 Nov 5;263(31):15906–15914. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Kaczorowski G. J., Garcia M. L., Kaback H. R. Active transport in membrane vesicles from Escherichia coli: the electrochemical proton gradient alters the distribution of the lac carrier between two different kinetic states. Biochemistry. 1980 Dec 9;19(25):5692–5702. doi: 10.1021/bi00566a005. [DOI] [PubMed] [Google Scholar]
- Roepe P. D., Zbar R. I., Sarkar H. K., Kaback H. R. A five-residue sequence near the carboxyl terminus of the polytopic membrane protein lac permease is required for stability within the membrane. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3992–3996. doi: 10.1073/pnas.86.11.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. A., Levy S. B. Interdomain hybrid Tet proteins confer tetracycline resistance only when they are derived from closely related members of the tet gene family. J Bacteriol. 1990 May;172(5):2303–2312. doi: 10.1128/jb.172.5.2303-2312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin-Tóth M., Kaback H. R. Cysteine scanning mutagenesis of putative transmembrane helices IX and X in the lactose permease of Escherichia coli. Protein Sci. 1993 Jun;2(6):1024–1033. doi: 10.1002/pro.5560020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckler R., Möröy T., Wright J. K., Overath P. Anti-peptide antibodies and proteases as structural probes for the lactose/H+ transporter of Escherichia coli: a loop around amino acid residue 130 faces the cytoplasmic side of the membrane. Biochemistry. 1986 May 6;25(9):2403–2409. doi: 10.1021/bi00357a016. [DOI] [PubMed] [Google Scholar]
- Seckler R., Wright J. K., Overath P. Peptide-specific antibody locates the COOH terminus of the lactose carrier of Escherichia coli on the cytoplasmic side of the plasma membrane. J Biol Chem. 1983 Sep 25;258(18):10817–10820. [PubMed] [Google Scholar]
- Seckler R., Wright J. K. Sidedness of native membrane vesicles of Escherichia coli and orientation of the reconstituted lactose: H+ carrier. Eur J Biochem. 1984 Jul 16;142(2):269–279. doi: 10.1111/j.1432-1033.1984.tb08281.x. [DOI] [PubMed] [Google Scholar]
- Stochaj U., Bieseler B., Ehring R. Limited proteolysis of lactose permease from Escherichia coli. Eur J Biochem. 1986 Jul 15;158(2):423–428. doi: 10.1111/j.1432-1033.1986.tb09770.x. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Vogel H., Wright J. K., Jähnig F. The structure of the lactose permease derived from Raman spectroscopy and prediction methods. EMBO J. 1985 Dec 16;4(13A):3625–3631. doi: 10.1002/j.1460-2075.1985.tb04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. K., Weigel U., Lustig A., Bocklage H., Mieschendahl M., Müller-Hill B., Overath P. Does the lactose carrier of Escherichia coli function as a monomer? FEBS Lett. 1983 Oct 3;162(1):11–15. doi: 10.1016/0014-5793(83)81039-4. [DOI] [PubMed] [Google Scholar]
- van Iwaarden P. R., Pastore J. C., Konings W. N., Kaback H. R. Construction of a functional lactose permease devoid of cysteine residues. Biochemistry. 1991 Oct 8;30(40):9595–9600. doi: 10.1021/bi00104a005. [DOI] [PubMed] [Google Scholar]