Summary
Sleep and sleep disturbances are increasingly recognized as determinants of women’s health and well-being, particularly in the context of the menstrual cycle, pregnancy, and menopause. At present, however, little is known about whether fertility is affected by sleep quantity and quality. That is, to what degree, and by what mechanisms, do sleep and/or its disturbances affect fertility? The purpose of this review is to synthesize what is known about sleep disturbances in relation to reproductive capacity. A model is provided, whereby stress, sleep dysregulation, and circadian misalignment are delineated for their potential relevance to infertility. Ultimately, if it is the case that sleep disturbance is associated with infertility, new avenues for clinical intervention may be possible.
Keywords: sleep, sleep disturbance, infertility, insomnia, circadian, reproductive health, women’s health, reproductive hormones
Introduction
The relationship between sleep and fertility is largely unknown. This paucity of research is surprising, given that sleep is such a critical component to one’s physical and emotional health and well-being. Among both women and men, it is well established that sleep disorders, particularly insomnia, contribute to, or are associated with, myriad health conditions, including cardiovascular disease, hypertension, glucose dysregulation, depression, and anxiety disorders. Specific to women, sleep disturbances coincide with premenstrual dysphoria, pregnancy, postpartum depression, and the menopausal transition (1). While there appears to be a relationship between sleep disturbance and reproductive health, little is known about which form of sleep disturbance is related to reproductive capacity and which specific aspects of reproductive capacity are particularly affected. With respect to sleep disturbance, the relevant domains could include sleep fragmentation, sleep continuity disturbance, short or long sleep duration, circadian dysrhythmia, and/or hypoxia. With respect to reproductive capacity, the relevant domains could include problems with fertility, conception, implantation, gestation, delivery, and/or neonatal health. Finally, it is possible that the relationship between these domains is reciprocal in nature, such that sleep disturbances and their associated sequelae may not only ensue from, but also interfere with, reproductive processes.
To date, the majority of evidence for the association between sleep disturbance and diminished reproductive capacity has been within the area of shift work (2-6). In general, adverse reproductive health outcomes were observed (e.g., menstrual irregularities, dysmenorrhea, increased time to, and reduced rates for, conception, increased miscarriages, lower birth weights) and were taken to implicate the negative effects of circadian misalignment, and/or the sleep disturbance that coincides with shift work. There is a more limited literature with respect to sleep disordered breathing and infertility. Polycystic Ovary Syndrome (PCOS) is known to reduce reproductive potential and is believed to be one of the most common causes of female infertility. Two key studies demonstrate the association between sleep disordered breathing and PCOS. One seminal study showed that premenopausal women with PCOS were 30 times more likely to suffer from SDB than controls (7). Another key study similarly showed OSA was prevalent in 44% of obese women with PCOS, compared to 6% of age- and weight-matched reproductively normal women (8). OSA is believed to contribute to the metabolic abnormalities (insulin resistance and decreased glucose tolerance) in women with PCOS (9). Therefore, it is possible that OSA contributes to one form of female factor infertility. Finally, with respect to sleep continuity disturbance, only two studies have evaluated the direct association between sleep continuity disturbance and infertility. First, Pal et al. found that sleep disturbance, assessed using the single item query “do you experience disturbed sleep?”, occurred in 34% of infertile women (10). In addition, women with diminished ovarian reserve were found to be 30 times more likely to have disturbed sleep, while controlling for race, BMI, and vasomotor symptoms. Second, Lin and colleagues (11) found that greater than 35% of women receiving intrauterine insemination reported disturbances in their sleep. While these studies are among the first to examine sleep disturbance in relation to successful pregnancy outcomes and in populations of infertile women, sleep disturbance was assessed globally (i.e., is phrased in such a way to be all-inclusive and non-specific with respect to individual sleep disorders). Further, the direction of the findings suggests that the observed sleep disturbance is a consequence of infertility. The purpose of the present paper is to examine the reciprocal proposition, that sleep disturbance may adversely affect fertility.
In order to lay the foundation for the possibility that sleep disturbance contributes to infertility, several domains of information are provided. First, the prevalence and significance of infertility are highlighted. Second, three proposed pathways by which sleep disturbance could contribute to infertility are presented. Theoretical and empirical support for each is reviewed. Third, clinical relevance and implications, not only for extending sleep medicine practices to this population, but also for addressing infertility from a behavioral sleep medicine perspective, are discussed.
Infertility
Definition and Prevalence
Infertility is defined as “the failure to achieve a successful pregnancy after 12 months or more of appropriate, timed unprotected intercourse or therapeutic donor insemination. Earlier evaluation and treatment may be justified based on medical history and physical findings and is warranted after 6 months for women over age 35 years” (12). An estimated 72.4 million women worldwide currently encounter infertility, with approximately 6.1 million having difficulty becoming pregnant or carrying a pregnancy to term in the United States (12-14), Among a sample of 7,643 females, infertility rates in the US approximated 15.5% (95% CI 8.6%–27.5%) for all women and 24.3% (95% CI 12.4%–43.5%) for nulliparous women (15).
Consequences
The American Society for Reproductive Medicine considers infertility a disease, rather than a mere quality of life issue. In addition, a recent US Supreme Court opinion expressed that conditions interfering with reproduction should be considered a disability as defined under the Americans with Disabilities Act since reproduction is a major life activity, which when disturbed, can be severely debilitating (16). As such, infertility, in and of itself, presents a profound challenge to one’s emotional and social well-being (16). For example, anticipatory anxiety of “failed” attempts, repeated disappointments, uncomfortable physical procedures, relationship constraints, and unfulfilled life values can produce high levels of psychosocial distress. It is not surprising that this level of psychological distress constitutes risk for psychiatric disorders. Anxiety is among the most common form of psychological distress followed by depression, with the prevalence of anxiety and depression exceeding that of their fertile counterparts (16). The fact that anxiety and depression are associated with disorders of initiating and maintaining sleep make it even more likely that sleep disturbance will occur in this context. Taken together, insomnia should be highly prevalent in this population; yet to date, there is a paucity of research on the prevalence of insomnia among women with infertility, let alone whether or not there is a reciprocal relationship between sleep disturbance and infertility.
Causes of Infertility
Infertility may be attributed to, or a consequence of, organic (e.g., anovulation, tubal obstruction), iatrogenic (e.g., adverse effects of chemotherapy or radiation), and/or lifestyle (e.g., weight, nutrition, and substance use) factors (see Figure 1). As discussed above, the psychological stress involved with infertility can also be extensive, leading one to further question whether stress/distress itself contributes to infertility (16, 17). While it is certainly the case that stress produces sleep continuity disturbance, normal sleep and sleep disturbances have yet to be investigated for their capacity to impede and/or facilitate reproductive function.
Figure 1.
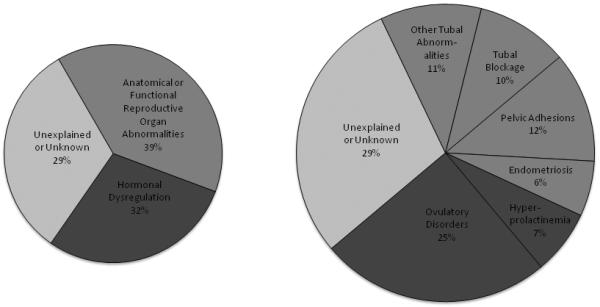
Causes of Female Infertility
a. Prevalence data presented are based on the World Health Organization (WHO) task force on Diagnosis and Treatment of Infertility (90)
b. The chart on the left represents three broad classifications of the etiologies (hormonal dysregulation; anatomical or functional reproductive organ abnormalities; unexplained or unknown causes); the chart on the right denotes sub-classifications of these categories.
c. To our knowledge, the prevalence of the causes of female factor infertility have yet to be comprehensively and concurrently delineated. For example, a comprehensive accounting of population prevalence would include at least the following factors: hormonal dysregulation, anatomical or functional reproductive organ abnormalities, unexplained or unknown conditions, lifestyle and environmental influences, disease and iatrogenesis.
How Might Sleep Affect Fertility?
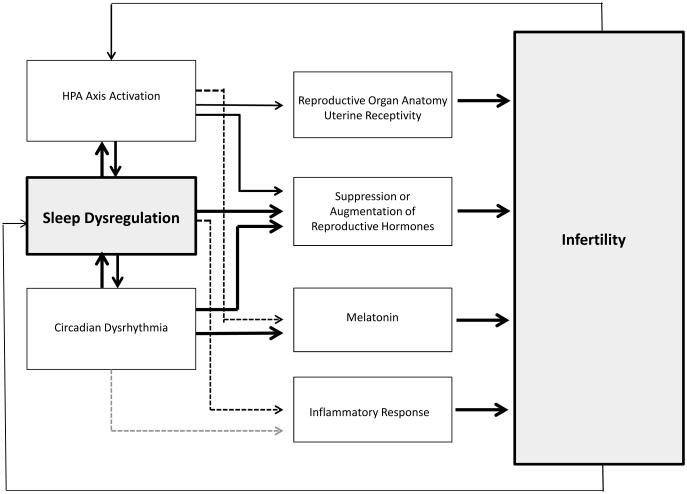
Provided here is both (a) a synthesis of literature that relates sleep and/or sleep disturbance to reproductive indices and (b) a framework encompassing the pathways by which sleep disturbance can interfere with fertility (see Figure 2). There are at least three possible pathways by which sleep disturbance may be related to infertility: 1) the HPA activation that precipitates sleep disturbance may also interfere with reproduction; 2) altered sleep duration and/or sleep continuity disturbance may, in and of itself, interfere with reproduction or result in further increased HPA activation; 3) circadian dysrhythmia, independent of (or in interaction with) HPA axis activation, sleep duration and/or sleep continuity disturbance, may result in infertility.
Figure 2. Stress, sleep dysregulation, and circadian dysrhythmia as potential pathways for infertility.
Note: The model is focused on the three factors explicated in this article. Other pathways are likely, if not probably, including, lifestyle, environmental, and disease factors.
1. HPA activation may interfere with reproductive capacity
As depicted in Figure 2, HPA activation is identified as both an independent pathway towards infertility and as a triggering factor for sleep dysregulation, which in turn, may independently affect reproductive capacity. While the central focus of this paper is to demonstrate how sleep dysregulation in association with, or independent of, stress may be related to infertility, it is important to first note what is known about stress’s effects on reproductive capacity.
A number of neurobiological pathways demonstrate how stress can heighten risk of a woman’s infertility (16-18). Four potential associations are highlighted here. First, HPA axis may directly exert its effect on reproductive hormones (19), and potentially interfere with normal follicle development (20), menstruation (21), and fecundity (22). For example, Nakamura and colleagues (17) have found that stress-related alteration of progesterone secretion may increase the risk of abortive responses. Second, stress, as measured by increases in physical activity and fasting, has been shown to acutely raise melatonin levels. While the role of melatonin and reproduction is complex (see below), sustained increases in melatonin have been linked to amenorrhea (18). Further, altered melatonin secretion is associated with GnRH suppression and altered ovulation (17, 22). Third, heightened stress has been shown to reduce innervation to the reproductive organs and this effect may influence ovary and follicle size (16, 18). Fourth, stress may reduce the likelihood of conception through decreased uterine receptivity (23).
In sum, stress has the potential to impair fertility during critical periods involving regular menstruation (21, 24) or successful ovulation, implantation, and placental growth and development (17). The degree, however, to which stress accounts for infertility is yet to be determined. For example, Campagne (19) notes that among studies that do not show a relationship between traditional stress markers and fertility may be a function of lack of specificity in their markers, not necessarily a null finding of the relationship, and further recommends choosing biomarkers that are particularly relevant to reproductive processes or hormones. Moderating factors, such as coping and individual differences, are likely to interact with this response system, to ultimately determine for whom and under what conditions stress will influence reproduction (19). In addition, the duration and chronicity of stress, may also moderate the degree to which changes in hormonal milieu affect one’s overall physiological health, in general, and fertility, in specific (19). The effect of stress on a woman’s fertility may not be universal, and examination of moderating factors may be more revealing about the conditions under which or for whom stress may affect fertility. Finally, it is also important to recognize that not every species experiences reproductive challenge in the face of stress. Wingfield and Saplosky (25) provide examples of successful breeding among fish and mammals in resistance to environmental and social stress.
2. Sleep dysregulation may result in infertility
As depicted in Figure 2, sleep dysregulation can occur in the absence of HPA activation and may nonetheless lead to infertility without being precipitated or perpetuated by HPA activation. For example, it is likely that RLS/PLMs and narcolepsy are not triggered by HPA activation, though may be responsible for inducing such activation subsequently. Even in the case of insomnia, it may be that acute insomnia is triggered, but not perpetuated, by HPA activation (26). In order to address sleep dysregulation comprehensively both in terms of, and independent of, HPA activation, three pathways are presented here: a) Insomnia may co-occur with, or result in, HPA activation, thereby leading to infertility; b) Sleep dysregulation (sleep fragmentation, sleep continuity disturbance, short or long sleep duration) may independently alter successful conception through the suppression or augmentation of reproductive hormones; and c) Sleep loss may also affect conception via compromised immunity.
2a. Insomnia may co-occur with, or result in, HPA activation, thereby leading to infertility
The pathophysiology of chronic insomnia is commonly understood as a disorder of hyperarousal, (27) which results from the interaction of both psychological and biological factors. At the psychological level, perceived stress and behavioral practices are thought to interact to produce a chronic form of the disorder. At the biological level, autonomic activation includes abnormal neuroendocrine responses (27). For example, Vgontzas and colleagues (28) have shown elevated stress hormone responses in patients with chronic insomnia as opposed to good sleepers. Given that CRH and cortisol, released by HPA axis activation, produce arousal and sleeplessness, they hypothesized that individuals with chronic insomnia would demonstrate increased levels of plasma ACTH and cortisol, and the severity of insomnia would indicate increased activation. Indeed, compared to controls, individuals with chronic insomnia had increased levels of ACTH and cortisol, while controlling for BMI. Objective sleep disturbance predicted higher cortisol levels. Thus, it is possible that stress affects fertility, and that chronic insomnia, to the extent that it produces stress responses, also affects fertility.
Assuming that chronic insomnia results in persistent activation of the HPA axis and hypersecretion of stress related neurohormones, then it would come as no surprise if chronic insomnia and infertility were correlated. The correlation however, in this scenario, would be the result of insomnia and impaired fertility sharing a common biological substrate (i.e., a common biological antecedent). It is also plausible that the relationship occurs in a more direct manner. If normal sleep plays a role in the regulation of reproductive hormones, and/or other related physiologic processes, then it stands to reason that disturbed sleep should correspond to a dysregulation along these same dimensions. This direct relationship would then be expected to add to and magnify the correlation between insomnia and infertility above and beyond that which occurs on the basis of stress alone.
2b. Sleep dysregulation may alter successful conception through the suppression or augmentation of reproductive hormones
It is well established that sleep modulates a variety of hormonal functions. Less well established is the extent to which sleep positively or negatively affects fertility and reproduction. Before summarizing what is known about the association between sleep and reproductive hormones, it is important to acknowledge that very few studies directly address such issues and that the data that exist often appear to lack consistency. With respect to the former, it is often the case that a particular hormone of interest is measured as part of a much larger set of assays and/or in the context of questions that may not pertain to sleep and/or reproductive capacity. Thus, while often not a focus of the investigations, such data exist and provide support for the concept that sleep, sleep disturbance, sleep deprivation, and/or circadian factors may be associated with reproductive capacity in general, and fertility, in specific. With respecttothelatter, it might be expected that good sleep (appropriate duration and timing, without sleep disorders) would be associated with healthy, normal levels of hormonal function, and conversely, that poor sleep would be associated with non-healthy levels of hormonal function. This is not, however, always the case. Further, when multiple sources of information about sleep are available (e.g., correlations with sleep duration, experimental protocols with partial or full sleep deprivation, group analyses comparing sleep disordered vs. healthy subjects) there is often inconsistency across the sources (e.g., a hormone may be found to be positively correlated with sleep duration and yet unaffected by partial or full sleep deprivation). With these caveats in mind, what is known is summarized below (see Table 1).
Table 1.
The association between stress, sleep dysregulation, and circadian dysrhythmia and reproductive hormones.
| HORMONES | ||||||||
|---|---|---|---|---|---|---|---|---|
| TSH | LH | FSH | PRL | Androgens | Estradiol | AMH | Progesterone | |
| Role in Fertility |
↑levels:: anovulation, miscarriages |
↑LH:: Infertility |
↑FSH:: poor ovarian reserve; reproductive aging; ↓luteal phase dysfunction |
↑ PRL indicates anovulation, PCOS, endometriosis |
↑androgens: PCOS |
Irregular estradiol secretion::ano vulation |
AMH::DOR |
Progesterone:: Implantation; ↓levels linked to infertility |
| Sleep/Wake Changes |
TSH ↑ around sleep onset; ↑ throughout night; ↓during daytime |
Sleep:: ↓ LH pulses; Awakenings:: ↑ LH pulses in early follicular phase |
Short sleep:: ↓ FSH |
Sleep onset:: PRL ↑ Awakenings:: PRL ↓ |
Sleep onset:: ↑testosterone following sleep onset; Sleep duration in men:: ↑testosterone |
? |
? |
Second half of night: ↑ progesterone |
| PATHWAYS | ||||||||
| 1. Stress | ? | Stress:: ↓ LH | Stress:: ↓FSH | ? | ? | ? | ? | Stress:: ↓ progesterone |
| 2. Sleep Dysregulation |
Acute SD:: ↑ TSH; Chronic SD: ↓ TSH lnsomnia::↓ TSH |
PSD and TSD:: ↑ LH amplitude |
PSD :: no change in FSH |
Narcolepsy:: ↓PRL; Night eating syndrome:: ↑ PRL |
PCOS:: testosterone ; ↑ OSA risk; TSD in men and women with depression:: ↓ testosterone |
PSD:: ↑ estradiol Variable sleep schedules:: ↓ estradiol |
↑ sleep disturbance :: DOR |
SDB, PCOS :: ↓ Progesterone |
| 3. Circadian Rhythmicity |
+ | −/+ ultradian pulsatility |
− | + | + in men | − | + subtle | ? |
|
Dysrhythmia |
− ? |
Shift work:: ↑ LH during daytime and nighttime sleep |
Shift workers:: ↑ FSH during daytime sleep and nighttime sleep |
Shift work::↑ PRL in shift workers |
? |
Shift workers: ↑ estradiol in those who did not nap; E1C unchanged |
? |
? |
+ There is evidence to support that this hormone has a circadian rhythm
− There is evidence to support that this hormone does not have a circadian rhythm
:: associated with/is related to
SD Sleep Deprivation
PSD Partial Sleep Deprivation
TSD Total Sleep Deprivation
SDB Sleep Disordered Breathing
OSA Obstructive Sleep Apnea
DOR Diminished Ovarian Reserve
Thyroid Stimulating Hormone (TSH)
High levels of TSH, as seen in hypothyroidism, can cause anovulation, recurrent miscarriages, amenorrhea and menstrual irregularities (29). Additionally, high TSH can increase PRL, which can also lead to infertility (30-32). TSH appears to increase prior to sleep onset, and continues to increase over the course of the sleep period/night, and then decreases during the day (33). Under acute sleep deprivation TSH surges, whereas under extended sleep deprivation, TSH may become diminished (33). For example, during partial, acute sleep deprivation among healthy young women in the follicular phase, TSH increased significantly, (34) and acute sleep loss was associated with increased TSH (35, 36). Kessler, Nedeltcheva (37) investigated the effects of TSH and T4 among healthy middle aged men and women in a comparison of 5.5 vs. 8.5 hours per night sleep conditions to approximate chronic partial sleep deprivation. In contrast to previous work on TSH, which demonstrated increases during acute sleep loss, women in the sample evidenced significant reductions in thyroid profiles TSH and T4. With respect to sleep disorders, insomnia ratings have been found to be negatively correlated with TSH levels (38).
Luteinizing Hormone (LH)
Rises or elevation in LH surges or premature LH surges can interfere with reproduction (39, 40). Sleep interacts with the menstrual cycle phase in determining its modulation on LH secretory pulses and amplitude (33). There is evidence that sleep decreases LH pulses in the early follicular phase and that awakenings are related to increased LH pulse amplitude (41). Sleep also diminishes the frequency of these pulses (41). Both the amplitude and frequency of LH pulses further decreases during the mid-follicular phase, where sleep exerts a less noticeable modulation on LH pulses. Toward the middle to end of the luteal phase, the amplitude of LH is decreased and not modulated by sleep. LH amplitude increases with partial and total sleep deprivation (34).
Follicle Stimulating Hormone (FSH)
High FSH levels during the early follicular phase are also considered an indicator of low ovarian reserve (42) and reproductive aging (29). Abnormally low FSH levels may indicate hypothalamic or pituitary dysfunction in the setting of irregular cycles or amenorrhea. Alternatively, low follicular phase FSH levels may indicate luteal phase dysfunction, which most commonly presents as short luteal phase (43). Partial sleep deprivation does not appear to result in changes in FSH (34). FSH, however, has, in one study, been found to be 20% lower among women who are short sleepers compared to long sleepers (>8 hrs per night), where age and BMI were controlled (44).
Prolactin (PRL)
Secreted through the pituitary gland, prolactin stimulates lactation in women and also plays a role in reproduction. For example, hyperprolactinemia is associated with anovulation and PCOS, and endometriosis (45). PRL surges upon sleep onset and is maximal throughout the night. PRL is inhibited by transient awakening and is profoundly suppressed by sleep deprivation (33). PRL has been studied in several sleep disordered populations and in patients taking hypnotics. Individuals with night eating syndrome had increased levels of PRL (46) which was interpreted to represent a stress-regulatory response to cortisol. Women with narcolepsy (with or without sleep apnea) had lower levels of sleep-related PRL release (47). Patients with obstructive sleep apnea (OSA) who received CPAP therapy exhibited a decrease in elevated PRL measures (48). This last finding may have implications for the management of PCOS. Finally, sleep inducing medications such as zolpidem, triazolam and Ramelteon, (49) also produce hypersecretions in PRL. In sum, because the observed associations are inconsistent, all that can be concluded for the time being is that sleep disturbance may modulate PRL secretion and thereby may lead to dysregulation with regard to this hormonal parameter.
Testosterone
Testosterone serum concentrations are generally low in healthy reproductive age women (29). Testosterone appears to be a follicular regulator, which may explain the relationship between elevated testosterone and fertility problems in PCOS (50). That is, hyperandrogenism may be linked to PCOS, and thereby infertility (29). While testosterone is secreted in women and men, it has mostly been studied in men. Sleep duration positively correlates with testosterone in men, controlling for age, body fat, and activity levels (51). Among women, Sowers and colleagues (52) found that lower baseline testosterone levels were modestly correlated with longer WASO duration years later, during menopause. Following total sleep deprivation, testosterone decreases in men and women with depression (53). Interestingly, women with PCOS have higher testosterone levels and are at greater risk for developing sleep apnea (54).
Estradiol
Estradiol is secreted by the granulosa cells in the ovarian follicles and regulates FSH and LH, playing a critical role in ovulation. When estrogen does not rise and fall appropriately, FSH and LH may not be able to stimulate ovulation (29). While many studies have examined sleep disturbance in response to declining levels of estrogen, particularly among middle-age and aging women, studies on the effects of sleep and sleep deprivation on estradiol in reproductive age women are limited. Estradiol has been shown to increase during partial sleep deprivation in women of reproductive age (34). Women with more variable sleep schedules had higher levels of estradiol than women with more regular schedules (55). Women with regular sleep schedules had 60% lower levels of estradiol than women with more variability (55). High levels of estradiol were also related to poorer sleep quality (52). One study that is inconsistent with these findings showed poor sleep quality to be associated with low levels of estradiol among late reproductive age women (56). Furthermore, both pre- and post-menopausal women with sleep-disordered breathing have lower estradiol levels compared to age and cycle phase matched controls (57). Estrogen therapy has been shown to improve sleep in women with sleep-disordered breathing (57).
Anti-Mullerian Hormone (AMH)
AMH is a marker of ovarian reserve (58). To our knowledge, AMH has not been studied in the context of sleep disorders. As noted above, one group of investigators examined the relationship between diminished ovarian reserve and sleep disturbance. Interestingly, Pal et al. (10) found that among a sample of infertile women diminished ovarian reserve was related to sleep disturbance, as measured by a single item ‘do you experience disturbed sleep?’. They hypothesized that ovarian hormone declines in estrogen and testosterone may underlie this relationship. Alternatively, and to the extent that the subjects in this protocol were aware that they had low ovarian reserve, this may have been anxiogenic, and this alone could result in sleep continuity disturbance.
Progesterone
Progesterone is related to luteal function and is necessary for implantation and maintenance of pregnancy. Abnormally low levels of progesterone may be an index of luteal phase dysfunction (29). Progesterone’s effects on sleep have been relatively well documented (e.g. on sleep architecture and sleep-related breathing), (59) yet sleep’s effect on progesterone is limited. Low levels of progesterone may account for increased sleep disordered breathing in general (59) and among women with PCOS (60).
In sum, there are multiple instances whereby sleep quantity or quality, sleep deprivation, and/or disordered sleep, can exert an effect on a number of different hormones involved with reproduction. Given the synchrony of hormones needed for successful ovulation, conception, and implantation, it would follow that sleep continuity disturbance could play a significant role in interfering with the hormonal milieu necessary for reproduction.
2c. Sleep loss may affect conception via compromised immunity
It is also plausible, albeit speculative, that sleep loss or insomnia might affect conception via compromised immunity. For example, cytokine and immune inflammatory responses marked by TNF and IL-6, have been observed to increase under conditions of sleep loss (61-63); likewise poor sleep quality and continuity has been related to elevated C-reactive protein (CRP) in young women (64). Irwin et al (65) similarly demonstrated immune parameters to be compromised among individuals with insomnia. Interestingly, IL-6 levels were posited to play a role in unexplained infertility; women with infertility showed significantly higher IL-6 levels when compared with the fertile group (66). With regard to TNF, infertile women had a greater intracellular (IC) production of TNF- α, and higher ratios of TNF- α/IL-2, TNF- α/IL-4, TNF- α/ IFN-γ when compared to the fertile group (67). Idiopathic infertile and immune-infertile groups showed greater TNF- α concentrations from cervical mucus (but not from sera) compared to fertile groups, with immune-infertile groups having greater TNF- α concentrations compared to the idiopathic groups (68).
3. Circadian dysrhythmia may interfere with fertility
Evidence that circadian dysrhythmia has the potential to affect fertility outcomes is drawn from four lines of research showing that: a) fertility-related hormones exhibit a circadian pattern under the conditions of normal sleep; b) circadian dysrhythmia (as observed in shift workers) is linked to altered secretion of reproductive hormones; c) circadian dysrhythmia is associated with adverse reproductive outcomes; and d) melatonin, a key circadian hormone, is associated with fertility.
3a. Fertility related hormones exhibit a circadian pattern under normal conditions of sleep
Even in the absence of known sleep or circadian effects on fertility, it remains possible that reproductive hormones by virtue of having a circadian or ultradian rhythm, may have deleterious effects on reproductive capacity when those rhythms are disrupted. Accordingly, summarized here are the findings with respect to rhythmicity and relevant reproductive hormones. Both TSH and PRL have been found to exhibit a circadian pattern. Plasma TSH is low during the day, peaks within 1-2 hours of sleep onset, and thereafter, declines until reaching the diurnal minimal levels beginning at about 10:00 a.m. (33). In contrast, daytime sleep does not parallel the nighttime sleep response; it does not appear to attenuate the TSH response, and thereby suggests an interaction between circadian and sleep effects. PRL secretion increases at the beginning of the sleep period, peaks in the latter half of the sleep period, and decreases to low levels by mid-morning. This clear ultradian rhythm appears to be fundamentally related to sleep as PRL secretion is largely suppressed by sleep deprivation, as noted above. AMH shows a subtle, though not clinically significant, circadian pattern (69). Progesterone begins to rise in the second half of the night and peaks around 8:00 am (69) though the diurnal distribution and variation over time is unclear. LH does not appear to have a circadian pattern, but does exhibit ultradian pulsatility, that is, a variation in amplitude over time with about a 2 hour period (70). Testosterone follows a distinct diurnal rhythm in men in which there is a surge of testosterone following sleep onset, which peaks in the early morning hours (54). To our knowledge, there is no research on diurnal or circadian variation of testosterone in women. Neither FSH (70) nor estradiol appear to have a circadian or ultradian rhythm (69).
3b. Circadian dysrhythmia is linked to altered secretion of reproductive hormones
Increased LH and FSH was observed in shift workers during daytime sleep (compared to nighttime sleep) and were unchanged on nights off (nighttime sleep); likewise increased LH and FSH during nighttime sleep was observed in shift workers compared to day workers (71). Among shift workers, PRL is increased compared to non-shift workers (72). With regard to sleep disturbances among shift workers, Merklinger-Gruchala and colleagues, (55) theorized that increased exposure to light at night during sleep deprivation or night shift-work suppresses endogenous melatonin levels, which in turn could increase estrogen levels. Consistent with this notion, higher estradiol levels were observed among shift workers who do not take naps (73). Another study, however, showed that the estrogen E1C was unchanged among shift workers and non-shift workers (71). What remains to be determined is whether these abnormalities have consequences with respect to reproductive capacity.
3c. Circadian dysrhythmia is associated with adverse reproductive outcomes
The evidence for circadian influence on reproduction, while not well studied, has primarily shown a link between shift work and infertility. Menstrual irregularities and dysmenorrhea are elevated among women engaging in shift work (2, 74, 75). For example, according to Labyak et al.’s (6) survey of nurses of reproductive age, 53% reported menstrual changes while engaging in shift work; such changes were a marker of sleep disturbance, difficulty concentrating, and general malaise. Specific to reproductive health, women who engage in shift work are at an increased risk for infertility, spontaneous abortions, pre-term birth, and impaired fetal development (3, 4, 74, 75). Another study that measured fertility in male and female shift workers found that women who were exposed to shift work showed indicators of sub-fecundity, as measured by increased time to conception (5).
Finally, circadian misalignment circadian dysrhythmia may result in infertility through insulin resistance and/or increased inflammation. First, insulin resistance is implicated in the development of PCOS, and in turn, may play a role in the infertility that often accompanies this disorder (76) Second, it has been demonstrated that circadian misalignment results in inflammation, as ascertained by increases in C- reactive protein (77). Given that inflammation may exacerbate endometriosis, this is another pathway whereby fertility may be compromised by circadian misalignment (78). Note: Circadian misalignment may or may not necessarily be orthogonal to sleep disturbance. On the one hand, sleeping during one’s non- preferred phase may entail difficulty initiating and maintaining sleep and/or short sleep duration. On the other hand, some individuals may have normal sleep continuity and duration despite sleeping outside their preferred phase.
3d. Melatonin, a key circadian hormone, is associated with fertility
As reviewed by Tamura et al. (79) melatonin, while primarily thought to be related to circadian function, is also thought to affect multiple biological functions, including the reproductive system. Early studies showed that increased levels of melatonin were associated with amenorrhea and functional, hypothalamic hypogonadism (see Macchi & Bruce (80) for review). In the 1990’s melatonin was touted as a potential contraceptive (81, 82) due to its potential inhibitory effect on ovulation. It is interesting to note that melatonin was higher among women on oral contraceptives compared to naturally cycling women (83).
Melatonin’s direct effects, however, on reproductive hormones have not been straightforward, and their clinical significance is in question. A few examples are noted here. Davis and colleagues (71) demonstrated that night shift workers, compared to their non-shift-working counterparts, showed lowered melatonin levels, and associated increases in LH and FSH (but not E1C, an estrone conjugate), during night work and during daytime sleep. Cagnacci and colleagues (84) showed that melatonin administration had an enhancing effect on LH and FSH and possibly through GnRH at the follicular, but not the luteal phase, indicating that melatonin’s effect on reproductive hormones may vary depending on cycle phase. Bispink and colleagues (85) showed that melatonin modulated prolactin secretion, thereby potentially influencing reproductive function. Finally, Richardson and Wang-Weigand (49) studied the effect of Ramalteon, a melatonin agonist, on reproductive hormones. They did not observe changes in LH, FSH, estradiol, and testosterone, but did observe marginal increase in prolactin. It is plausible that melatonin influences, or is influenced by, reproductive hormones. The degree to which these changes result in reproductive hindrance or enhancement needs further study.
As stated by Reiter and colleagues (86) “Melatonin is per se neither antigonadotrophic nor progonadotrophic.”(pg 445). It may be better conceptualized as a seasonal indicator that provides information to the HPG axis. They further present evidence of the beneficial effects of melatonin on reproductive function among animals and humans; melatonin is closely associated with seasonal breeding mammals and is believed to facilitate oocyte quality and development, ovulation, luteal function, and embryo development (86, 87). Melatonin’s usefulness in pregnancy rates among women are reviewed, concluding that melatonin be further investigated as a means to enhance IVF and embryo transfer. Recent conceptualizations involve melatonin’s anti-oxidative properties (79) and its potential to enhance fertility. Eryilmaz and colleagues (88) showed that exogenously administered melatonin fostered oocyte and embryo quality among IVF patients with disturbed sleep. Though sleep quality was not improved, the mechanism proposed was potentially through melatonin’s antioxidant properties. In another study among women with PCOS, elevated levels of melatonin and oxidative stress have been observed among women with PCOS relative to women who did not have PCOS, while the circadian rhythm in each group remained stable. The PCOS group also demonstrated lower sleep efficiencies. The authors also hypothesize that the elevated levels of melatonin may be demonstrative of its anti-oxidant properties in response to the elevated levels of oxidative stress. They point to these unique associations as possible, but not yet confirmed, mechanisms in the pathophysiology of PCOS (89).
While the exact mechanisms by which melatonin may influence and modulate and perhaps facilitate reproduction continue to be delineated, the regulatory secretion of melatonin is of particular interest here. Dampening or augmentation of its secretion during sleep or when sleep is disrupted could potentially moderate one’s success at conception.
Clinical and Research Implications
Insomnia disorder disproportionately affects women compared to men. Reproductive transitions (menstrual, pregnancy, menopause) are thought to precipitate sleep continuity disturbance, hence heightening women’s risk for sleep problems throughout their lifespan. Traditional ways of thinking are that the endocrinology, mood dysregulation, and/or lifestyle changes that accompany reproductive transitions present a vulnerability to insomnia. It is posited here that the converse may also be true. Sleep continuity disturbance may influence fertility, and do so in one of the several ways enumerated above. At a more global level of construal, if insomnia represents a response to real or perceived life threat, it may be that insomnia serves as a biological cue for less than optimal circumstances for reproduction. From an evolutionary point of view, such an association would be adaptive. These assertions, while plausible, require data to (a) determine if, and/or to what degree, sleep continuity disturbance may interfere with, modulate, or prevent reproductive success; and (b) to test the extent to which sleep interventions may reverse these associations.
Quantification and qualification of sleep disturbance in this vulnerable population may serve to sensitize and inform clinicians about sleep problems as potentially common conditions comorbid with infertility. At minimum, treatment of sleep disorders as comorbid conditions can be expected to enhance the quality of life of women suffering from infertility. At maximum, one or more sleep disorders represent moderating factors for infertility or response to treatment. If this proves to be the case, treatment for sleep disorders may serve to increase the potential for natural conception, response to fertility treatment, and/or successful carriage to term.
Practice Point.
1. Targeted treatment for sleep dysregulation (e.g., sleep continuity disturbance, abnormal sleep duration and/or sleep disordered breathing), or circadian misalignment may contribute to enhanced reproductive capacity, and barring this, will certainly enhance quality of life for individuals struggling with infertility.
2. It is possible that stress affects fertility and that chronic insomnia, to the extent that it produces stress responses, also affects fertility. Thus, targeted treatment for insomnia may be useful for enhancing infertility.
Research Agenda.
1. Cross-sectional and prospective studies are needed to determine if there is strong evidence for the association between stress, sleep dysregulation, and circadian misalignment, alone or in combination, with the various causes of infertility.
2. While follow-up studies will likely be warranted for all three of the pathways, of particular importance to the sleep community will be to determine which specific aspects of sleep dysregulation are associated with which specific types of infertility.
3. If the cross-sectional and prospective data strongly suggest that sleep and circadian factors affect fertility, intervention studies may be warranted to determine if improved sleep enhances fertility outcomes and successful full term pregnancies.
Acknowledgements
First, we would like to acknowledge Caterina Mosti and Diana D’Argenio for their work on literature searches. Second, we like to thank Dr. Mary Spiers for her feedback on an earlier draft of this manuscript. Third, we would like to thank the reviewers for their considerate and comprehensive feedback. This manuscript is immeasurably better because of their thoughtful review. Finally, we would like to thank Mahendra De Silva, Ph.D for helping to inspire this idea so many years ago.
This work was partially supported by grant NIA R01 AG041783.
Abbreviations
- ACTH
Adrenocorticotropic Hormone
- AMH
Anti-Mullerian Hormone
- BMI
Body Mass Index
- CRH
Corticotropin-releasing Hormone
- CRP
C-reactive Protein
- FHA
Functional Hypothalamic Amenorrhea
- FSH
Follicle-stimulating Hormone
- GCS
Glucocorticoids
- GnRH
Gonadotropin-releasing Hormone
- HPG
Hypothalamic-pituitary-gonadal
- IL-6
Interleukin-6
- IVF
In Vitro Fertilization
- LH
Luteinizing Hormone
- OSA
Obstructive Sleep Apnea
- PCOS
Polycystic Ovarian Syndrome
- PRL
Prolactin
- T4
Thyroxine
- TSH
Thyroid Stimulating Hormone
- TNF
Tumor Necrosis Factor
- WASO
Wake After Sleep Onset
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Kloss JD, Nash CO. Women’s sleep through the lifespan. In: Spiers MV, Geller PA, Kloss JD, editors. Women’s Health Psychology. Wiley; New York: 2013. [Google Scholar]
- 2.Mahoney MM. Shift work, jet lag, and female reproduction. Int J of Endocrinol. 2010;2010:1–1. doi: 10.1155/2010/813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axelsson G, Rylander R, Molin I. Outcome of pregnancy in relation to irregular and inconvenient work schedules. Br J Ind Med. 1989;46:393–393. doi: 10.1136/oem.46.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald AD, McDonald JC, Armstrong B, Cherry NM, Cote R, Lavoie J, et al. Fetal death and work in pregnancy. Br J Ind Med. 1988;45:148–148. doi: 10.1136/oem.45.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W. Shift work and subfecundity: a European multicenter study. European study group on infertility and subfecundity. J Occup Environ Med. 1996;38:352–352. doi: 10.1097/00043764-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int. 2002;23:703–703. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- 7.*Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J of clin endocrinol metab. 2001;86:517–517. doi: 10.1210/jcem.86.2.7185. [DOI] [PubMed] [Google Scholar]
- 8.Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP. Increased Prevalence of Obstructive Sleep Apnea Syndrome in Obese Women with Polycystic Ovary Syndrome. J clin endocrinol metab. 2001;86:1175–1175. doi: 10.1210/jcem.86.3.7316. [DOI] [PubMed] [Google Scholar]
- 9.*Tsali E, Van Cauter E, Ehrmann DA. Polycystic Ovary Syndrome and Obstructive Sleep Apnea. Sleep medicine clinics. 2008;3:37–37. doi: 10.1016/j.jsmc.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.*Pal L, Bevilacqua K, Zeitlian G, Shu J, Santoro N. Implications of diminished ovarian reserve (DOR) extend well beyond reproductive concerns. Menopause. 2008;15:1086–1086. doi: 10.1097/gme.0b013e3181728467. [DOI] [PubMed] [Google Scholar]
- 11.Lin JL, Lin YH, Chueh KH. Somatic symptoms, psychological distress and sleep disturbance among infertile women with intrauterine insemination treatment. J Clin Nurs. 2013;23:1677–1677. doi: 10.1111/jocn.12306. [DOI] [PubMed] [Google Scholar]
- 12.Practice Committee of the American Society for Reproductive Medicine: Definitions of infertility and recurrent pregnancy loss. Fertil Steril. 2008;90:1–1. [Google Scholar]
- 13.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005:23. [PubMed] [Google Scholar]
- 14.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1506. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 15.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1324. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prince LB, Domar AD. The stress of infertility. In: Spiers MV, Geller PA, Kloss JD, editors. Women's Health Psychology. John Wiley & Sons; Hoboken: 2013. pp. 328–54. [Google Scholar]
- 17.Nakamura K, Sheps S, Arck PC. Stress and reproductive failure: past notions, present insights and future directions. J Assist Reprod Genet. 2008;25:47–47. doi: 10.1007/s10815-008-9206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenker JG, Meirow D, Schenker E. Stress and human reproduction. Eur J Obstet Gynecol Reprod Biol. 1992;45:1–1. doi: 10.1016/0028-2243(92)90186-3. [DOI] [PubMed] [Google Scholar]
- 19.Campagne D. Should fertilization treatment start with reducing stress? Hum Reprod. 2006;21:1651–8. doi: 10.1093/humrep/del078. [DOI] [PubMed] [Google Scholar]
- 20.Ferin M. Clinical review 105: Stress and the reproductive cycle. J clin endocrin metab. 1999;84:1768–1768. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- 21.Gordon CM. Functional hypothalamic amenorrhea. N Engl J Med. 2010;363:365–365. doi: 10.1056/NEJMcp0912024. [DOI] [PubMed] [Google Scholar]
- 22.Louis GM, Lum KJ, Sundaram R, Chen Z, Kim S, Lynch CD, et al. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril. 2011;95:2184–2184. doi: 10.1016/j.fertnstert.2010.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondoh E, Okamoto T, Higuchi T, Tatsumi K, Baba T, Murphy SK, et al. Stress affects uterine receptivity through an ovarian-independent pathway. Hum Reprod. 2009;24:945–945. doi: 10.1093/humrep/den461. [DOI] [PubMed] [Google Scholar]
- 24.Berga SL. Stress and amenorrhea. Endocrinologist. 1995;5:416–416. [Google Scholar]
- 25.Wingfield JC, Sapolsky RM. Reproduction and resistance to stress: when and how. J Neuroendocrinol. 2003;15:711–711. doi: 10.1046/j.1365-2826.2003.01033.x. [DOI] [PubMed] [Google Scholar]
- 26.Perlis M, Shaw P, Cano G, Espie C. Models of insomnia. Principles and practice of sleep medicine. 2011;5:850–850. [Google Scholar]
- 27.*Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–19. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 28.*Vgontzas AN, Bixler EO, Lin H, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocr Metab. 2001;86:3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 29.Speroff L, Fritz MA. Clinical gynaecologic endocrinology and infertility. 8th Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 30.Arredondo F, Noble LS. Endocrinology of recurrent pregnancy loss. Semin Reprod Med. 2006;24:33–33. doi: 10.1055/s-2006-931799. [DOI] [PubMed] [Google Scholar]
- 31.Olooto WE, Amballi AA, Banjo TA. A review of Female Infertility; important etiological factors and management. J of Microbiol & Biotechnol Res. 2012;2:379–379. [Google Scholar]
- 32.Verma I, Sood R, Juneja S, Kaur S. Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on infertility. Int J Appl Basic Med Res. 2012;2:17–17. doi: 10.4103/2229-516X.96795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.*Van Cauter E, Tsali E. Endocrine physiology in relation to sleep and sleep disturbances. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5 Elsevier; 2011. [Google Scholar]
- 34.Baumgartner A, Dietzel M, Saletu B, Wolf R, Campos-Barros A, Graf KJ, et al. Influence of partial sleep deprivation on the secretion of thyrotropin, thyroid hormones, growth hormone, prolactin, luteinizing hormone, follicle stimulating hormone, and estradiol in healthy young women. Psychiatry Res. 1993;48:153–153. doi: 10.1016/0165-1781(93)90039-j. [DOI] [PubMed] [Google Scholar]
- 35.Parker DC, Rossman LG, Pekary AE, Hershman JM. Effect of 64-hour sleep deprivation on the circadian waveform of thyrotropin (TSH): further evidence of sleep-related inhibition of TSH release. J clin endocrinol metab. 1987;64:157–157. doi: 10.1210/jcem-64-1-157. [DOI] [PubMed] [Google Scholar]
- 36.Brabant G, Prank K, Ranft U, Schuermeyer T, Wagner TO, Hauser H, et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J clin endocrin metab. 1990;70:403–403. doi: 10.1210/jcem-70-2-403. [DOI] [PubMed] [Google Scholar]
- 37.Kessler L, Nedeltcheva A, Imperial J, Penev PD. Changes in serum TSH and free T4 during human sleep restriction. Sleep. 2010;33:1115–1115. doi: 10.1093/sleep/33.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poirier MF, Loo H, Galinowski A, Bourdel MC, Remi-Bouissiere P, Piketty ML, et al. Sensitive assay of thyroid stimulating hormone in depressed patients. Psychiatry Res. 1995;57:41–41. doi: 10.1016/0165-1781(95)02307-i. [DOI] [PubMed] [Google Scholar]
- 39.Messinis IE, Messini CI, Dafopoulos K. Luteal-phase endocrinology. Reprod Biomed Online. 2009:15–29. Luteal-phase endocrinology. Reprod Biomed Online. [PubMed] [Google Scholar]
- 40.Nitzschke M, Ruvalcaba L, Stetson S. Alternative treatment approach based on clomiphene citrate for patients with low ovarian reserve. IVF Lite. 2014;1:17. [Google Scholar]
- 41.Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J clin endocrin metab. 2005;90:2050–2050. doi: 10.1210/jc.2004-2033. [DOI] [PubMed] [Google Scholar]
- 42.Iliodromiti S, Nelson SM. Biomarkers of ovarian reserve. Biomark Med. 2013;7:147–147. doi: 10.2217/bmm.12.97. [DOI] [PubMed] [Google Scholar]
- 43.The clinical relevance of luteal phase deficiency: A committee opinion. Practice Committee of the American Society of Reproductive Medicine Fertility Sterility. 2012 doi: 10.1016/j.fertnstert.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 44.Touzet S, Rabilloud M, Boehringer H, Barranco E, Ecochard R. Relationship between sleep and secretion of gonadotropin and ovarian hormones in women with normal cycles. Fertil Steril. 2002;77:738–738. doi: 10.1016/s0015-0282(01)03254-x. [DOI] [PubMed] [Google Scholar]
- 45.Radwanska E, Henig I, Dmowski WP. Nocturnal prolactin levels in infertile women with endometriosis. J Reprod Med. 1987;32:605–605. [PubMed] [Google Scholar]
- 46.Birketvedt GS, Geliebter A, Kristiansen I, Firgenschau Y, Goll R, Florholmen JR. Diurnal secretion of ghrelin, growth hormone, insulin binding proteins, and prolactin in normal weight and overweight subjects with and without the night eating syndrome. Appetite. 2012;59:688–688. doi: 10.1016/j.appet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 47.Clark RW, Schmidt HS, Malarkey WB. Disordered growth hormone and prolactin secretion in primary disorders of sleep. Neurology. 1979;29:855–855. doi: 10.1212/wnl.29.6.855. [DOI] [PubMed] [Google Scholar]
- 48.Macrea MM, Martin TJ, Zagrean L. Infertility and obstructive sleep apnea: the effect of continuous positive airway pressure therapy on serum prolactin levels. Sleep Breath. 2010;14:253–253. doi: 10.1007/s11325-010-0373-0. [DOI] [PubMed] [Google Scholar]
- 49.Richardson G, Wang-Weigand S. Effects of long-term exposure to ramelteon, a melatonin receptor agonist, on endocrine function in adults with chronic insomnia. Hum Psychopharmacol. 2009;24:103–103. doi: 10.1002/hup.993. [DOI] [PubMed] [Google Scholar]
- 50.Haning RV, Jr., Hackett RJ, Flood CA, Loughlin JS, Zhao QY, Longcope C. Testosterone, a follicular regulator: key to anovulation. J clin endocrin metab. 1993;77:710–710. doi: 10.1210/jcem.77.3.8370694. [DOI] [PubMed] [Google Scholar]
- 51.Goh VH, Tong TY. Sleep, sex steroid hormones, sexual activities, and aging in Asian men. J Androl. 2010;31:131–131. doi: 10.2164/jandrol.109.007856. [DOI] [PubMed] [Google Scholar]
- 52.Sowers MF, Zheng H, Kravitz HM, Matthews K, Bromberger JT, Gold EB, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–1339. [PMC free article] [PubMed] [Google Scholar]
- 53.Baumgartner A, Graf KJ, Kurten I, Meinhold H, Scholz P. Neuroendocrinological investigations during sleep deprivation in depression. I. Early morning levels of thyrotropin, TH, cortisol, prolactin, LH, FSH, estradiol, and testosterone. Biol Psychiatry. 1990;28:556–556. doi: 10.1016/0006-3223(90)90394-h. [DOI] [PubMed] [Google Scholar]
- 54.Andersen ML, Alvarenga TF, Mazaro-Costa R, Hachul HC, Tufik S. The association of testosterone, sleep, and sexual function in men and women. Brain Res. 2011:80–104. doi: 10.1016/j.brainres.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 55.Merklinger-Gruchala A, Ellison PT, Lipson SF, Thune I, Jasienska G. Low estradiol levels in women of reproductive age having low sleep variation. Eur J Cancer Prev. 2008;17:467–467. doi: 10.1097/CEJ.0b013e3282f75f67. [DOI] [PubMed] [Google Scholar]
- 56.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98:391–391. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 57.Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, et al. Hormone replacement therapy and sleep-disordered breathing. American journal of respiratory and critical care medicine. 2003;167:1186–1186. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 58.Steiner AZ. Biomarkers of ovarian reserve as predictors of reproductive potential. Semin Reprod Med. 2013;31:437–437. doi: 10.1055/s-0033-1356479. [DOI] [PubMed] [Google Scholar]
- 59.Balserak BI, Lee K. Sleep disturbances and sleep-related disorder in pregnancy. In: Kryger M, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. 5 Elsevier; 2011. [Google Scholar]
- 60.Nitsche K, Ehrmann DA. Obstructive sleep apnea and metabolic dysfunction in polycystic ovary syndrome. Best Pract Res Clin Endocrinol Metab. 2010;24:717–717. doi: 10.1016/j.beem.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.*Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1756. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 62.*Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J clin endocrinol metab. 2004;89:2119–2119. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 63.von Kanel R, Dimsdale JE, Ancoli-Israel S, Miller PJ, Patterson TL, McKibbin CL, et al. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D dimer in older caregivers of people with Alzheimer’s disease. J Geriatr Society. 2006;54:431–7. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 64.*Okun ML, Coussons-read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain, Behav, and Immun. 2009;23:351–351. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.*Irwin MR, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 66.Demir B, Guven S, Guven ES, Atamer Y, Gul T. Serum IL-6 level may have role in the pathophysiology of unexplained infertility. Am J Reprod Immunol. 2009;62:261–261. doi: 10.1111/j.1600-0897.2009.00734.x. [DOI] [PubMed] [Google Scholar]
- 67.Horka P, Jarosova R, Malickova K, Janatkova I, Mareckova H, Zima T, et al. Intracellular cytokine production in peripheral blood lymphocytes: a comparison of values in infertile and fertile women. Am J Reprod Immunol. 2011;65:466–466. doi: 10.1111/j.1600-0897.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 68.Naz RK, Butler A, Witt BR, Barad D, Menge AC. Levels of interferon-gamma and tumor necrosis factor-alpha in sera and cervical mucus of fertile and infertile women: implication in infertility. J Reprod Immunol. 1995;29:105–105. doi: 10.1016/0165-0378(95)00936-f. [DOI] [PubMed] [Google Scholar]
- 69.Bungum L, Jacobsson AK, Rosen F, Becker C, Yding Andersen C, Guner N, et al. Circadian variation in concentration of anti-Mullerian hormone in regularly menstruating females: relation to age, gonadotrophin and sex steroid levels. Hum Reprod. 2011;26:678–678. doi: 10.1093/humrep/deq380. [DOI] [PubMed] [Google Scholar]
- 70.Klingman KM, Marsh EE, Klerman EB, Anderson EJ, Hall JE. Absence of circadian rhythms of gonadotropin secretion in women. J clin endocrin metab. 2011;96:1456–1456. doi: 10.1210/jc.2010-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis S, Mirick DK, Chen C, Stanczyk FZ. Night shift work and hormone levels in women. Cancer Epidem Biomar. 2012;21:609–609. doi: 10.1158/1055-9965.EPI-11-1128. [DOI] [PubMed] [Google Scholar]
- 72.Miyauchi F, Nanjo K, Otsuka K. Effects of night shift on plasma concentrations of melatonin, LH, FSH and prolactin, and menstrual irregularity. Sangyo Igaku. 1992;34:545–545. doi: 10.1539/joh1959.34.545. [DOI] [PubMed] [Google Scholar]
- 73.Bracci M, Copertaro A, Manzella N, Staffolani S, Strafella E, Nocchi L, et al. Influence of night-shift and napping at work on urinary melatonin, 17-beta-estradiol and clock gene expression in pre- menopausal nurses. J Biol Regul Homeost Agents. 2013;27:267–267. [PubMed] [Google Scholar]
- 74.Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep med. 2007;8:613–613. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 75.*Shechter A, James FO, Boivin DB. Circadian rhythms and shift working women. Sleep med clin. 2008;3:13–13. [Google Scholar]
- 76.Pauli J, Raja-Khan N, Wu X, Legro R. Current perspectives of insulin resistance and polycystic ovary syndrome. Diabetic Med. 2011;28:1445–1445. doi: 10.1111/j.1464-5491.2011.03460.x. [DOI] [PubMed] [Google Scholar]
- 77.Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1860. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004;1034:300–300. doi: 10.1196/annals.1335.032. [DOI] [PubMed] [Google Scholar]
- 79.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–280. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 80.Macchi MM, Bruce JN. Human pineal physiology and functional significance of melatonin. Front Neuroendocrinol. 2004;25:177–177. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Silman RE. Melatonin: a contraceptive for the nineties. Eur J Obstet Gynecol Reprod Biol. 1993;49:3–3. doi: 10.1016/0028-2243(93)90099-x. [DOI] [PubMed] [Google Scholar]
- 82.Voordouw BC, Euser R, Verdonk RE, Alberda BT, de Jong FH, Drogendijk AC, et al. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J clin endocrin metab. 1992;74:108–108. doi: 10.1210/jcem.74.1.1727807. [DOI] [PubMed] [Google Scholar]
- 83.Kostoglou-Athanassiou I, Athanassiou P, Treacher DF, Wheeler MJ, Forsling ML. Neurohypophysial hormone and melatonin secretion over the natural and suppressed menstrual cycle in premenopausal women. Clin Endocrinol (Oxf) 1998;49:209–209. doi: 10.1046/j.1365-2265.1998.00504.x. [DOI] [PubMed] [Google Scholar]
- 84.Cagnacci A, Paoletti AM, Soldani R, Orru M, Maschio E, Melis GB. Melatonin enhances the luteinizing hormone and follicle-stimulating hormone responses to gonadotropin-releasing hormone in the follicular, but not in the luteal, menstrual phase. J clin endocrin metab. 1995;80:1095–1095. doi: 10.1210/jcem.80.4.7714075. [DOI] [PubMed] [Google Scholar]
- 85.Bispink G, Zimmermann R, Weise HC, Leidenberger F. Influence of melatonin on the sleep- independent component of prolactin secretion. J Pineal Res. 1990;8:97–97. doi: 10.1111/j.1600-079x.1990.tb00669.x. [DOI] [PubMed] [Google Scholar]
- 86.Reiter RJ, Tan DX, Manchester LC, Paredes SD, Mayo JC, Sainz RM. Melatonin and reproduction revisited. Biol Reprod. 2009;81:445–445. doi: 10.1095/biolreprod.108.075655. [DOI] [PubMed] [Google Scholar]
- 87.Carlomagno G, Nordio M, Chiu TT, Unfer V. Contribution of myo-inositol and melatonin to human reproduction. Eur J Obstet Gynecol Reprod Biol. 2011;159:267–267. doi: 10.1016/j.ejogrb.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 88.Eryilmaz OG, Devran A, Sarikaya E, Aksakal FN, Mollamahmutoglu L, Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet. 2011;28:815–815. doi: 10.1007/s10815-011-9604-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shreeve N, Cagampang F, Sadek K, Tolhurst M, Houldey A, Hill CM, et al. Poor sleep in PCOS; is melatonin the culprit? Hum Reprod. 2013;28:1348–1348. doi: 10.1093/humrep/det013. [DOI] [PubMed] [Google Scholar]
- 90.WHO Technical Report Series Recent advances in medically assisted conception. 1992;820:1–1. [PubMed] [Google Scholar]