Abstract
BACKGROUND
Management of asymptomatic early stage chronic lymphocytic leukemia (CLL) centered on expectant surveillance for active disease warranting chemotherapy. In CLL, elevated serum β-2 microglobulin (β2M) levels were associated with more rapid disease progression and shorter survival (OS).
METHODS
An early intervention trial was designed to assess response, time-to-progression (TTP), and OS after immunotherapy with standard-dose rituximab (375 mg/m2 intravenously weekly for 8 consecutive weeks) in 34 asymptomatic untreated early stage CLL with β2M level ≥ 2 mg/dL.
RESULTS
Long-term follow-up and results are reported. The overall response rate in 34 patients was 82% (9% complete [CR]), median TTP in the 28 responders was 23 months, the median time to subsequent treatment was 43 months, and the 8-year OS rate was 74% (median follow-up, 102 months).
CONCLUSIONS
Early treatment with rituximab was well tolerated and safe. Further studies are needed to determine if this intervention can decrease CLL-related morbidity and mortality.
Keywords: chronic lymphocytic leukemia, rituximab, therapy, early stage
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease with an extremely variable course.1 Patients with “smoldering” CLL appear to have a life span similar to age-matched controls in the general population.2,3 Similarly, early stage asymptomatic CLL (Rai stage 0-II without active disease as defined by the National Cancer Institute Working Group [NCI-WG] Criteria) is associated with a favorable prognosis with a reported median overall survival (OS) that ranges from 8 to 12 years.2,4 Currently, management of asymptomatic early stage CLL centers on expectant surveillance for active disease warranting chemotherapy. However, survival outcomes vary considerably within this subset depending on prognostic factors at presentation. Early stage patients with unfavorable features are at higher risk for disease progression, and, therefore, may benefit from early intervention rather than the traditional watch-and-wait approach.
The large randomized clinical trials conducted by the French Cooperative Group in the 1980s for asymptomatic early stage CLL were limited to potentially toxic chemotherapeutics (alkylating agents).5,6 Early treatment with daily chlorambucil or intermittent chlorambucil/prednisone was associated with slower disease progression, but not longer survival when compared with the observation arms. The lack of survival benefit was attributed to selection of resistant CLL clones due to early exposure to chlorambucil, limited availability at that time of effective salvage regimens, and excess deaths from secondary epithelial cancers. With regard to the clinical course of the disease, these randomized trials showed that at least 40% of patients with early stage CLL eventually require therapy for progressive disease, and that approximately 25% ultimately die of disease-related causes.
One recognized risk factor for progression in CLL is elevated serum β-2 microglobulin (β2M), a 12-kDa light chain protein that binds to the α-chain of major histocompatability class 1 antigens and dissociates into extracellular fluids after degradation of the complex. The prognostic relevance of serum β2M has been well-established in various hematologic malignancies, particularly CLL.7–9 We had previously assessed β2M levels in 190 untreated early stage CLL patients, and noted shorter median OS for β2M ≥ 2 mg/dL (75 versus 115+ months, P = .02). This indicated that serum β2M levels ≥ 2 mg/dL could be used to identify a subset of early stage CLL with a shorter median survival that could potentially benefit from early intervention.
Rituximab is a relatively well-tolerated chimerical monoclonal antibody directed at surface CD20 with significant activity in previously-treated indolent non-Hodgkin lymphoma (NHL), except for the markedly inferior response rate of 12% in the International Working Formulation A subset (tissue equivalent of CLL).10 Overall response (OR) rates improved to 36% with dose-escalation or to 45% with increased dose-intensity of rituximab.11–13 In patients with untreated CLL with indications for therapy, the OR rate after 4 weeks of standard-dose rituximab was 51% (complete [CR] rate 4%).14 Herein, we report the long-term outcomes of the first prospective pilot study evaluating early intervention with extended dosing single-agent standard-dose rituximab in patients with asymptomatic, early stage disease and high β2M CLL.
PATIENTS AND METHODS
Patients
Between March 2000 and October 2001, 34 patients with previously untreated early stage (Rai 0-II) CLL with β2M levels ≥ 2 mg/dL without indications for therapy per the NCI-WG guidelines participated in this trial (Patients characteristics are summarized in Table 1). Median age was 66 years and median time from diagnosis to the start of rituximab treatment was 25 months. The protocol was approved by the Institutional Review Board at MD Anderson Cancer Center. Informed consent for participation was obtained in accordance with institutional guidelines and the Declaration of Helsinki.
Table 1.
Characteristics of Patients With Early Stage Higher Risk CLL Treated With Single-Agent Extended Dosing Rituximab
| Parameter | Median (range) |
|---|---|
| Age, y | 66 (44–82) |
| Median months from diagnosis (range) | 25 (1–219) |
| Hematological | |
| WBC × 109/L | 40 (9.2–181) |
| Hemoglobin (g/dL) | 13 (10–16)a |
| Platelet × 109/L | 160 (116–321) |
| Beta-2 microglobulin (mg/dL) | 3 (2–12.8) |
| No. (%) | |
|---|---|
| Sex | |
| Male | 18 (53) |
| ECOG performance status | |
| 0 | 8 (24) |
| 1 | 26 (76) |
| Rai stage | |
| 0 | 7 (21) |
| I | 15 (44) |
| II | 12 (27) |
CLL indicates chronic lymphocytic leukemia; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell count; IgH, immunoglobulin heavy.
Anemia in 1 patient was attributed to concomitant rheumatologic disorder; note that study antedated standardized IgH somatic mutation or ZAP-70 assays.
Study Design
Eligibility criteria
To be eligible to participate in this study, patients were required to have untreated CLL, Rai stage 0 to II disease, serum β2-M level of 2 mg/L or higher, without indications for treatment as per 1996 National Cancer Institute-Working Group (NCI-WG) guidelines (4). Other eligibility requirements were an Eastern Cooperative Oncology Group performance status of 0 to 2, absence of active infection, and maintained organ function, which was defined as a total bilirubin level less than 3 mg/dL, serum hepatic transaminases less than 3 times upper normal level, and a creatinine level less than 3 mg/dL.
Treatment
Rituximab was administered intravenously at a dose of 375 mg/m2 once a week for 8 consecutive weeks. All patients received single doses of dyphenidramine (25 mg) and acetaminophen (650 mg) before rituximab administration. No antibiotic or anti-viral prophylaxis was given during this study.
Response criteria
We used the 1996 NCI-WG guidelines for response (4) to assess the activity of rituximab. Responses were assessed at 3, 6, and 12 months from the start of treatment and yearly thereafter. Toxicity reporting was determined as per National Cancer Institute Common Toxicity Criteria (version 3.0).
Statistical Design
Response duration, time to disease progression, and patient survival were calculated according to the Kaplan-Meier method. Median overall survival rate was estimated by a Kaplan-Meier curve for all patients who received at least 1 dose of rituximab, and survival duration was measured from the initiation of therapy. Progression-free survival rate was measured from the time the patient first received rituximab to the time of disease progression, or the date of the last clinical exam, or the date of last available data if disease progression was not documented. Response rate was one of the end-point, and a response rate of 40% to 50% was considered to be of interest and warren further investigation in this patient population. The statistical design was a two-stage design by Simon.
RESULTS
Response and toxicity data were available for the entire patient population of 34 patients. Complete remission (CR) was observed in 3 patients (9%), nodular partial remission (nodular PR) in 7 patients (21%), and partial remission (PR) in 18 patients (53%), yielding an overall response (OR) rate of 82%. Six patients did not respond to treatment (18%). Responses are summarized in Table 2.
Table 2.
Responses of Patients With Early Stage Higher Risk CLL Treated With Single-Agent Extended Dosing Rituximab
| Response | No. (%) |
|---|---|
| Overall (OR) | 28 (82) |
| Complete(CR) | 3 (9) |
| Nodular partial | 7 (21) |
| Partial | 18 (53) |
| No response | 6 (18) |
| Flow cytometry, bone marrow (CD19+CD5+) (n=29) | |
| <5% | 5 (15) |
| <1% | 2 (6) |
| OR, CR by beta-2 microglobulin (mg/dL)a | |
| <3 | 15 (88), 2 (12) |
| ≥3 | 13 (76), 1 (6) |
P value, not significant.
The amount of residual marrow disease was quantified by immunoflow cytometry at the time of response evaluation in 29 patients. The neoplastic subpopulation of cells (defined as lymphocytes with CD5/CD19 co-expression and light chain restriction) accounted for less than 5% of the total population of analyzed cells in 5 patients and less than 1% in 2 patients (Table 2).
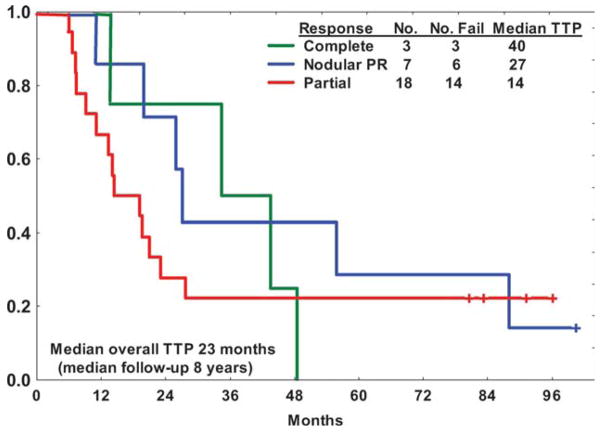
The median overall TTP and time to retreatment (TTR) in the 28 responding patients were 23 and 43 months, respectively (Fig. 1). The median TTP and TTR were longer for patients who achieved CR or nodular PR compared with PR (40 and 63 months for CR, 27 and 56 months for nodular PR, 14 and 31 months for PR, respectively, P = .03 for TTP, no statistically significant difference for TTR). The median TTP was longer for pretreatment β2M levels below the median (3 mg/dL) (30 vs 18 months, P = .02).
Figure 1.
Time-to-progression (TTP) in 28 responders stratified by quality of response to extended therapy with single-agent rituximab. CR, complete remission; nPR, nodular partial remission; PR, partial remission.
All but 3 patients completed the planned therapy (treatment was discontinued for progression/lack of response after 2, 4, and 7 weekly doses of rituximab in each patient, respectively). Treatment related toxicity was limited to expected mild-moderate infusional side effects. None of the patients discontinued treatment early because of toxicity and no infection complications were observed.
Twenty-five patients (74%) received subsequent therapy. Twelve patients (48%) received chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) with an OR rate of 92% (CR rate 67%). Nine patients (36%) were retreated with rituximab (± Granulocyte/macrophage colony stimulating factor [GM-CSF]) with an OR rate of 56%. The remaining 4 patients were treated with single agent alemtuzumab (1 patient), fludarabine (1 patient), chlorambucil (1 patient), or lenalidomide (1 patient).
The 8-year OS rate was 74%; 8 patients died (all after subsequent therapy); 4 from disease-related causes. With median follow-up of 102 months (range, 84–120 months), the median OS was 110+ months.
DISCUSSION
In this study, a small group of asymptomatic patients with Rai stage 0 to II CLL and elevated serum β2M level (≥ 2 mg/dL) were treated with standard-dose rituximab for 8 consecutive weeks. Use of β2M as the prognostic feature to guide selection for early intervention was based on our prior retrospective data analysis which showed higher baseline levels were predictive of shorter survival in this group of patients, and potential application to clinical practice with availability of rapid standardized assays for β2M measurement. Subsequently to the conduction of this trial, the large prospective randomized CLL4 trial of initial therapy for CLL confirmed that higher pretreatment β2M levels (> twice the upper level of normal) were associated with shorter TTP and OS irrespective of treatment assignment supporting the relevance of β2M levels as prognostic indicators.15 Furthermore, this study was designed in 1999 when the prognostic relevance of genomic aberrations (as evidenced by fluorescent in situ hybridization [FISH] analysis), IgVH mutation status, and expression of ZAP-70 had not been yet reported. The βeta2M levels, instead had been available at our center for years allowing us to review the survival expectations based on this marker.
In these patients without indications for chemotherapy, immunotherapy was believed to be an appropriate intervention owing to the activity of rituximab in untreated indolent NHL and the favorable toxicity profile unlikely to promote resistance to subsequent therapy or to affect hematopoietic stem cells. An extended dosing schedule was selected to potentially increase efficacy.
The OR rate observed in this study is comparable to the French early intervention experience (86% for daily chlorambucil; 69% for chlorambucil/prednisone). Flow cytometric quantitation of residual disease in the bone marrow suggested that even single-agent therapy with rituximab could induce significant reduction in disease burden. The overall TTR was shorter than the 63 months reported with chlorambucil in the French randomized trials, but this parameter is influenced by frequency of monitoring, subjective interpretations of “active” disease, and potential for retreatment with rituximab (or other targeted agents) in situations not mandating definitive chemotherapy.
At the time of this report, the median OS is 110+ months and this compared favorably with our previous experience (median OS, 75 months) for age-matched patients with asymptomatic early stage CLL and elevated β2M who did not undergo early intervention.
A more recent study was reported by Zent et al with combined treatment with rituximab and alemtuzumab in 30 patients with CLL and without standard indications for treatment, but at least 1 high risk feature (17p del, 11q del or unmutated IgvH concomitantly with the presence of expression of ZAP-70 or CD-38). In this trial, the OR rate (90%) and CR rate (37%) associated with both antibodies used in combination was higher than those observed in our study. However, duration of response and TTR appeared shorter than our single agent rituximab experience with a median duration of response of 14 months and a median TTP of 12.5 months. This approach was also associated with a rate of CMV reactivation of 10% and an occurrence of neutropenia of 17%. However, the results of these 2 clinical trials cannot be directly compared in view of the differences in patient selection (higher risk patients in the population studied by Zent et al) and therapy (inclusion of alemtuzumab).
Other ongoing early intervention trials are using fludarabine, rituximab with alemtuzumab, lenalidomide, or other agents (eg, omega-3, GM-CSF) for high-risk CLL as defined by adverse genomic abnormalities (17p or 11q deletions) or unmutated IgvH.
In our experience, early therapy with rituximab did not significantly compromised efficacy of subsequent rituximab-based therapy. The CR rate in the small subset that underwent subsequent treatment with FCR more closely resembled the results in therapy-naive CLL as opposed to the salvage experience.16–18
In conclusion, the results of our study indicate that early treatment with rituximab is well tolerated and safe. Larger studies will need to be conducted to evaluate whether there is a survival benefit to early intervention with rituximab, and to assess the cost-effectiveness of this approach, which currently remains investigational in nature.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Research funding provided by Genentech, Inc, and Biogen Idec.
References
- 1.Horner MJ, Ries LAG, Krapcho M, et al. SEER cancer statistics review, 1975–2006. Bethesda, MD: National Cancer Institute; 2009. Available at: http://seer.cancer.gov/csr/1975_2006 Based on November 2008 SEER data submission, posted to the SEER website. [Google Scholar]
- 2.Effects of chlorambucil and therapeutic decision in initial forms of chronic lymphocytic leukemia (stage A): results of a randomized clinical trial on 612 patients. The French Cooperative Group on Chronic Lymphocytic Leukemia. Blood. 1990;75:1414–1421. [PubMed] [Google Scholar]
- 3.Molica S. Progression and survival studies in early chronic lymphocytic leukemia. Blood. 1991;78:895–899. [PubMed] [Google Scholar]
- 4.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 5.Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med. 1998;338:1506–1514. doi: 10.1056/NEJM199805213382104. [DOI] [PubMed] [Google Scholar]
- 6.Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst. 1999;91:861–868. doi: 10.1093/jnci/91.10.861. [DOI] [PubMed] [Google Scholar]
- 7.Hallek M, Wanders L, Ostwald M, et al. Serum beta(2)-microglobulin and serum thymidine kinase are independent predictors of progression-free survival in chronic lymphocytic leukemia and immunocytoma. Leuk Lymphoma. 1996;22:439–447. doi: 10.3109/10428199609054782. [DOI] [PubMed] [Google Scholar]
- 8.Wierda WG, O’Brien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–4685. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 9.Wierda WG, O’Brien S, Wang X, et al. Characteristics associated with important clinical end points in patients with chronic lymphocytic leukemia at initial treatment. J Clin Oncol. 2009;27:1637–1643. doi: 10.1200/JCO.2008.18.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 11.Piro LD, White CA, Grillo-Lopez AJ, et al. Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1999;10:655–661. doi: 10.1023/a:1008389119525. [DOI] [PubMed] [Google Scholar]
- 12.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 14.Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Oscier D, Wade R, Orchard J, et al. Biological markers: data from the LRF CLL4 trial. Leuk Lymphoma. 2007;48(suppl 1):S4–S5. [Google Scholar]
- 16.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 17.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]