Abstract
Animal models are a necessary component of systems neuroscience research. Determining which animal model to use for a given study involves a complicated calculus. Some experimental manipulations are easily made in some animal models but impossible in others. Some animal models are similar to humans with respect to particular scientific questions, and others are less so. In this review, I discuss work done in my laboratory to investigate the neural mechanisms of color vision in the rhesus macaque. The emphasis is on the strengths of the macaque model, but shortcomings are also discussed.
Keywords: Color, Vision, Monkey
A broad goal of neuroscience is to understand how the brain operates in states of health and disease. This goal is sufficiently lofty, and the brain sufficiently complex, that no single line of research can achieve it alone. Instead, scientific progress is made through inquiry into a variety of specific questions, using a variety of techniques and, frequently, animal models. In this review, I discuss research into the color vision system of macaques, focusing on recent work from my own lab as one example of how this model can be used to reveal how neural signals guide behavior.
Mammalian vision begins with the absorption of light by photosensitive cells at the back of the eye. In most mammals, daytime vision is mediated by two types of cone photoreceptor: one sensitive to short wavelengths, the other sensitive to longer wavelengths. The fact that the cone types are maximally sensitive to different wavelengths gives mammals an important ability: to dissociate light intensity from spectral content. When a light varies in intensity – say, when a cloud passes in front of the sun–activity in all cones changes (approximately) proportionally. This change is relayed to the brain and is interpreted as a change in luminance. If the spectral content of a light changes–say, as the setting sun’s rays are filtered progressively by the atmosphere–activity can change across cone types disproportionally. Disproportionate changes in cone activities often lead to colored percepts. Color vision is thus a perceptual experience associated with a comparison of signals across different classes of receptor. Similar comparisons are likely to occur elsewhere in the nervous system, and understanding how such comparisons mediate color vision may guide our understanding of neural signal processing more generally.
Humans have three distinct types of cone photoreceptor (as do other cattarhine primates; for a scholarly review of the evolution of primate cones, the interested reader is referred to Jacobs 2009). These three cone types are named after the wavelengths to which they are maximally sensitive: the L-, M-, and S-cones are maximally sensitive to long, medium, and short wavelengths, respectively. By comparing signals across all three cone types, the trichromatic visual system supports a rich gamut of color experiences. Some non-primate species may enjoy an even richer color experience: most birds and reptiles have four or five cone types, and the mantis shrimp has 12.
The similarity between the human and macaque visual systems goes beyond trichromacy. The absorption spectra of the three types of cone in macaques and humans are very similar (Bowmaker 1990). Macaque and human visual systems are anatomically similar at the level of the retina (Dacey 2000), lateral geniculate nucleus (LGN) (Garey, Dreher et al. 1991), striate cortex (V1) (Casagrande and Kaas 1994, Bernard, Lubbers et al. 2012), and early extrastriate cortex (Orban, Van Essen et al. 2004). Area V1 of macaques and humans stain similarly (but not identically) for cytochrome oxidase (Horton and Hubel 1981, Horton and Hedley-Whyte 1984, Preuss, Qi et al. 1999), an enzyme whose expression correlates with activity level and, for reasons that are not understood, domains that appear particularly relevant to color processing (Livingstone and Hubel 1984, Ts’o and Gilbert 1988). Chromatic detection and discrimination thresholds are similar in humans and macaques (De Valois, Morgan et al. 1974, Merigan 1989, Kalloniatis and Harwerth 1991, Gagin, Bohon et al. 2014). Damage to homologous areas of the macaque and human temporal lobe result in similar visual deficits, including deficits in color processing (Heywood, Gaffan et al. 1995). Collectively, this body of work demonstrates the utility of the macaque model for studying color vision and supports the idea that what we learn about color processing in the macaque will transfer directly, or nearly so, to the human.
Over the past several decades, a standard model of the first stages of color processing has emerged (De Valois and De Valois 1993, Lee 1996). According to this model, signals from the L- and M-cones are combined roughly additively and transmitted through the magnocellular layers of the LGN. This rapidly conducting pathway plays an important role for the perception of luminance, especially luminance-defined patterns in motion. In parallel with the magnocellular pathway, the parvocellular pathway combines L- and M-cone signals antagonistically to convey chromatic information and fine-scale luminance information to the LGN. Finally, at least two more anatomically distinct channels carry signals from the S-cones. These S-cone-dominated channels also receive inputs from a combination of L- and M-cones with opposite sign, although exactly what contribution each cone type makes to this combination is still being worked out (Tailby, Solomon et al. 2008, Martin and Lee 2014, Miyagishima, Grunert et al. 2014).
Why S-cone signals are processed differently from L- and M-cone signals is presumably related to the fact that L- and M-cones diverged from a common ancestral cone type relatively recently. Prior to this divergence, all mammals were probably dichromats, with a set of S-cones and another set of cones that was sensitive to longer wavelengths. By comparing signals between these two cone types, these animals would have been able to distinguish lights with a greater proportion of long or short wavelengths. The addition of a third cone type allowed for a finer spectral analysis; it is in part due to this finer spectral analysis that nearby wavelengths in the yellow part of the spectrum are readily distinguishable to us. Color vision, as we experience it, is thus the product of a relatively new pathway, which pits L- and M-cone signals against each other, laid down on top of an evolutionary older pathway, which compares S-cone signals to a combination of L- and M-cone signals. By studying how signals from the old and new pathways interact, we have an exciting opportunity in color vision to learn how evolution shapes neural signal processing and the perception that results from it.
Cone signal combination is relatively well understood in the retina and LGN, but is less well understood at the next stage of visual processing, in area V1. This is the frontier that my lab has been working on. One approach we have taken is to probe V1 neurons with low contrast stimuli. We reasoned that color processing in V1, though quite complicated at high contrasts, may be simpler at low ones (many nonlinear systems behave approximately linearly in response to small perturbations). For example, human color vision behaves nonlinearly at high contrasts, but psychophysical chromatic detection thresholds can be described with a simple, linear mathematical model. According to this model, chromatic detection occurs whenever the difference between L- and M-cone signals reaches a threshold or whenever a difference between S-cone signals and a sum of L- and M-cone signals reach a threshold. The crossing of these two different thresholds is associated with different chromatic percepts in humans (Mullen and Kulikowski 1990). These, and related observations, has led to the idea that signals carried by the new and old color pathways are detected by entirely separate populations of neurons. Our data, however, are inconsistent with this hypothesis (Hass and Horwitz 2013).
We tested this hypothesis with an experiment that exploited two advantages of the macaque model in addition to those previously mentioned. First, macaques can be trained to perform chromatic detection tasks, and in such tasks the old and new color pathways appear to contribute to performance similarly to the way they do in humans (Krauskopf, Williams et al. 1982, Stoughton, Lafer-Sousa et al. 2012). Second, using extracellular electrodes inserted directly into the brain, we can record the electrical activity of individual neurons during task performance, giving us the opportunity to correlate neuronal events with behavioral ones.
We recorded from V1 neurons as a rhesus macaque reported the location of a faint chromatic flash. To our surprise, some individual V1 neurons responded to threshold-contrast signals whether carried by the new or the old pathway, in other words whether the contrast was between the L- and M-cones, or between the S-cones and the other two (Figure 1). This result shows that even at low contrasts, the new and old pathways converge onto individual V1 neurons.
Figure 1.
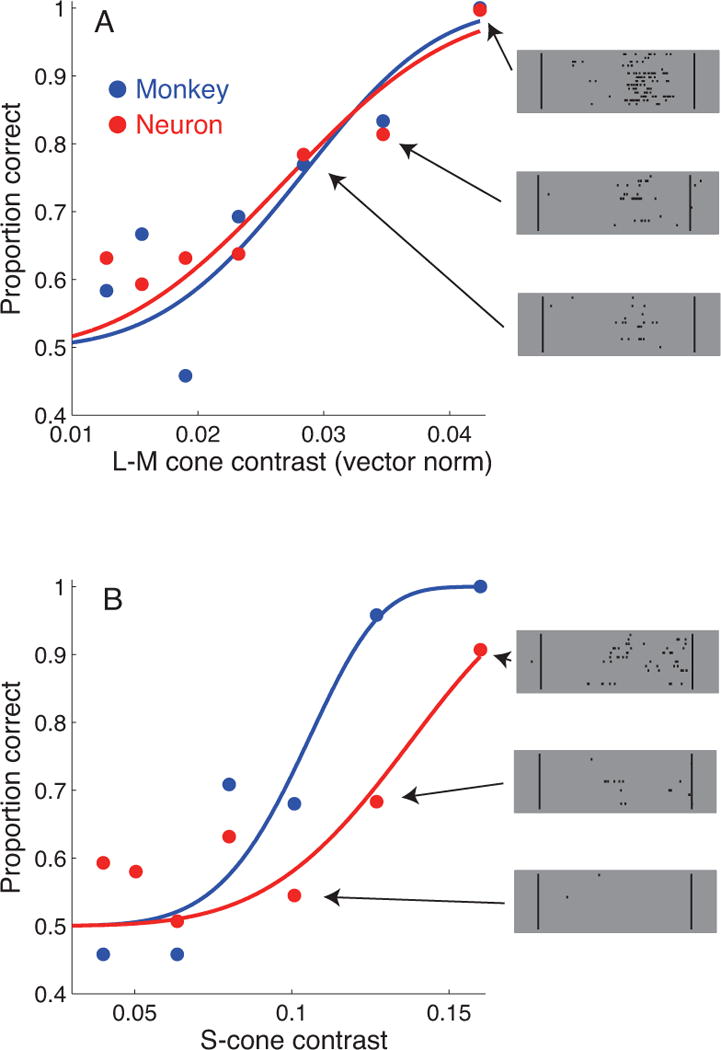
Chromatic detection functions for a single V1 neuron (red) and the macaque (blue). See (Hass and Horwitz 2013) for methodological details. In randomly interleaved trials, stimulus modulations were antiphase modulations of the L- and M-cones (A) or modulations of the S-cones alone (B). Neurometric sensitivity was quantified by an ideal observer analysis on the measured spike counts. Example rasters for the three highest contrast stimuli are shown (right).
We followed up this result by recording the responses of V1 neurons to stimuli that were identical to the ones we had used in the psychophysical experiments, but varied over a wider range of color and contrast. In these experiments, we presented visual stimuli and recorded neuronal responses but did not require the macaques to make psychophysical judgments. The only behavior required was visual fixation on a small dot, a behavior that a trained macaque can perform, with brief breaks, for hours.
Consistent with the neuronal sensitivity we had observed previously, we found a population of V1 neurons that were very broadly tuned for color. When represented in a 3D cone contrast space, the collection of stimuli that excited these neurons (at an arbitrary level set by the experimenter) traced out an ellipsoid (Figure 2A). This suggested that some V1 neurons compute the functional equivalent of a sum of squared LGN signals (Horwitz and Hass 2012). This quantity contains much information about the contrast of a stimulus, but relatively little about its color. Accordingly, neurons of this type could support performance on a chromatic detection task, but are unlikely to contribute significantly to hue perception. Color vision is not a monolithic entity, but rather a collection of distinct but related abilities, some of which (e.g. detection) are easier to study in the macaque than others (e.g. color naming).
Figure 2.
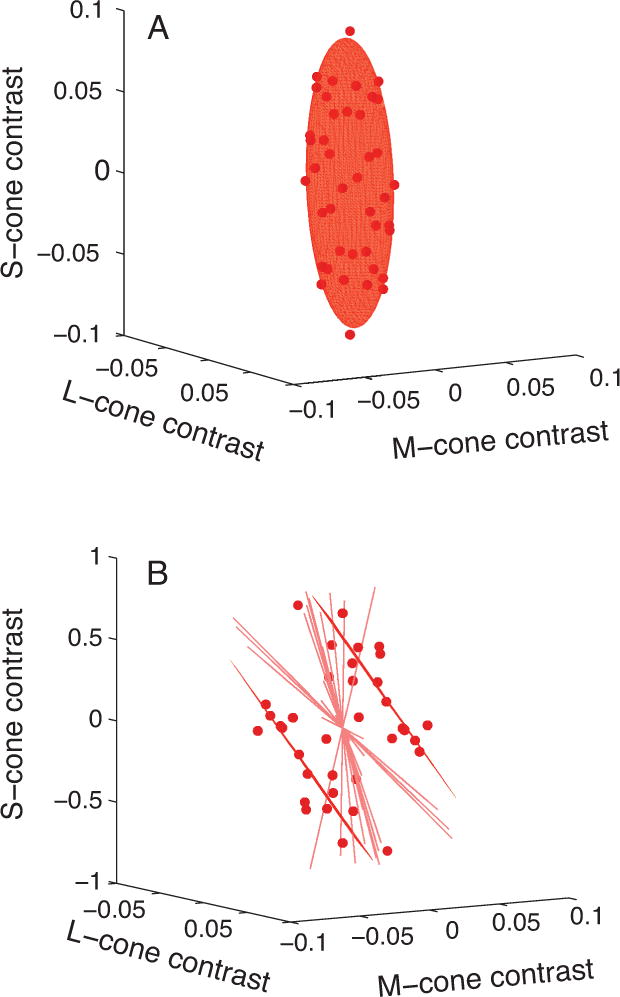
Isoresponse surfaces for two V1 neurons. See (Horwitz and Hass 2012) for methodological details. In each plot, the origin represents a blank gray screen, and distance from the origin represents contrast. Points represent stimuli that caused the neuron to discharge a criterion number of spikes. Points and surface fits are constrained to be symmetric about the origin because the stimuli used in the experiment were symmetric modulations through gray. A: Isoresponse surface from a neuron that responded in every color direction tested. B: Isoresponse surface from a neuron that responded to the sum of L- and M-cone contrasts. Radial lines in B represent directions in the stimulus space in which a criterion response could not be measured. Note the difference in y-axis scale between A and B.
Other V1 neurons that we investigated appeared to compute a sum of cone signals without the squaring–in other words, the set of stimuli that excite them at a common level occupy a plane in cone contrast space. A particularly interesting population of these neurons responded to a sum of L- and M-cone contrasts (Figure 2B). Such a sum of L- and M-cone contrasts is essentially the definition of “luminance”, a photometric quantity that describes the spectral sensitivity of the average human observer under a specific set of spatiotemporal conditions. The human visual system presumably contains a population of neurons that combines signals from the L- and M-cones in a nearly additive way to mediate luminance perception, and it is reasonable to assume that the macaque visual system does too. The L+M-sensitive population of V1 neurons that we recorded are good candidates for participation in this process, but with a small but significant difference. They appeared to receive a small S-cone input with the same sign as L- and M-cone inputs, whereas luminance in humans does not have an S-cone component.
Reasoning from this observation, we asked whether macaque luminance perception differs from human luminance perception in the way suggested by the spectral sensitivity of these neurons. If so, the V1 neurons we recorded from may participate in the macaque’s sense of luminance. To find out whether macaques and humans differ in their luminance sensitivity, we took advantage of the fact that luminance sensitivity is greater than chromatic sensitivity at high temporal frequencies. We adjusted the color and contrast of a rapidly flickering stimulus in these experiments using algorithms similar to those used in the previous set of experiments. In the new set of experiments, instead of finding stimuli that excited a neuron at a particular level, we found stimuli that were just barely detectable to a macaque observer. The stimulus selection algorithms were designed to reduce the number of trials needed to make the measurements, but data collection from the macaque still required 1680 detection trials spread over two days. Through these trials, the macaque had to remain a consistent, sensitive psychophysical observer for the algorithms to work properly and for the data to be interpretable – variability in the macaque’s performance could easily conceal the patterns we hoped to find.
As we had expected from classic human psychophysics, stimuli at the macaque’s detection threshold lay close to a plane in cone-contrast space (Figure 3A & 3B). The orientation of this plane was similar to the isoresponse planes we had measured from some V1 neurons, and both differed from the prediction given by the formal definition of luminance. This result indicates that the macaque (and its neurons) have a different spectral sensitivity than that of the average human observer. We confirmed a lack of S-cone input to luminance in humans under the conditions of our experiment by repeating these measurements in a human subject (Figure 3C & 3D).
Figure 3.
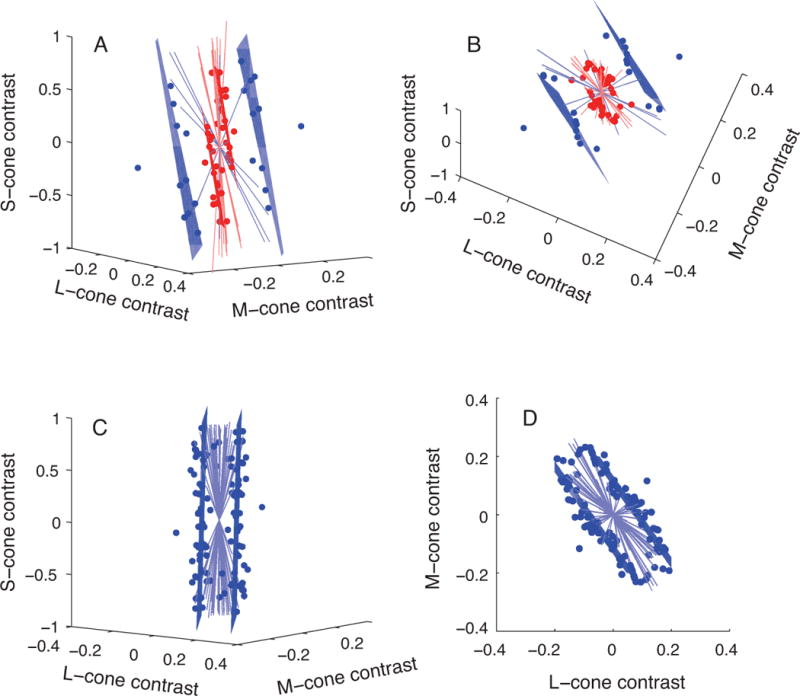
Isodetectability planes for a macaque (A & B) and a human (C & D). Blue points represent stimuli at the observer’s detection threshold, blue lines are color directions in which detection thresholds could not be measured, and planes are fits to these data. Red points and planes are redrawn from Figure 2B to illustrate the similarity in orientation between neuronal isoresponse planes and macaque isodetectability planes. Data in A & B are identical but are shown from different viewing angles (and similarly, C & D). The stimulus used in the psychophysical experiments modulated at 25 Hz for the macaque and at 15 Hz for the human.
The macaque and human visual systems are not identical. Differences are expected, and when they arise we should investigate them, not brush them under the rug (see Preuss 2000 for an important perspective on this point). Fortunately, we are in a good position to do this. The monkey and human visual systems are well understood in part because they are good models for each other – what we learn about one can inform us about the other. The symbiosis between human psychophysics and macaque neurophysiology has a long history and contributes greatly to the value to the macaque model.
To illustrate this symbiosis, consider the difference in spectral sensitivity between the human and macaque (Figure 3). One interpretation of these data is that S-cones contribute to luminance in macaques but not in humans. More parsimonious alternatives exist, however, and to find such alternatives, we need only to look to the literature on photopic human spectral sensitivity. Under the conditions of our experiment, short wavelength sensitivity depends on light absorption by the lens, and precise measurements of the human lens optical density spectrum are available in the literature (Stockman, Sharpe et al. 1999). To account for the data in Figure 3, we found that the optical density of the macaque lens would have to be ~60% of the average human lens. Such a difference is well within the possible range: macaques used in our experiments are quite a bit younger and smaller than the average human observer, and these factors are expected to reduce the macaque’s lens optical density by approximately this amount (Werner 1982, Lindbloom-Brown, Tait et al. 2014).
The macaque is an unmatched model system for understanding human trichromatic vision. Humans and macaques have the same three cone types, and signals from these are combined in similar if not identical ways at the earliest stages of postreceptoral processing. These similarities do not, of course, imply that macaques experience color in exactly the same way we do, but such a leap of faith is even less justified in animals whose visual systems differ more.
Techniques for measuring and manipulating electrical activity in the macaque visual system are well established, which is an advantage of this model, but unfortunately crude, which is a profound disadvantage. In genetically tractable animal models, electrical (and other types of) activity can be measured and controlled in relatively refined ways. Importantly, these manipulations can be targeted to particular cell populations of interest, defined on the basis of gene expression. Such manipulations, as currently implemented, are not currently feasible in macaques because they require large birth litters and a short latency from conception to sexual maturity. Developing alternative means to achieve the same end is an important direction for visual neuroscience. One promising approach is to engineer replication-deficient viral vectors that transduce a targeted cell type or to carry constructs that express only in targeted cells (Huang and Zeng 2013, Packer, Roska et al. 2013). Alternatively, transgenic macaques might become available to the point of practicality through the use of new genome editing techniques (Niu, Shen et al. 2014). Finally, experimental preparations could be developed that combine genetic tractability, a trichromatic visual system, and a wide behavioral repertoire (Tailby, Szmajda et al. 2008, Sasaki, Suemizu et al. 2009, Mitchell, Reynolds et al. 2014).
Highlights.
The macaque is valuable for neurophysiological studies of trichromatic vision
The visual systems of macaques and humans are similar but not identical
Understanding these differences facilitates data interpretation
Genetically targeted neural activity manipulations would be useful in macaques
Acknowledgments
Thanks to C. Hass, J.P. Weller, E. Grover, Z. Lindbloom-Brown, and L. Tait for invaluable assistance with monkey training and data collection. The work was supported by The McKnight Foundation and NIH grants RR000166, P30EY01730, and EY018849.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Bernard A, Lubbers LS, Tanis KQ, Luo R, Podtelezhnikov AA, Finney EM, McWhorter MM, Serikawa K, Lemon T, Morgan R, Copeland C, Smith K, Cullen V, Davis-Turak J, Lee CK, Sunkin SM, Loboda AP, Levine DM, Stone DJ, Hawrylycz MJ, Roberts CJ, Jones AR, Geschwind DH, Lein ES. Transcriptional architecture of the primate neocortex. Neuron. 2012;73(6):1083–1099. doi: 10.1016/j.neuron.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker JK. Advances in Photoreception: Proceedings of a Symposium on Frontiers of Visual Science. 1990. Cone visual pigments in monkeys and humans. (N. R. C. Committee on Vision: 19–30). [Google Scholar]
- Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: PA, RK, editors. Cerebral Cortex. Vol. 10. New York: Plenum; 1994. pp. 201–259. [Google Scholar]
- Dacey DM. Parallel pathways for spectral coding in primate retina. Annu Rev Neurosci. 2000;23:743–775. doi: 10.1146/annurev.neuro.23.1.743. [DOI] [PubMed] [Google Scholar]
- De Valois RL, De Valois KK. A multi-stage color model. Vision Res. 1993;33(8):1053–1065. doi: 10.1016/0042-6989(93)90240-w. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Morgan HC, Polson MC, Mead WR, Hull EM. Psychophysical studies of monkey vision. I. Macaque luminosity and color vision tests. Vision Res. 1974;14(1):53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- Gagin G, Bohon KS, Butensky A, Gates MA, Hu JY, Lafer-Sousa R, Pulumo RL, Qu J, Stoughton CM, Swanbeck SN, Conway BR. Color-detection thresholds in rhesus macaque monkeys and humans. J Vis. 2014;14(8) doi: 10.1167/14.8.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ, Dreher B, Robinson SR. The organization of the visual thalamus. In: Dreher B, Robinson SR, editors. Vision and Visual Dysfunction. Vol. 3. Boca Raton: CRC Press; 1991. pp. 176–234. [Google Scholar]
- Hass CA, Horwitz GD. V1 mechanisms underlying chromatic contrast detection. Journal of Neurophysiology. 2013;109(10):2483–2494. doi: 10.1152/jn.00671.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood CA, Gaffan D, Cowey A. Cerebral achromatopsia in monkeys. Eur J Neurosci. 1995;7(5):1064–1073. doi: 10.1111/j.1460-9568.1995.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hedley-Whyte ET. Mapping of cytochrome oxidase patches and ocular dominance columns in human visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984;304(1119):255–272. doi: 10.1098/rstb.1984.0022. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hubel DH. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981;292(5825):762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Hass CA. Nonlinear analysis of macaque V1 color tuning reveals cardinal directions for cortical color processing. Nat Neurosci. 2012;15(6):913–919. doi: 10.1038/nn.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Zeng H. Genetic approaches to neural circuits in the mouse. Annu Rev Neurosci. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. Evolution of colour vision in mammals. Philos Trans R Soc Lond B Biol Sci. 2009;364(1531):2957–2967. doi: 10.1098/rstb.2009.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalloniatis M, Harwerth RS. Effects of chromatic adaptation on opponent interactions in monkey increment-threshold spectral-sensitivity functions. J Opt Soc Am A. 1991;8(11):1818–1831. doi: 10.1364/josaa.8.001818. [DOI] [PubMed] [Google Scholar]
- Krauskopf J, Williams DR, Heeley DW. Cardinal directions of color space. Vision Res. 1982;22(9):1123–1131. doi: 10.1016/0042-6989(82)90077-3. [DOI] [PubMed] [Google Scholar]
- Lee BB. Receptive field structure in the primate retina. Vision Res. 1996;36(5):631–644. doi: 10.1016/0042-6989(95)00167-0. [DOI] [PubMed] [Google Scholar]
- Lindbloom-Brown Z, Tait LJ, Horwitz GD. Spectral sensitivity differences between rhesus monkeys and humans: Implications for neurophysiology. Journal of Neurophysiology. 2014 doi: 10.1152/jn.00356.2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Anatomy and physiology of a color system in the primate visual cortex. J Neurosci. 1984;4(1):309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Lee BB. Distribution and specificity of S-cone (“blue cone”) signals in subcortical visual pathways. Vis Neurosci. 2014;31(2):177–187. doi: 10.1017/S0952523813000631. [DOI] [PubMed] [Google Scholar]
- Merigan WH. Chromatic and achromatic vision of macaques: role of the P pathway. J Neurosci. 1989;9(3):776–783. doi: 10.1523/JNEUROSCI.09-03-00776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Reynolds JH, Miller CT. Active vision in marmosets: a model system for visual neuroscience. J Neurosci. 2014;34(4):1183–1194. doi: 10.1523/JNEUROSCI.3899-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagishima KJ, Grunert U, Li W. Processing of S-cone signals in the inner plexiform layer of the mammalian retina. Vis Neurosci. 2014;31(2):153–163. doi: 10.1017/S0952523813000308. [DOI] [PubMed] [Google Scholar]
- Mullen KT, Kulikowski JJ. Wavelength discrimination at detection threshold. J Opt Soc Am A. 1990;7(4):733–742. doi: 10.1364/josaa.7.000733. [DOI] [PubMed] [Google Scholar]
- Niu Y, Shen B, Cui Y, Chen Y, Wang J, Wang L, Kang Y, Zhao X, Si W, Li W, Xiang AP, Zhou J, Guo X, Bi Y, Si C, Hu B, Dong G, Wang H, Zhou Z, Li T, Tan T, Pu X, Wang F, Ji S, Zhou Q, Huang X, Ji W, Sha J. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell. 2014;156(4):836–843. doi: 10.1016/j.cell.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8(7):315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16(7):805–815. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Taking the measure of diversity: comparative alternatives to the model-animal paradigm in cortical neuroscience. Brain Behav Evol. 2000;55(6):287–299. doi: 10.1159/000006664. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Qi H, Kaas JH. Distinctive compartmental organization of human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96(20):11601–11606. doi: 10.1073/pnas.96.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, Shiozawa S, Maeda T, Ito M, Ito R, Kito C, Yagihashi C, Kawai K, Miyoshi H, Tanioka Y, Tamaoki N, Habu S, Okano H, Nomura T. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459(7246):523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Stockman A, Sharpe LT, Fach C. The spectral sensitivity of the human short-wavelength sensitive cones derived from thresholds and color matches. Vision Res. 1999;39(17):2901–2927. doi: 10.1016/s0042-6989(98)00225-9. [DOI] [PubMed] [Google Scholar]
- Stoughton CM, Lafer-Sousa R, Gagin G, Conway BR. Psychophysical chromatic mechanisms in macaque monkey. J Neurosci. 2012;32(43):15216–15226. doi: 10.1523/JNEUROSCI.2048-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby C, Solomon SG, Lennie P. Functional asymmetries in visual pathways carrying S-cone signals in macaque. J Neurosci. 2008;28(15):4078–4087. doi: 10.1523/JNEUROSCI.5338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby C, Szmajda BA, Buzas P, Lee BB, Martin PR. Transmission of blue (S) cone signals through the primate lateral geniculate nucleus. J Physiol. 2008;586(Pt 24):5947–5967. doi: 10.1113/jphysiol.2008.161893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ts’o DY, Gilbert CD. The organization of chromatic and spatial interactions in the primate striate cortex. J Neurosci. 1988;8(5):1712–1727. doi: 10.1523/JNEUROSCI.08-05-01712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human ocular media. Journal of the Optical Society of America. 1982;72(2):247–258. doi: 10.1364/josa.72.000247. [DOI] [PubMed] [Google Scholar]


