INTRODUCTION
The three decades since the adoption of MRI as a mainstream imaging modality have seen enormous advances in both image quality and the range of contrast mechanisms available via MRI. By today’s standards, image quality in the early years of MRI was poor, and the ability to generate novel contrasts through clever manipulation of the NMR signal from different tissues was not fully appreciated. However, as basic MRI systems composed of superconducting polarizing magnets, 3-axis gradient systems, and simple RF excitation and receive systems were refined during the 1980s, imaging scientists and engineers found an enormously rich platform replete with opportunities for improvement and innovation. The imaging capabilities of current MRI machines were made possible not by innovations in a single component area, but rather by decades of improvement in a variety of areas. These include innovations in acquisition and reconstruction strategies, development of contrast agents, advances in main field and gradient hardware, and advances in radiofrequency (RF) coil design.
Innovations in image acquisition and reconstruction algorithms have continually improved image quality and contrast, often without requiring any change or improvement to existing hardware. New contrast mechanisms have appeared; MRI is now routinely used to measure the diffusion properties of tissues, flow velocities and volumes, tissue temperature, blood oxygenation level, and even susceptibility differences across tissues. The development and testing of intravascular contrast agents such as gadolinium significantly advanced MR angiography, and made possible dynamic contrast-enhanced imaging techniques now widely used in a variety of clinical and research applications. Advances in gradient hardware have enabled higher resolutions, shorter echo times, faster imaging via steady-state techniques, and improved contrast.
While each of these advances has contributed to make MRI the rich modality it is today, the importance of RF coil design (for both excitation and reception of the NMR signal) should not be underestimated. There are a limited number of ways to improve image quality for many of the mature MRI techniques in common usage today. SNR theoretically improves linearly with main polarizing field strength. The increased magnetic polarization achieved at 3 Tesla vs. 1.5 Tesla improves SNR by a factor of approximately two, all other things being equal1; hence the increasing popularity of high-field magnets. Once a pulse sequence has been optimized for a given application, there is little that can be done to sequence parameters (beyond increasing the number of averages) to improve SNR. However, there are often relatively large potential improvements in image quality to be gained using an RF coil optimized for the application or anatomy of interest. As an example, a recent paper demonstrated a 260% increase in mean SNR in the breast using an optimized 16-channel bilateral breast array when compared to a high-quality 8-channel commercial breast array coil at 3 Tesla. The optimized design did nothing more than cleverly arrange array elements on a bra-like structure so that they more closely matched the contours of the breast, more than tripling mean SNR in the process [1].
Several factors are driving a resurgent interest in RF coil design and optimization. First, as the incremental improvements in image quality from algorithmic and other hardware developments slow, attention has turned to ensuring that the signal is excited and detected as efficiently as possible. Second, some emerging techniques make very specific demands on the RF coil. Parallel imaging, for example, requires multiple receive coils, and the degree of acceleration achievable depends at least in part on coil topology and performance. Multi-nuclear imaging requires very sensitive RF coils tuned to specific frequencies other than that of the hydrogen nucleus. Third, the physics of the RF coil design problem change in fundamental ways at higher fields (3 Tesla and now 7 Tesla) as the wavelength of the MR signal decreases. Finally, the RF power deposited in the subject (RF heating) increases as the square of the main field strength, introducing new design constraints on high-field excitation coils to minimize patient heating.
In this paper, we will first describe some of the fundamental concepts needed to understand and evaluate the performance of RF coils, and briefly describe the progression and history of RF coil development for MRI systems. We will then focus specifically on the state-of-the-art in RF coil development for musculoskeletal applications, introducing some of the exciting current developments in the area.
A Primer on RF Coils for MRI
Coils play a major role in overall image quality. They are the fundamental magnetic interface between the MR imaging system and the human subject. All high frequency RF signals transmitted to and received from the human subject are done through one or more RF coils. The RF coil must perform the electromagnetic conversion with minimal electrical resistive loss to maintain good image quality. The RF coil must also have the proper geometrical design in order to image the particular anatomy of interest.
It is important to understand several basic trends in MRI to place the discussion of RF coil technology that follows in context.
Higher field strengths: The magnetic polarization responsible for the MR signal increases linearly with field strength. As a consequence, we can expect a near-linear increase in SNR (all other factors being equal) with field strength. In the 1990s, the vast majority of clinical scanner deployed had a field strength of 1.5 Tesla. The past decade has seen a rapid deployment of 3-Tesla systems. A limited number of scanners have been installed at 4.0, 7.0, and even 9.4 Tesla, but they are primarily limited to academic research institutions. While higher field strengths can produce images with higher SNR at higher spatial and temporal resolutions, there are significant challenges to be overcome as well, many affecting RF coil design.
Larger numbers of simultaneous receivers: The last decade has seen the number of available simultaneous receive channels on many systems increase from 4 to as high as 128. This presents both opportunities and challenges to the coil designer. Large-channel coil arrays can improve image quality and provide new functionality, but they also add significant complexity and manufacturing cost.
Multinuclear imaging and MR spectroscopy: Improvements in all aspects of the MRI system (higher field strengths, better field uniformity, more capable gradients, better RF coils, and novel pulse sequences) are making feasible non-hydrogen imaging and MR spectroscopy in vivo. Applications of sodium, carbon, phosphorus, and fluorine imaging are all under investigation. These techniques require special RF coils, additional hardware such as broadband RF amplifiers, and specialized pulse sequences, all of which can add significant cost to a system.
Need for RF Coil Development
As new applications and methodologies emerge using MRI, the need for new coils has become more apparent. Coils are now routinely designed to match the anatomical regions of interest and optimized for the pulse sequences used in a given application. Most MR research centers employ RF coil experts to develop and optimize research coils. Modifications to existing designs and even entirely new designs have emerged to operate at the higher RF frequencies dictated by higher field strengths. Newer systems are beginning to support higher numbers of receive channels, and new phased array designs that employ 32 or more receivers are appearing. The improved performance of these arrays enables systems to produce images at higher spatial resolution, higher temporal frame rates, or both.
Parallel imaging techniques such as SENSE [2] and SMASH [3] make use of multiple receive coils (or receive coil arrays) to reduce image sampling requirements and accelerate image acquisition. Parallel transmit techniques make use of transmit coil arrays to reduce the duration of multidimensional RF pulses [4], and can potentially provide a high degree of control over excitation area, minimizing RF power deposition. These techniques benefit from optimized coil designs that lower the g-factor while maximizing the acceleration factor achievable for a given application.
In higher field systems it is now possible to image other non-hydrogen nuclei such as sodium or carbon at resolutions and in scan times that would not previously have been possible. Non-hydrogen imaging applications require highly sensitive coils designed to operate at specific RF frequencies different than that of the hydrogen nucleus. In vivo quantitative sodium imaging, for example, shows promise in assessing cartilage health and quantifying very early degenerative changes in cartilage.
In many applications, we are approaching the limits of performance in pulse sequence acquisition efficiency at a given field strength. One of the natural remaining avenues to improve image quality is through improvements in the RF coil configurations used. Smaller coils tailored to specific anatomy have more attractive noise properties than larger coils, as discussed below.
Positioning coil elements closer to the anatomy of interest can help significantly. A “one size fits all” coil strategy is inherently less SNR efficient than having access to a variety of coils, each tailored for anatomy and patient size. The multiple-coil approach can clearly become expensive and cumbersome, but modern scanners are increasingly equipped with a broad array of application-specific coils.
Coil Performance
As mentioned, radiofrequency coil performance is of critical importance to image acquisition. Unfortunately, there is no single metric that can be used to gauge coil performance. The uniformity of radiofrequency excitation across the volume of interest, the reception sensitivity of the coil system to the NMR signal, and the amount of RF energy deposited in the subject must all be considered. The importance of each of these factors can vary with pulse sequence, field strength, resolution and SNR requirements, and even scan subject.
Uniformity of excitation
For many applications and pulse sequences, the uniformity of radiofrequency excitation (also called transmit field uniformity) provided by the coil is of critical importance. Variations in transmit field uniformity are manifest as variations in the flip angle achieved across the volume of interest. Basic imaging sequences such as spin echo (SE) and fast spin echo (FSE) work best when the actual flip angle matches the prescribed flip angle, and are thus sensitive to inhomogeneity in the transmit field. On most 1.5 Tesla and 3 Tesla systems, a large radiofrequency coil just smaller than the bore of the magnet (the “body coil”) is built into the system. The body coil provides a high degree of transmit field uniformity, but can deposit significant RF energy in the scan subject and suffers from low receive sensitivity (both discussed below). The body coil is often used for excitation in applications and pulse sequences where patient heating is not a concern due to its excellent transmit uniformity. Most 7 Tesla whole body scanners do not have a body coil, since such a coil would deposit excessive RF energy in the body as discussed below.
Receive sensitivity
The reception sensitivity of the coil system to the NMR signal has a direct impact on image SNR. A higher receive sensitivity contributes to higher image SNR, although other considerations, such as the sensitive volume (or field of view) of the coil, also play an important role. In most MRI applications, the coils are large enough to be in a regime where image noise is dominated by so-called “body noise”. Body noise arises from random electrical fluctuations in the body. A coil with a large sensitive volume picks up noise from a larger volume. This tends to degrade SNR. As a consequence, to maximize SNR it is sometimes desirable to use the smallest coil possible that provides a sufficient field of view. However, the receive sensitivity of a coil (or coil array) is spatially varying, which causes “shading” in an image (spatially-dependent image intensity or SNR). Small surface coils tend to yield the highest SNR within their sensitive area, but are not very uniform. Sometimes trade-offs in image uniformity versus SNR must be made when selecting a coil.
RF energy deposition
The radio-frequency pulses used to excite the NMR-signal-yielding nuclei deposit energy in the subject, leading to tissue heating. The specific absorption rate, or SAR, is a metric that assesses the potential for tissue heating due to these RF pulses. SAR is defined as the radiofrequency power absorbed by the body per unit mass, and is typically communicated in watts per kilogram. Large transmit coils (e.g., body coils) can deposit RF energy across large portions of the body, leading to high SAR, but they do so relatively uniformly. Smaller coil configurations with a large degree of transmit field inhomogeneity can lead to higher local SAR, or local “hot spots”. Accurate assessment of patient heating is extremely challenging; models are typically developed for standard coils, and then radio frequency irradiation kept at the lowest possible level to avoid possible patient heating problems. Some areas of the body, such as the head, are more sensitive to RF heating than others, such as the extremities; the SAR limits imposed by the FDA and European regulators are dependent on the part of the body being exposed to the RF fields. SAR is highly pulse sequence dependent; the sequence RF duty cycle, flip angle, and type all influence total SAR. RF energy deposition increases as the square of field strength. For example, the same 90 degree RF pulse played on a 1.5 Tesla scanner will deposit approximately four times the RF power if played on a 3 Tesla scanner with a similarly-configured coil and similarly-sized patient. This is why most 7 Tesla whole body scanners do not have a body coil; the RF power deposited across the patient by a whole-body coil at 7 Tesla raises patient heating concerns.
Challenges in RF Coil Design
We summarize some of the challenges in RF coil design below.
Physical constraints
Whole body scanners are prevalent in most modern clinical MRI settings. Typical whole-body scanners have a bore opening of between 55 and 70 cm that accommodates most human body sizes. These scanners typically have spherical imaging volumes of 40-50 cm in diameter. With a subject present in the scanner, there is limited space for coil enclosures. RF coils must be in a protective housing for mechanical stability. These housings must be lightweight, non-magnetic, and non-conductive. They also must be non-flammable and somewhat biocompatible so as not to cause skin irritation to the subject. It can also be challenging to get the sensitive volume of the coil close to the anatomy of interest.
Patients present a variable that must be taken into account in coil design. Patient height, weight, head, and limb size may vary significantly and require different size variants of a coil design to accommodate the range. Patient size may also place restrictions on the space available between the patient and magnet bore for the RF coil. In some cases large configurable coil arrays may be used for a range of patient sizes by selecting a group of coil elements in the array appropriate to the patient. Patient size may also affect the electrical loading or exact tuning of a coil, although there are some design techniques that partially address this issue.
Implementation complexity
Older, simpler coils were merely plugged into the MRI system by the operator, and it was then up to the operator to choose parameters appropriate to the attached coil. Most modern systems use automatic coil identification to configure and limit system usage to proper and safe configurations and to warn of or prevent problems associated with loose connectors or faulty coil circuitry. While there are many advantages to the modern systems, such as reducing the possibility of RF burns and damaging coils, the added complexity imposes additional design and implementation challenges on the RF coil engineer.
Ease of use
Coils must be kept simple and quick to set up and position on the patient. They must be kept lightweight for easy removal and positioning by the MR operator. Cables and connectors need to be designed to be robust and to not interfere with patient comfort and positioning.
Cost
As the number of coil elements and available receive channels increases, coils are becoming more complicated, with more components and cable interconnects. Most large array coils now have low noise preamplifiers for each receive element built directly into the coil body. Coil ID devices, transmit/receive switches, hybrid splitters, and other components all add cost and complexity, and tend to reduce the reliability of modern coils.
Other RF considerations
The RF coil design problem becomes more challenging as main polarizing field strengths increase. As mentioned earlier, RF power deposition increases as the square of field strength (all other things being equal), sometimes precluding the use of a large body transmit coil. An additional problem arises from the short wavelengths of the radiofrequency NMR signal at higher field. At 1.5 Tesla, the 1H resonant frequency is approximately 64 MHz, yielding a wavelength of approximately 5 m in free space and roughly 55-80 cm in the body. At 3 Tesla, the 1H resonant frequency increases to ~127MHz: a 2.5 m wavelength in free space and ~27-40 cm wavelength in the body. These wavelengths are often of the same order as the dimensions of the anatomy to be imaged, complicating the RF coil design problem. We begin to encounter RF penetration and standing wave issues with certain coil sizes and configurations at higher fields. While still manageable with many coil designs at 3 Tesla, the challenges are significant at 7 Tesla where wavelengths in body tissue can get as low as ~12 cm.
Overview of RF Coils
Improvements in RF coil technology have produced significant advances in functionality and image quality since MRI became a mainstream imaging modality in the early 1980s. The introduction of the quadrature birdcage coil in 1985 (Figure 1), for example, was an early major advancement that increased signal to noise ratio and produced more uniform RF fields. The quadrature birdcage design achieved higher efficiency than previous linear saddle coil designs [5, 6]. The move in the early 1990s from large volume head and body coils to localized surface coils and multi-coil arrays for reception also enabled major improvements in image quality. The newer configurations achieved improved sensitivity while maintaining large fields of view by employing multiple simultaneous receivers [7].
Figure 1.

A prototype 3 Tesla Body Coil. Image courtesy of Stanford University Department of Radiology
Body coils
Large volume whole body RF coils have been around since the beginning of MRI. These coils usually operate over the maximum imaging volume of the main magnet and gradient coils. They typically achieve excellent transmit field homogeneity. However, the large noise volume visible to these coils and the relatively low (albeit uniform) receive sensitivity severely limit the image quality that can be obtained using the body coil for signal reception. Image quality can be significantly improved by using the body coil for transmit only, using smaller coils or arrays of coils that are placed very near the anatomy of interest for local reception of the MR signal. The birdcage coil is used in the majority of systems, and was originally designed to operate at 64 MHz at 1.5 Tesla. In recent years birdcages have been designed to operate at higher field strengths such as 3 Tesla and 7 Tesla [8, 9]. Since the introduction of commercial body coils at 3T there has been a rapid increase in clinical usage of very high field MR. Other designs such as TEM coils have also been proposed for large volume head and body coils [10].
Structure of RF Coils
At a very basic level, RF coils consist of a conductor geometry that forms a magnetically inductive structure that can generate and/or receive RF magnetic fields polarized in a plane transverse (perpendicular) to the direction of the main static polarizing field. Capacitor elements are then added to this inductive structure to make it resonant to a specific narrow band of frequencies centered near the Larmor frequency of the nucleus of interest at the specific static polarizing field strength. This resonant coil is then impedance-matched to an RF transmission line or cable with an RF network. In many cases an active PIN diode switch or passive diode limiter is employed to disable the coil resonance during RF transmit or receive mode depending on the coil type.
Some coil structures are relatively uniform over a contained volume, such as solenoid and birdcage structures, but have a lower general sensitivity compared to smaller local surface coils. As previously mentioned, small surface coils have a high sensitivity and see a smaller noise volume, yielding relatively high SNR. However, these coils have a correspondingly small sensitive area and are typically not very uniform; intensity corrections are sometimes used in conjunction with surface coils, which can lead to spatially varying noise across the image.
It is common to employ a large volume coil such as the body coil for transmit, and high sensitivity local surface coils or arrays for receive. For example, phased-array knee coils are becoming increasingly popular. These coils typically rely on the body coil to achieve a uniform excitation across the knee, with the relatively small coil elements in the phased array receiving the MR signal. Receive-only phased array coils that are relatively small compared to the body coil have an added advantage in the simplicity of not containing or being in close proximity to a transmit coil. However, in high SAR sequences (i.e. sequences with the potential to deposit significant RF power in the subject) transmission or excitation with the body coil may not be possible due to patient heating limitations.
Other knee coil configurations combine a local birdcage volume transmit coil with a multi-coil receive-only array. This has several advantages. First, these transmit coils require less power due to the small coil volume, and as a consequence can provide higher peak RF transmit intensity (leading to shorter RF pulses). Second, RF power deposition (or SAR) considerations are somewhat mitigated since RF heating is confined to the local anatomic region and not the entire body. Third, instead of both knees being excited, only the knee of interest is locally excited, which in turn helps in reduction of aliasing. At 7 Tesla, this is a common technique since large volume transmit body coils are typically not available.
New Coils for Musculoskeletal MRI
Below is a survey of some interesting recent coil designs for musculoskeletal MRI. Note that this list is certainly not exhaustive, but is rather meant to give the reader a feel for interesting current developments in RF coil design for musculoskeletal applications.
28-channel 7 Tesla knee coil
As mentioned, the coil design challenges at 7 Tesla are considerable given the lack of a uniform body coil for transmit and the higher frequency (and hence shorter wavelength) of the RF radiation. A local transmit and high-channel receive knee coil with multiple rows of elements in the S/I direction was recently presented at 3 Tesla [11].
This geometry yields high receive sensitivity and allows higher parallel imaging acceleration factors, reducing both pulsation flow artifacts and scan time. The design was used as the basis for a state-of-the-art 7 Tesla 28-channel receive-only array combined with a partially shielded transmit birdcage [12]. Again, the local transmit capability provided by the partially shielded birdcage was essential, since most 7 Tesla scanners do not have built in body coils.
The coil is shown in Figure 2 and Figure 3. The 28 receive channels allow high acceleration factors to be used with parallel imaging, and the excellent receive sensitivity enables very high anatomic resolution. A clever partial-shielding concept was developed for the transmit birdcage to minimize radiation losses and contain the leakage field so as not to excite the adjacent knee. The shield reduces the total inductance of the coil structure, reducing the local electric fields that can increase local SAR. While not achieving the same degree of shielding as a fully continuous shield, the partial shield (which resembles the actual birdcage itself) provides adequate shielding while also allowing access to the inner coil structure for manufacturing and service. The space constraints associated with providing adequate patient comfort using a coil that integrates a 12-rung birdcage transmit coil, the partial shield, and 28 receive channels necessitated the use of ultra-compact, low noise pre-amplifiers. A sample image obtained using the coil is shown in Figure 4.
Figure 2.

QED 28-Channel Knee Coil (Courtesy of Quality Electrodynamics, OH, USA; Ravinder Regatte, NYU Langone Medical Center, NY, USA; Siegfried Trattnig, the Medical University of Vienna, Vienna, Austria)
Figure 3.
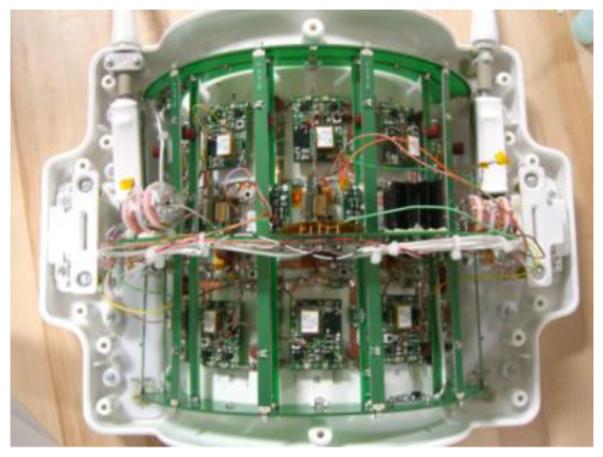
QED 28-Channel Knee Coil (Courtesy of Quality Electrodynamics, OH, USA; Ravinder Regatte, NYU Langone Medical Center, NY, USA; Siegfried Trattnig, the Medical University of Vienna, Vienna, Austria)
Figure 4.
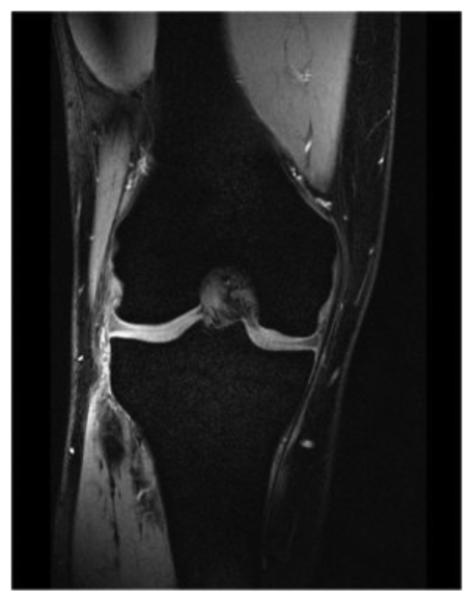
Coronal MR Image of healthy volunteer (From: Finnerty, M., et al., A 7-Tesla High Density Transmit with 28-Channel Receive-Only Array Knee Coil, in Intl. Soc. Mag. Reson. Med. (ISMRM) 2008: Toronto.)
Dual-tuned configurations for MSK applications (1H/23Na)
Advances in gradient hardware, the availability of whole-body scanners with high polarizing field strengths, and the development of efficient pulse sequences with extremely short echo times [13, 14] have made feasible sodium MRI in vivo in reasonable scan times. As a consequence, there has been a renewal of interest in using sodium MRI to assess cartilage health. Sodium MRI has been shown to be an indicator of proteoglycan content in cartilage [15-17], and as such may be sensitive to the very earliest degenerative changes in cartilage that portend the onset of osteoarthritis.
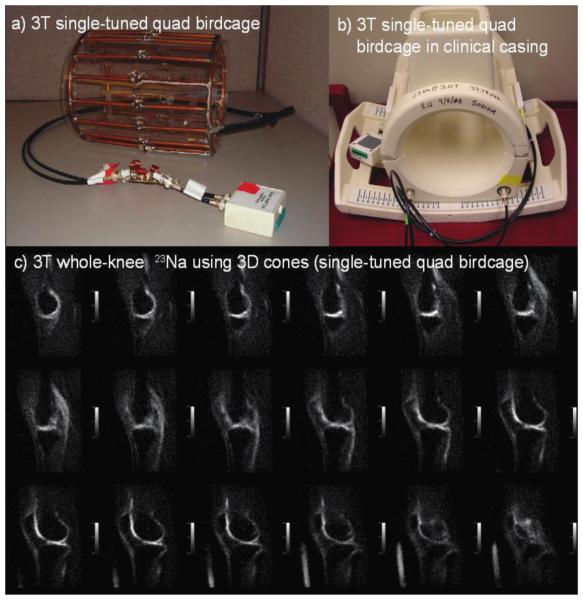
Two example single-tuned 3 Tesla sodium birdcage knee coils are shown in Figure 5, along with corresponding 3D whole-knee sodium MRI images. While these single-tuned quadrature birdcage coils yield excellent results, dual-tuned 1H/23Na coil configurations (i.e., coils that resonate at both sodium and hydrogen frequencies) are desirable so that sodium image acquisition can be performed in conjunction with a standard 1H exam without the need to move the patient or change coils. This is particularly advantageous when accurate registration of sodium and proton images is required. We discuss several dual-tuned 1H/23Na knee coil configurations below.
Figure 5.
(a) Custom quadrature sodium-tuned 3.0T knee coil (inside diameter 17cm). (b) A similar custom sodium-tuned 3.0T coil enclosed in a patient-safe clinical casing. (c) Series of sagittal 3D whole-joint knee images at 3.0T using the quad birdcage design. Sodium images area at a resolution of 1.25x1.25x4.0mm, with healthy cartilage SNR of 22. Courtesy of Stanford University Radiology Department
Dual-tuned 4-ring birdcage for 1H/23Na imaging
A traditional approach to dual-tuning employs frequency-specific block traps, allowing selective routing of different frequencies through either spate rungs or rings in a birdcage structure. The drawback of this approach is significant resistive losses (due to wire resistance in the inductor) and magnetic field losses (due to inductors coupling to other structures or radiating in air), lowering coil electrical Q and SNR performance [18]. Several dual-tuned coil designs have been developed capable of imaging both 1H and 23Na without some of the losses in the sodium structure inherent in the traditional frequency block trap design [19, 20]. One version of such a coil is a multiple end ring design that incorporates a short z-FOV sodium quad low-pass birdcage in the central section of a split z-axis proton quad high-pass birdcage [21, 22]. This type of coil structure does not need frequency traps and provides very efficient high SNR and high uniformity sodium imaging, while producing proton images of adequate quality for anatomic registration of the sodium images. However, due to the geometric split in the proton section this coil cannot produce clinical quality proton images. A flared end-ring version of this design to accommodate larger patients while maximizing sodium SNR is shown in Figure 6. A split casing design is shown in Figure 7.
Figure 6.

Flared 4-ring dual-tuned 1H/23Na birdcage knee coil. Image courtesy of Stanford University Department of Radiology, University of Utah Department of Radiology, and Brigham Young University Department of Electrical and Computer Engineering.
Figure 7.

Split 4-ring dual-tuned 1H/23Na birdcage knee coil. Image courtesy of Stanford University Department of Radiology.
PIN diode switched dual-tuned coils:
Another strategy for dual frequency coils uses PIN diodes (Figure 8) to switch additional capacitors in and out of the coil circuit. This scheme eliminates the need for the trap circuit and the associated losses resulting from the self-resistance of the trap circuit inductors; it also overcomes problems previously associated with attempts at achieving optimal tuning and matching of both frequencies [23].
Figure 8.
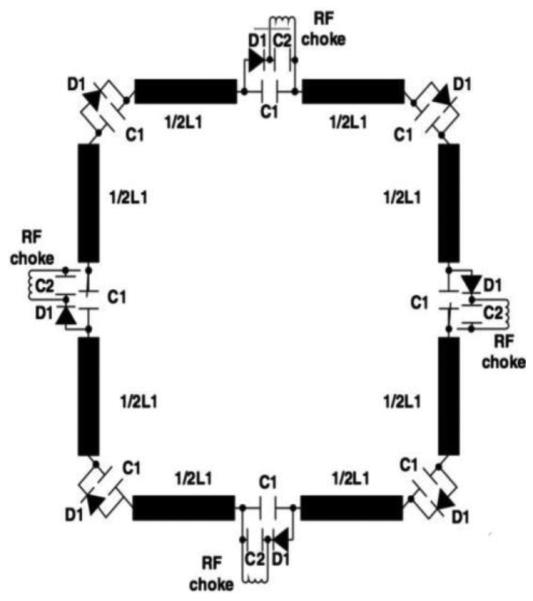
A Schematic of a PIN diode switch tuned surface coil capable of electrically switching between two or more frequencies. From: Ha, S., et al. (2010) A PIN diode controlled dual-tuned MRI RF coil and phased array for multi nuclear imaging (Physics in Medicine and Biology)
A similar method of switching capacitors in a volume coil has been demonstrated by Shen (Figure 9). This design uses PIN diodes to switch a low-pass end ring into a band-pass coil, changing its frequency and operation from band-pass to low-pass [24].
Figure 9.
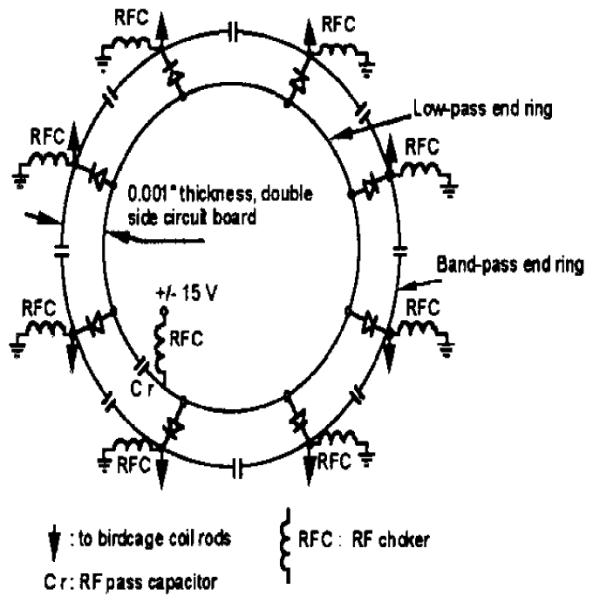
A dual frequency birdcage coil capable of switching between band pass and low pass modes at two different frequencies. From: Shen, G.X. (1999) Quadruple tuned (23Na, 7Li, 31P,1H) Band/Lowpass Birdcage Coil at 3.0T (ISMRM)
Split TEM volume knee coils
Traditionally, high-field RF coils in the form of rigid cylinders have been used for scanning the human head and limbs. There are several limitations when using these types of coils. To be scanned, the patient has to either slide into the coil or the coil made to slide over the patient. For patients with impaired or limited mobility this can make positioning difficult. The ability to both place and visualize the location of the array is also limited when receive-only arrays are used within the transmission coil. A split “two-piece” coil can be used to overcome these limitations.
Split unshielded birdcage coils have been described for field strengths up to 3 Tesla. Due to the presence of end ring RF currents these coils require a continuous electrical connection between the two halves of the coil. Furthermore, most high-field (>3 Tesla) head-sized RF coils use a shield surrounding the entire coil to decrease radiation losses. For shielded RF coils, both the birdcage coil and the shield must be separated and reliably reconnected electrically during each use. This complicates the fabrication process of the coil, complicates usage, potentially produces inefficiencies due to imperfect connections and the altered resonant structure geometry, and reduces coil reliability.
Transverse electromagnetic (TEM) RF coils can be used to eliminate the need for electrical connections between the split halves of the coil by utilizing through-space inductive coupling between resonant elements. The TEM design has been applied to head and body coils up to 7 Tesla. The TEM coil inductance depends on the ratio of the RF shield diameter to the inside diameter of the coil rather than on the absolute value of the coil diameter such as in the birdcage. This design decreases radiation losses and improves current distributions (no end ring current) [25]. An example is shown in Figure 10.
Figure 10.
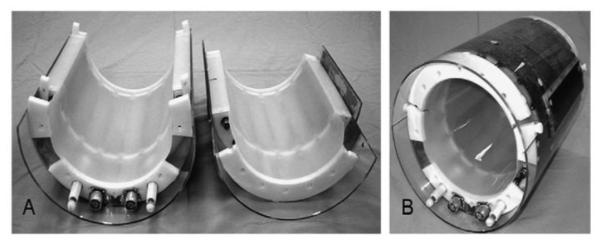
Pictures of assembled and disassembled (A), (B) knee split TEM volume coils. From: Avdievich, N.I., et al. (2007) 4T split TEM volume head and knee coils for improved sensitivity and patient accessibility (Journal of Magnetic Resonance)
Dedicated phased array 3-Tesla ankle coil:
Recently, some very nice dedicated phased array coils have begun to appear for specific MSK applications. Figure 11 shows one such coil developed to maximize SNR when imaging the thin articular cartilage of the ankle joint. This small-field-of-view eight-channel high-resolution coil exploits very close placement of the coil elements to the anatomy of interest to achieve impressive sensitivity, and the multiple receive elements enable parallel imaging applications. The coil is shaped like a boot that slides down over the foot and ankle and can be tilted in 5° increments [26].
Figure 11.
Dedicated Phase Array Coil. From: Welsch, G.H., et al. (2008) High-resolution morphological and biochemical imaging of articular cartilage of the ankle joint at 3.0 T using a new dedicated phased array coil: in vivo reproducibility study (Skeletal Radiology)
HTS high temperature superconducting coils
At least one group is producing RF coils based on Yttrium Barium Copper Oxide (YBa2CuO7, or simply YBCO) which is a crystalline material that can achieve superconducting state below 93° K, allowing it to be operated in liquid Nitrogen at 77° K [27]. This nearly eliminates all coil conductor noise allowing improved operation at relatively low magnetic fields. At higher magnetic fields inductive coupling is higher and sample (or body) noise usually dominates and overcomes coil conductor noise.
Nonoverlapping phased array coil:
Phased array coils often rely on geometric overlapping of coil elements to reduce coupling (and the accompanying crosstalk) between adjacent coil elements. An eight-channel, nonoverlapping phased array coil for 3 Tesla systems was recently demonstrated that achieves this decoupling between array elements through intercoil capacitive networks, requiring only standard system preamplifiers [28]. To overcome the mutual inductance that results from the nonoverlapping geometry, decoupling capacitance is installed between adjacent loops. By increasing the space between elements, SNR is improved. Scan time reduction from the improved SNR obtained in the GRAPPA hip scans reaches up to a factor of 4, which is highly significant for the imaging of trabecular structure in osteoporosis or cartilage in osteoarthritis.
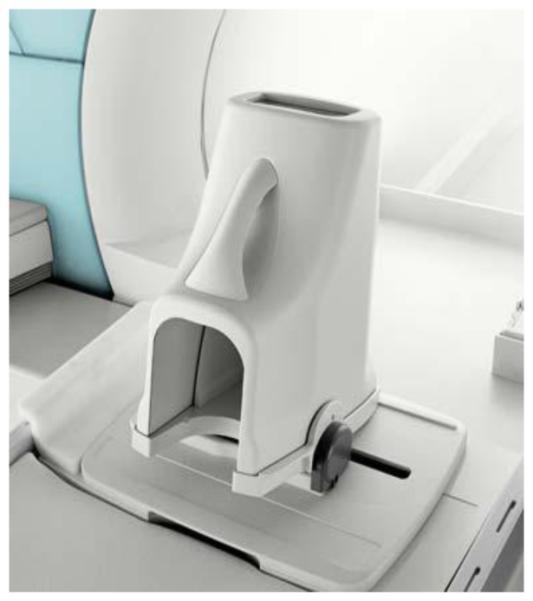
Dedicated limb scanners
Dedicated extremity scanners are available from several vendors. These systems use reduced size and weight magnets and electronics, making them convenient to locate in an outpatient imaging clinic or orthopedics office. These systems have a reduced imaging volume and are used primarily for imaging knees, ankles, wrists, and hands. They are typically low field (around 0.2-0.35 Tesla) and usually based on Neodymium Iron Boron permanent magnets (although at least one vendor has offered systems based on 1 Tesla or 1.5 Tesla superconducting magnets). These systems typically have a single built in volume RF coil that is used for both excitation and reception of the MR signal. An example is shown in Figure 12.
Figure 12.
Dedicated Extremity/Limb MRI Imager; Courtesy Time Medical Ltd. Singapore.
Geometry Embracing Method (GEM) coils
GE Healthcare and NeoCoil have recently developed a line of what they call Geometry Embracing Method (GEM) coils. These coils consist of interchangeable phased-array receive-only elements, each designed to suit imaging of various anatomic regions and applications. The operator can easily swap in the most appropriate array for the application. One example is the GEM Flex Coil system for 1.5 Tesla GE systems, shown in Figure 13. This system is comprised of 3 components: (1) several formable, flexible, and detachable 16-element receive arrays of different sizes, (2) an interface module containing the pre-amplifiers and other circuitry necessary to connect the receive arrays to the MR system, and (3) various accessory immobilization devices for patient comfort and stabilization. The 16-channel arrays can be used individually or can be combined for 32 channel scanning. Each array consists of 16 individual loop elements, arranged to achieve practical parallel imaging acceleration factors of 2-3. Although each array contains 16 receive elements, the system can be used with 8-channel MRI systems; combiner circuitry mixes element signals in this case to achieve the desired channel input to the MRI system. Such systems allow the flexibility of multiple receive arrays adapted to the anatomy to be imaged while eliminating the need for redundant interface circuitry with each array.
Figure 13.
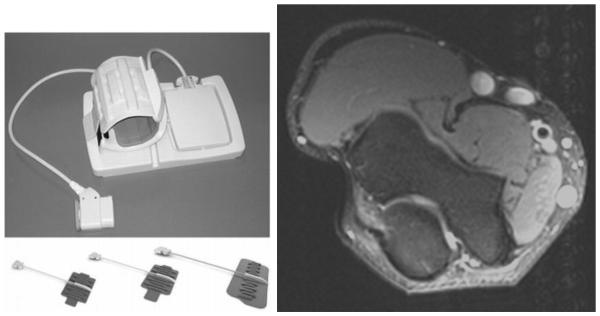
NeoCoil's 16 Channel GEM Flex Coil along with an elbow image obtained at 3.0T using this coil (Courtesy of NeoCoil, WI, USA).
CONCLUSION
As MRI technology has matured and main polarizing field strengths have increased, there has been a renaissance in RF coil design and development. The availability of a high number of receive channels, the advent of parallel imaging techniques, the proliferation of non-proton MRI techniques, and the changing RF environment at higher field strengths have all fueled this renaissance. There are currently a staggering variety of RF coils and coil systems available. They vary in size, weight, field strength of operation, complexity, and application. Each of these designs has specific advantages and disadvantages. Coils with large transmit elements typically achieve higher flip-angle uniformity, but can deposit significant RF energy in the scan subject (leading to patient heating concerns). Coils employing arrays of small receive coils positioned close to the anatomy of interest can achieve excellent SNR, but often have somewhat non-uniform receive sensitivity. Considerable effort is being put into the development of new and improved coil configurations for musculoskeletal applications. Better hardware, more efficient pulse sequencing and the availability of whole body scanners with high field strength have created a deviation from the practice of using proton-only coils, exploring and utilizing dualtuned coils such as 1H/23Na coils. The developments in MR imaging using these coils are making remarkable headway and are having significant impact on providing new insight to understanding early detection of MSK disease processes and providing clinicians greater access and flexibility for imaging bones and joints, ultimately leading to better patient care.
Footnotes
In practice, the doubling in SNR is typically not quite achieved.
REFERENCES
- 1.Nnewihe AN, et al. Custom-fitted 16-channel bilateral breast coil for bidirectional parallel imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011 doi: 10.1002/mrm.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pruessmann KP. Parallel imaging at high field strength: synergies and joint potential. Topics in magnetic resonance imaging : TMRI. 2004;15(4):237–44. doi: 10.1097/01.rmr.0000139297.66742.4e. [DOI] [PubMed] [Google Scholar]
- 3.Sodickson DK, Griswold MA, Jakob PM. SMASH imaging. Magnetic resonance imaging clinics of North America. 1999;7(2):237–54. vii-viii. [PubMed] [Google Scholar]
- 4.Katscher U, et al. Transmit SENSE. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2003;49(1):144–50. doi: 10.1002/mrm.10353. [DOI] [PubMed] [Google Scholar]
- 5.Hayes CE. The development of the birdcage resonator: a historical perspective. NMR in biomedicine. 2009;22(9):908–18. doi: 10.1002/nbm.1431. [DOI] [PubMed] [Google Scholar]
- 6.Hayes CE, et al. An Efficient, Highly Hokogeneous Radiofrequency Coil for Whole-Body NMR Imaging at 1.5 T. Journal of magnetic resonance imaging : JMRI. 1985;63:622–628. [Google Scholar]
- 7.Roemer PB, et al. The NMR phased array. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1990;16(2):192–225. doi: 10.1002/mrm.1910160203. [DOI] [PubMed] [Google Scholar]
- 8.Watkins RD, et al. Whole body RF coil for 3 Tesla MRI system; Proceedings of the 9th Annual Meeting of ISMRM; Glasgow, Scotland. 2001. [Google Scholar]
- 9.Watkins RDR. 300MHz Quadrature Shielded Birdcage Head Coil for 7 Tesla; Proceedings of the 11th Annual Meeting of the ISMRM; Toronto. 2003. K. W. [Google Scholar]
- 10.Vaughan JT, et al. Efficient high-frequency body coil for high-field MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;52(4):851–9. doi: 10.1002/mrm.20177. [DOI] [PubMed] [Google Scholar]
- 11.Finnerty M, et al. Intl. Soc. Mag. Reson. Med. (ISMRM) Toronto: 2008. A 3D Parallel Imaging Capable Transmit and 15-Channel Receive Array Knee Coil at 3T. [PMC free article] [PubMed] [Google Scholar]
- 12.Finnerty M, et al. A 7-Tesla High Density Transmit with 28-Channel Receive-Only Array Knee Coil, in Intl. Soc. Mag. Reson. Med. (ISMRM)2008: Toronto. [PMC free article] [PubMed] [Google Scholar]
- 13.Boada FE, et al. Fast three dimensional sodium imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1997;37(5):706–15. doi: 10.1002/mrm.1910370512. [DOI] [PubMed] [Google Scholar]
- 14.Staroswiecki E, et al. In vivo sodium imaging of human patellar cartilage with a 3D cones sequence at 3 T and 7 T. Journal of magnetic resonance imaging : JMRI. 2010;32(2):446–51. doi: 10.1002/jmri.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy R, et al. Sodium MRI of human articular cartilage in vivo. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1998;39(5):697–701. doi: 10.1002/mrm.1910390505. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro EM, et al. 23Na MRI accurately measures fixed charge density in articular cartilage. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2002;47(2):284–91. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheaton AJ, et al. Proteoglycan loss in human knee cartilage: quantitation with sodium MR imaging--feasibility study. Radiology. 2004;231(3):900–5. doi: 10.1148/radiol.2313030521. [DOI] [PubMed] [Google Scholar]
- 18.Shen GX, Boada FE, Thulborn KR. Dual-frequency, dual-quadrature, birdcage RF coil design with identical B1 pattern for sodium and proton imaging of the human brain at 1.5 T. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1997;38(5):717–25. doi: 10.1002/mrm.1910380507. [DOI] [PubMed] [Google Scholar]
- 19.Murphy-Boesch J, et al. Two configurations of the four-ring birdcage coil for 1H imaging and 1H-decoupled 31P spectroscopy of the human head. Journal of magnetic resonance. Series B. 1994;103(2):103–14. doi: 10.1006/jmrb.1994.1017. [DOI] [PubMed] [Google Scholar]
- 20.Tropp JSB, Derby Kevin A. Dual-tuned RF coil for MRI spectroscopy. Toshiba America MRI, Inc.; 1991. CA. (San Bruno, CA) So. San Francisco, CA. United States. [Google Scholar]
- 21.Peterson B, et al. Performance Comparison of a Hybrid Dual-Tuned 23Na/1H Birdcage to Single-Tuned 23Na Birdcage with Identical Geometry; Proceedings of the 18th Annual Meeting of the ISMRM; Stockholm. 2010. [Google Scholar]
- 22.Watkins RD, et al. High SNR Dual Tuned Sodium/Proton Knee Coil; 18th ISMRM Meeting2010; Stockholm. [Google Scholar]
- 23.Ha S, et al. A PIN diode controlled dual-tuned MRI RF coil and phased array for multi nuclear imaging. Physics in medicine and biology. 2010;55(9):2589–600. doi: 10.1088/0031-9155/55/9/011. [DOI] [PubMed] [Google Scholar]
- 24.Shen GX. Quadruple tuned (23Na, 7Li, 31P,1H) Band/Lowpass Birdcage Coil at 3.0T. in Intl. Soc. Mag. Reson. Med. (ISMRM) Philadelphia: 1999. [Google Scholar]
- 25.Avdievich NI, et al. 4T split TEM volume head and knee coils for improved sensitivity and patient accessibility. Journal of magnetic resonance. 2007;187(2):234–41. doi: 10.1016/j.jmr.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsch GH, et al. High-resolution morphological and biochemical imaging of articular cartilage of the ankle joint at 3.0 T using a new dedicated phased array coil: in vivo reproducibility study. Skeletal radiology. 2008;37(6):519–26. doi: 10.1007/s00256-008-0474-z. [DOI] [PubMed] [Google Scholar]
- 27.Ma QY, et al. Superconducting RF coils for clinical MR imaging at low field. Academic radiology. 2003;10(9):978–87. doi: 10.1016/s1076-6332(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 28.von Morze C, et al. An eight-channel, nonoverlapping phased array coil with capacitive decoupling for parallel MRI at 3 T. Concepts in magnetic resonance. Part B, Magnetic resonance engineering. 2006;31B:37–43. DOI: 10.1002/cmr.b.20078. [Google Scholar]