Summary
Individual mammalian cells exhibit large variability in cellular volume even with the same absolute DNA content and so must compensate for differences in DNA concentration in order to maintain constant concentration of gene expression products. Using single molecule counting and computational image analysis, we show that transcript abundance correlates with cellular volume at the single cell level due to increased global transcription in larger cells. Cell fusion experiments establish that increased cellular content itself can directly increase transcription. Quantitative analysis shows that this mechanism measures the ratio of cellular volume to DNA content, mostly likely through sequestration of a transcriptional factor to DNA. Analysis of transcriptional bursts reveals a separate mechanism for gene dosage compensation after DNA replication that enables proper transcriptional output during early and late S-phase. Our results provide a framework for quantitatively understanding the relationships between DNA content, cell size and gene expression variability in single cells.
Introduction
Within a population, individual mammalian cells can vary greatly in their volume, often independently of their position in the cell cycle (Bryan et al., 2014; Crissman and Steinkamp, 1973; Tzur et al., 2009). Biochemical reaction rates, however, depend on the concentration of reactants and enzymes. Thus, to maintain proper cellular function, most molecules must be present in the same concentration despite these volume variations, meaning that the absolute numbers of molecules would have to scale roughly linearly with cellular volume (see Marguerat and Bähler for an excellent review (Marguerat and Bähler, 2012)).
One critical molecule whose concentration need not scale with cellular volume, however, is DNA. Most mammalian cells have two or four copies of the genome per cell, and even cells with the same number of genomes can differ widely in size; thus, DNA concentration can vary dramatically from cell to cell. This poses a problem: if two otherwise identical cells with the same DNA content had different volumes, then the larger cell must somehow maintain a higher absolute number of biomolecules despite them being expressed from the same amount of DNA.
Previous efforts to resolve this puzzle have largely focused on analyzing bulk population measurements of size-altering mutants. A number of such studies have shown that the amount of both RNA and protein generally scales with cellular volume (Marguerat and Bähler, 2012; Marguerat et al., 2012; Schmidt and Schibler, 1995; Watanabe et al., 2007; Zhurinsky et al., 2010) and ploidy (Wu et al., 2010), with some further finding that transcription changes in mutants with larger or smaller cell volumes (Fraser and Nurse, 1979; Schmidt and Schibler, 1995; Zhurinsky et al., 2010). Most of these studies utilized yeast, with a few notable exceptions (Miettinen et al., 2014; Schmidt and Schibler, 1995; Watanabe et al., 2007).
These experiments do not, however, establish a causal relationship between cellular volume changes and transcript abundance. Causality could change the interpretation of gene expression measurements because if cellular volume changes can in and of themselves change global expression levels, observations of changes in global expression levels in response to various perturbations may actually be the indirect consequence of changes to cellular volume rather than resulting from direct global transcriptional responses to the perturbations per se.
Also unclear is how or even whether these mechanisms manifest in individual cells. Much recent evidence has shown that individual transcripts levels can vary from cell to cell due to stochastic effects in gene expression (Raj and van Oudenaarden, 2008; 2009; Sanchez and Golding, 2013) such as transcriptional bursts (Chubb et al., 2006; Golding et al., 2005; Raj et al., 2006; Suter et al., 2011; Zenklusen et al., 2008). Yet it remains unclear how differences in cellular volume affect the interpretation of such measurements, or whether transcriptional measurements can reveal further characteristics of homeostatic mechanisms.
We used single molecule RNA imaging and computational image analysis to measure transcript abundance and cellular volume simultaneously in individual human cells. Cell fusion experiments showed that cellular size can directly and globally affect gene expression by modulating transcription. Quantitative analysis of these experiments revealed that the mechanism underlying this global regulation does not merely sense cellular volume, but rather integrates both DNA content and cellular volume to produce the appropriate amount of RNA for a cell of a given size, consistent with a model in which a factor limiting for transcription is sequestered to the DNA, either through direct titration by DNA or restriction to the nuclear compartment. We also provide a quantitative framework for interpreting gene expression variability in single cells, and extended this framework to genome-wide single cell RNA-seq analysis, showing that cell-type specific genes are more variable than ubiquitously expressed genes.
Results
mRNA counts scale with cellular volume in single mammalian cells
We first looked at the number of mRNA molecules in individual primary fibroblast cells (human primary foreskin fibroblasts, CRL2097) within a population to see whether mRNA counts scale with cellular volume at the single cell level. We measured both mRNA abundance and volume simultaneously using single molecule multi-color mRNA fluorescence in situ hybridization (RNA FISH (Femino et al., 1998; Raj et al., 2008)), which allowed us to detect the positions of individual mRNAs in three dimensions as fluorescent spots in the microscope (Fig. 1A). We measured the abundance of a particular mRNA (e.g., TBCB) labeled with one color and then calculated the volume of the cell using the 3D positions of mRNA from a “volume guide” gene labeled with another color to define the cellular boundary (Fig. 1B, Methods). The cell volumes we measured varied over a six-fold range, agreeing with other estimates (Bryan et al., 2014; Tzur et al., 2009), and are robust to choice of guide gene, and the fixation procedure itself (Supplemental Fig. 1). We ultimately measured the abundance of 25-30 different mRNA species in both this primary fibroblast line and a lung cancer line (A549).
Figure 1. mRNA from many genes scales with cellular volume.
A. Single molecule RNA FISH. DAPI stain in blue, TBCB mRNA FISH probe in white. B. Representative outline of a primary fibroblast cell found using our volume calculation algorithm. C. mRNA vs. volume for EEF2, LMNA, TBCB. Each point represents one single cell measurement. Each data set is a combination of at least two biological replicates, with at least 30 cells per replicate. D. GAPDH mRNA and volume in primary fibroblast cells. Marginal histograms show volume and mRNA distributions. Colors indicate cell cycle stage determined by Cyclin A2 (CCNA2) mRNA count. Dashed diagonal line is the best linear fit of RNA vs. volume. Vavg indicates the average primary fibroblast volume. We determined volume-independent and -dependent transcript levels using the linear fit and Vavg. Data are a 15% subset of 1868 cells spanning >30 biological replicates. E. Fraction of volume-independent and -dependent RNA expression from the linear fit of RNA vs. volume for 21 genes in primary fibroblast cells (we omitted highly variable genes whose volume-independent fractions were less than zero). Data for each gene are a combination of at least two biological replicates, with at least 30 cells per replicate. F. GAPDH mRNA vs. volume in cycling and quiescent primary fibroblast cells. Dashed lines are best fit line for GAPDH in cycling cells. Data are an 8% subset of 1868 cells spanning >30 biological replicates for cycling cells, and 10% subset of 1105 cells for quiescent. We only analyzed quiescent cells that had less than 20 CCNA2 mRNA. G. Mean GAPDH mRNA count and H. concentration in different growth conditions for data from (f). See also supplemental figs. 1-3.
For most genes, mRNA counts and volumes in single cells exhibited a strongly positive, linear correlation (e.g. Fig. 1C; see Supplemental Fig. 2 for all genes examined). Because larger cells had proportionally more transcripts than smaller cells, the mRNA concentration remained relatively constant from cell to cell despite considerable variation in absolute mRNA numbers. This scaling property was not confined to high abundance mRNAs like GAPDH and EEF2–genes expressing as few as 10-20 mRNA per cell such as ZNF444 and KDM5A scaled similarly, as did rRNA (Supplemental Fig. 2). We also observed the same behavior for short lived mRNA such as UBC and IER2 mRNA, whose half-lives are 2.9 and 2.2 hours, respectively (Tani et al., 2012).
We checked whether the scaling of mRNA count with volume depended on cell cycle progression or cell growth. We co-stained cells with cell cycle markers (Eward et al., 2004; Levesque and Raj, 2013; Robertson et al., 2000; Whitfield et al., 2002) to classify them as being in the G1, S, or G2 phases of the cell cycle (Supplemental Fig. 3). Cell volume varied as much for cells in individual phases of the cell cycle as the population overall, with a shift in the distribution towards G2 cells being larger, and the linear relationship between mRNA count and volume did not depend on cell cycle phase (Fig. 1D), showing that mRNA count did not depend on DNA content of the cell. We also note that the primary fibroblast cells exhibit normal ploidy (Levesque and Raj, 2013), so our results are not simply explained by differences in ploidy. We also found that nuclear size increased somewhat with cellular volume, and that nuclear size increased in later stages of the cell cycle (Supplemental Fig. 3). To check if progression through the cell cycle or cell growth is responsible for maintaining scaling, we grew the primary fibroblasts for 7 days in medium lacking serum, making them quiescent. Despite growth and cell-cycle arrest, we found that mRNA count and volume still scaled strongly for all genes examined, showing that neither progression through cell cycle nor continual cell growth are required for mRNA count to scale with cellular volume (Fig. 1F). Interestingly, we found that while the mean count of mRNA decreased in quiescent cells as compared with proliferating cells, the cells maintained a similar concentration of GAPDH and other mRNA between the two conditions (Fig. 1G-H and Supplemental Fig. 1).
We also checked whether we could observe similar behavior in intact organisms. We looked at both RNA and DNA density in the heads and gonads of adult nematodes, comparing measurements from both wild-type worms and worms with mutations leading to decreased organismal size but with roughly the same number of cells (Watanabe et al., 2007) (Fig. 2A-B). We found that the RNA concentrations were roughly the same between the two strains, and that the number of RNA per molecule of DNA decreased by a factor similar to that of the volume differences between the strains (Fig. 2C-D), verifying that our observations can hold in vivo.
Figure 2. mRNA scales with volume in vivo.
A. Images of the two C. elegans strains. B. Quantification of the relative sizes of the two strains (N=24 for N2, N=20 for CB502). C. Number of mRNA molecules per cell in the gonad region for each type of worm for genes ama-1 and arf-3. We estimated the number of cells in each segment by counting nuclei stained with DAPI. Each bar is a compilation of 3 biological replicates, with >3 worms per replicate. D. Concentration of mRNA in the gonad region. All scale bars are 10μm. All error bars represent standard error of the mean.
It is important to note that while the mRNA abundance is strongly correlated with cellular volume, the y-intercept (a) of a line fit to the data (mRNA = a + b*V) was non-zero, indicating that mRNA count in individual cells has a volume-independent component in addition to the volume-correlated component. Quantifying the relative fractions of mRNA that are volume-correlated vs. volume-independent in a cell of average volume (Fig. 1E) (i.e., a/(a+b*Vavg) vs. b*Vavg/(a+b*Vavg)) revealed a range of values for different genes (Supplemental Fig. 1), although the volume-dependent fraction was dominant for most genes examined. Thus, the mRNA concentration is actually somewhat greater in smaller cells than in larger ones; for most genes, the smallest cells have an mRNA concentration 1.2-3 times greater than that of the largest cells (Supplemental Fig. 2). We later describe a mathematical model providing a potential explanation for this increased concentration based on nuclear volume measurements (see supplemental note).
Transcriptional activity, not mRNA degradation, scales globally with cellular volume
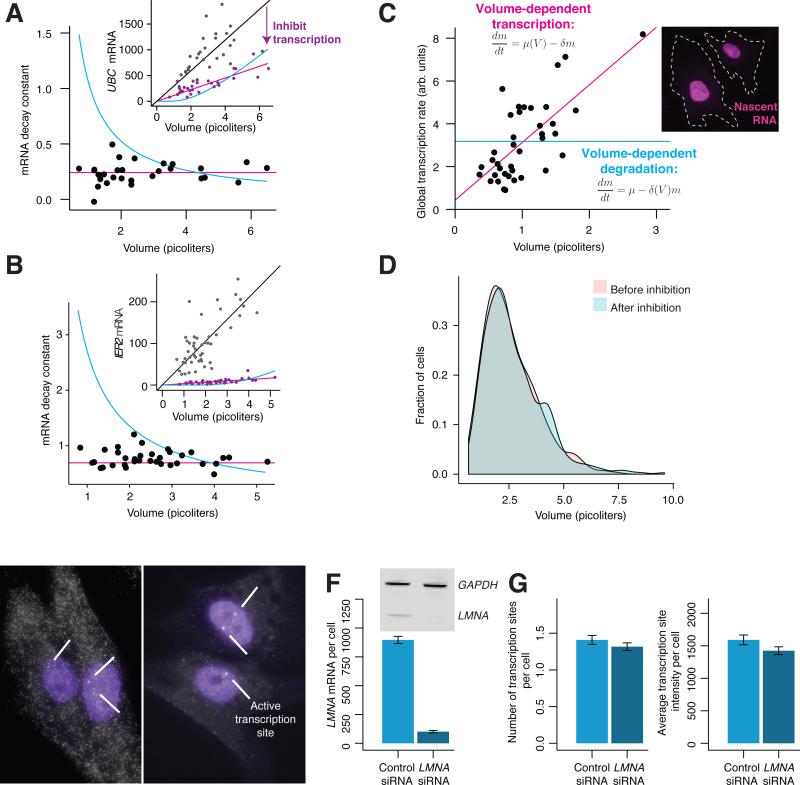
These data show that larger cells have a proportionally higher number of mRNA than smaller cells, even if they have the same absolute number of DNA molecules. To maintain this proportionality, larger cells must either transcribe more mRNA from the same number of DNA molecules or degrade those mRNA more slowly. To distinguish these possibilities, we determined the rate of mRNA degradation in cells of different sizes by measuring volumes and mRNA counts for UBC and IER2 after a period of 4 hours of transcriptional inhibition (transcriptional inhibition did not affect cell volume; Fig. 3D). By measuring mRNA counts before and after transcription inhibition (Fig. 3A-B, inset; see Methods for details), we calculated the effective decay constant for each measured cell (Fig. 3A-B). We found that the degradation rate was the same in cells of all volumes, showing that slower degradation is not responsible for the increased number of mRNA in larger cells.
Figure 3. Cells exhibit global volume-dependent transcriptional control over mRNA abundance.
A, B. We inhibited transcription in primary fibroblast cells using Actinomycin D for 4 hours and allowed UBC (A) and IER2 (B) mRNA to degrade. Inset shows mRNA before and after inhibition. Each point represents a single-cell measurement. We calculated the decay constant for each cell using the best-fit line before inhibition (see Methods). Blue line shows fit if degradation were volume-dependent; red line shows fit if transcription were volume-dependent. Data represent one of two biological replicates. C. We fluorescently labeled nascent RNA produced in one hour using the Click-iT eU assay in primary fibroblast cells, and quantified the total fluorescence intensity by imaging the nuclei of single cells. Inset shows raw micrograph data. Blue line shows fit for volume-dependent degradation; red line shows fit for volume- dependent transcription. Data shown is from quiescent cells, and is one of three biological replicates. D. Distribution of cell volumes before and after transcription inhibition. E. We performed siRNA treatment for 72 hours in primary fibroblast cells using either a control siRNA (left), or an siRNA targeting LMNA mRNA (right). DAPI stain is shown in purple, and LMNA mRNA FISH probe is shown in white. White arrows indicate active transcription sites. F. Quantification of cytoplasmic LMNA mRNA knockdown by RNA FISH. Inset shows protein knockdown. G. Comparison of the number of LMNA transcription sites and transcription site intensity in siRNA control and LMNA knockdown conditions. We detected transcription sites through intron/exon colocalization using RNA FISH. All error bars represent standard error of the mean. Data in D, E are a combination of two biological replicates, n = 323 cells for control siRNA, 284 cells for LMNA siRNA.
We next checked whether larger cells transcribe more than smaller cells (as observed in bulk populations (Fraser and Nurse, 1979; Schmidt and Schibler, 1995; Zhurinsky et al., 2010)). We inferred global transcription rate by incorporating a labeled uridine into all newly synthesized RNA produced during a 60 minute time window (Fig. 3C), which we then rendered fluorescent via click chemistry (Jao and Salic, 2008). The intensity of fluorescence is equivalent to the global transcription rate. We found that transcription rate is linearly proportional to volume, thus showing that individual cells vary in their overall transcription (Neves et al., 2010) and these variations correlate strongly with volume. We conclude that larger cells maintain proportionally higher levels of RNA by increased transcription rather than decreased degradation as compared to smaller cells. Also, quantification of fluorescence from probes targeting the internal transcribed spacer of the rRNA (the “intronic” sequence of rRNA) showed that transcription of rRNA also scales linearly with volume (Supplemental Fig. 2), indicating that RNA polymerase I transcription is also volume-dependent.
The scaling of transcription with cellular volume could be due to global factors regulating transcription of all genes in a volume-correlated manner, or it could be that gene regulatory networks sense deviations in each particular gene's protein concentration and modulate transcription to restore concentration. In the latter case, reducing protein concentration of any one gene would result in increased transcription to compensate, whereas in the global scenario, reducing the concentration of any one gene would not appreciably affect the cellular volume, thus leaving the gene's transcription unchanged. We tested this by reducing the level of Lamin A/C mRNA and protein in the cell via small interfering RNA (siRNA) (Fig. 3E-F); we chose Lamin A/C because its expression scales strongly with volume (Fig. 1C) and is thought to be tightly regulated (Swift et al., 2013). To measure the transcriptional response, we took advantage of the fact that transcription occurs in bursts (Chubb et al., 2006; Dar et al., 2012; Golding et al., 2005; Suter et al., 2011; Vargas et al., 2005; Zenklusen et al., 2008), and genes that are actively undergoing a transcriptional burst have bright accumulations of nascent RNA at the site of transcription itself (Levesque and Raj, 2013; Levsky et al., 2002; Raj et al., 2006; Zenklusen et al., 2008) (note that siRNA does not affect nuclear RNA (Maamar et al., 2013)). We measured both the fraction of cells with active Lamin A/C transcription sites and the intensity of those transcription sites, finding both metrics unchanged upon reduction of Lamin A/C protein levels (Fig. 3G). We conclude that increased mRNA counts in larger cells result from a global difference in transcription rather than the activity of a particular gene network that regulates the concentration of Lamin A/C. There may be other situations in which mRNA levels are regulated by specific networks.
A diffusible trans factor sensing DNA content and volume links cellular volume and transcription
What links volume and transcription? One possibility is that the total cellular content itself exerts a global influence on transcription, thus making transcription scale with cellular volume; alternatively, transcription may affect cellular volume. To distinguish these possibilities, we fused a small human melanoma cell that expressed GFP mRNA (WM983b-GFP-NLS) at constant density to the larger fibroblasts that do not express GFP (Fig. 4A) to form heterokaryons (Pomerantz et al., 2009). We found that absolute GFP mRNA counts increased in fused cells as compared to the original small cells (Fig. 4B), showing that increasing total cellular content is by itself sufficient to increase absolute mRNA abundance. Moreover, the GFP mRNA counts scaled with heterokaryon volume (Fig. 4C), suggesting that the rate of GFP transcription scaled with the ultimate volume of the fused cell. The fact that the nucleus from the WM983b-GFP-NLS cell could change its overall transcriptional activity showed that the modulation occurs via the activity of a diffusible trans factor.
Figure 4. A trans-acting limiting factor links gene expression to volume.
A. Representative image of fused cells (heterokaryon, left) and unfused cells (WM983b, primary fibroblast, right). DAPI stain is in orange, GFP mRNA is in green, and GAS6 mRNA is in white. White arrows indicate transcription sites. B. Quantification of GFP mRNA in unfused and fused cells. Box extends to first and third quartile, and whiskers extend to the maximum-distance points within 1.5 inter-quartile ranges of the box. Data are a combination of two biological replicates. C. GFP vs. volume for fused and unfused cells. Upper dashed line represents fit for unfused cells. Lower dashed line has a slope that is half of the upper fit line. D. Schematic of transcriptional output of fused cells if the scaling of expression with volume were mediated by a volume sensor or a volume/DNA sensor. All error bars represent standard error of the mean.
How might such a factor transmit volume information to the GFP gene in order to increase its transcription concordantly with the increase in cellular volume? There are two broad categories of mechanism: 1. The factor acts as a “volume sensor” and does not know about the amount of DNA in the cell. An example could be a modifiable global transcription factor protein whose degree of modification/activity is proportional to cellular size (Fig. 4D, left). 2. The factor acts as a “volume/DNA sensor” whose activity depends on both cellular volume and DNA content. One such mechanism is the existence of a general transcription factor of limiting abundance relative to the number of binding sites in the DNA (limiting factor). Here, the DNA “counts” how big the cell is by binding all available factor molecules (Fig. 4D, right), thus increasing transcription in bigger cells as more factor binds to DNA. Another possibility is sequestration of the factor to the nucleus, which can also achieve the same volume/DNA sensing behavior if nuclear volume is only weakly dependent on cellular volume (see supplemental note).
We distinguished these two alternatives by comparing the concentration of GFP mRNA in the fused and unfused cells (Fig. 4C). In the volume sensor scenario, the fusion cell will have the same concentration of GFP mRNA as the original small cells because the factor transmits the volume information to the GFP gene independent of the number of nuclei in the cell. In the volume/DNA sensor scenario, the fusion cell will have half the concentration of GFP mRNA because the factor senses both the increased volume and the two nuclei (for example, a limiting factor would be diluted between the two nuclei in the fused cell). We found that the concentration of GFP mRNA in the fused cells is strictly less than and very close to half the concentration in unfused cells. We conclude that the factor responsible for increased transcription in smaller cells is not a volume sensor, but responds to both the size and DNA content. These results also suggest that perturbations that change cell size will indirectly change global transcript counts per cell through this generic mechanism.
Transcriptional burst size increases in larger cells
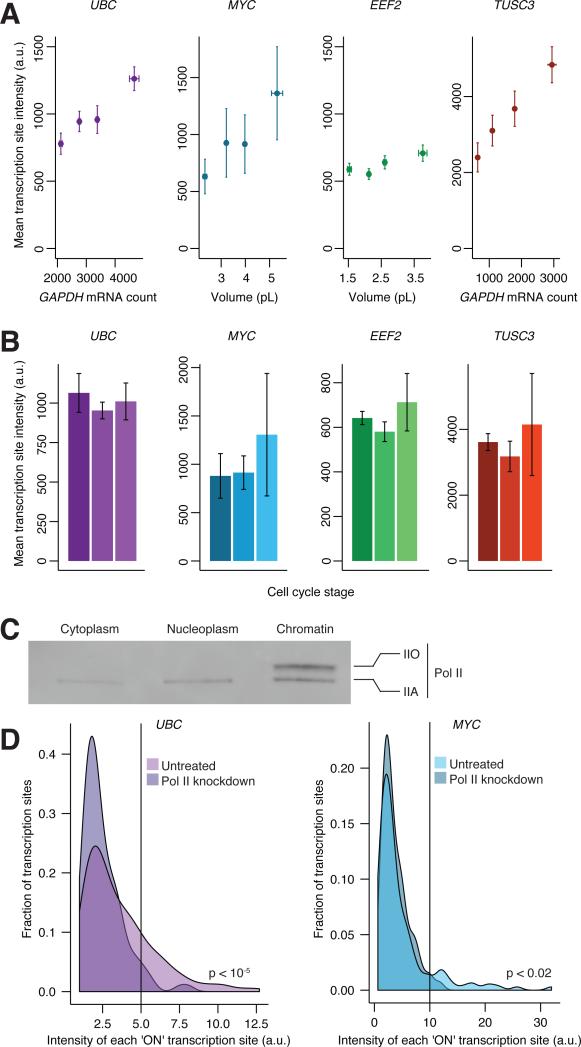
We next sought to characterize this mechanism further by examining the relationship between volume and transcription of individual genes. mRNA is produced in bursts, marked by bright accumulations of nascent mRNA at the site of transcription. We characterize this bursting behavior through burst size (how much RNA is produced during a single burst) and burst frequency (how often a gene is actively transcribing, which is related to burst frequency (Levesque and Raj, 2013)). To quantify burst size, we measured the intensity of transcription sites for four genes in our fibroblasts, and found higher intensity transcription sites in larger cells (Fig. 5A). We verified that the intensity of transcription sites reflected the degree of transcriptional activity by treating primary fibroblast cells with 100nM triptolide, which targets RNA polymerase II for degradation (Bensaude, 2011), reducing its levels (Fig. 5D). After one hour, we saw a reduction of bright transcription sites for two different genes, showing that transcription site intensity depends directly on the amount of transcriptional machinery available. This intensity is often proportional to burst size (Levesque and Raj, 2013) (Senecal et al., 2014). Interestingly, transcription site intensity did not depend on cell cycle stage (Fig. 5B). We concluded that the factor connecting gene expression and cellular volume affected mRNA abundance through modulation of transcriptional burst size.
Figure 5. Transcriptional burst size increases in larger cells.
A. Transcription site intensity and volume in primary fibroblast cells for genes UBC, MYC, EEF2, and TUSC3. Each data point represents the mean transcription site intensity per cell for a quartile of cells classified by volume or GAPDH. We detected transcription sites through intron/exon colocalization using RNA FISH. We calculated volume for EEF2 data using EEF2 as a guide, and volume for MYC data using GAPDH. We use GAPDH as a proxy for volume for UBC and TUSC3. B. Transcription site intensity and cell cycle stage in primary fibroblast cells. We determined cell cycle stage by Cyclin A2 and the histone 1H4E mRNA counts (see Methods and Supplemental Fig. 3). For intensity measurements, data for UBC, MYC, and EEF2 are from one of two biological replicates (EEF2: n = 190, UBC: n = 202, MYC: n = 103 transcription sites). Data for TUSC3 are combined from two biological replicates (n = 255 transcription sites). C. Western blot analysis reveals that >99% of the C-terminal domain hyper-phosphorylated form of RNA Polymerase II (IIO) is present in the chromatin fraction. The hypo-phosphorylated form of Pol II (IIA) is captured in all cellular fractions. We generated subcellular lysates from the same batch of primary fibroblast cells and probed with the F-12 antibody (Santa Cruz Biotechnology) that is directed against the N-terminal region of RPB1, the largest subunit of RNA polymerase II. We adjusted sample volumes so that Western blot signals of the subcellular fractions are comparable. D. Quantification of transcription site intensity before and after treatment with 100nM triptolide for one hour. P-value represents the probability of randomly finding the distributions of bright transcription sites (values to the right of the black line) in each condition. See also supplemental figs. 3-4.
What might this factor be? In order to formalize the possibilities, we developed a mathematical model for how this factor may act (see supplemental note), assuming that the factor is almost purely nuclear and that the total amount of factor in the cell is proportional to cellular volume. Our model of perfect linear scaling of transcription with volume requires that either 1. the volume of the nucleus remains fixed between cells irrespective of cytoplasmic volume or 2. the factor has such a high affinity for DNA that it is essentially titrated by DNA (or both). In both scenarios, either the nucleus or the DNA “counts” the amount of factor in the cell to modify transcription, thus making transcription proportional to the total amount, rather than concentration, of the factor. Further, in scenario 1, it predicts that if nuclear volume changes with cell size, it will manifest as a deviation from pure linear scaling of transcription with cellular volume. Our data cannot distinguish these two possibilities, but our analysis did predict that in scenario 1, the slight increase in nuclear volume we saw in larger cells (Supplemental Fig. 3) could lead to a slight decrease in mRNA concentration, manifesting as a non-zero intercept when fitting RNA abundance to volume, as observed in Fig. 1D. Indeed, the quantitative magnitude of the increased concentration at low cell volumes matches what our model predicts based on our measured relationship between nuclear volume and cellular volume (see supplemental note), perhaps favoring the model in which transcriptional scaling results from sequestration of this factor to the nucleus over a pure titration mechanism. It is possible that the factor is some component of the general transcriptional machinery, which is 94% nuclear based on fractionation (Fig. 5C) (Mayer et al. 2015).
A DNA-linked cis-acting factor reduces transcription frequency, not burst size, immediately after DNA replication
We have shown that cells in the G1 and G2 phases of the cell cycle can have the same volume and same mRNA density, although cells in G2 have twice the DNA content as those in G1. How then do two cells of the same size produce the same amount of mRNA, despite having different amounts of DNA?
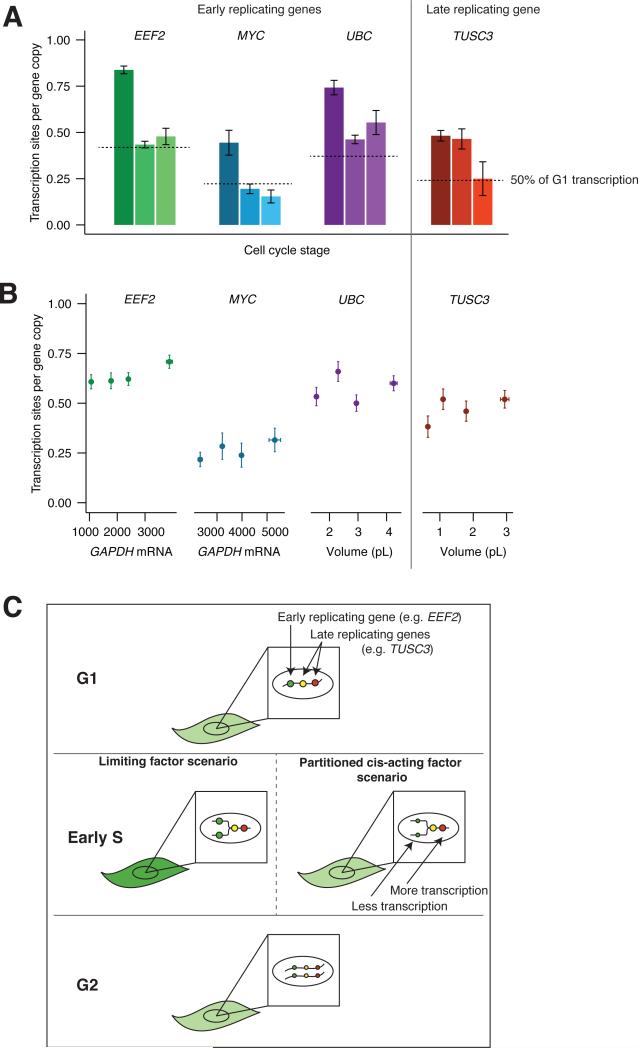
We already found that transcriptional burst size does not change dramatically between phases of the cell cycle (Fig. 5B), so we now measured burst frequency. To measure transcriptional burst frequency, we counted active transcription sites in each cell and divided by the total number of gene copies for each stage of the cell cycle (two copies in G1, four copies in G2). For all of the genes we measured, the frequency per gene copy in G2 was approximately half of that in G1 (Fig. 6A). This is not due to repression of replicated copies of DNA, as we observed G2 cells with four transcription sites (Supplemental Fig. 4). This showed that the cell has a mechanism to precisely reduce transcription frequency in G2 to keep overall transcription constant across the G1 and G2 phases of the cell cycle. Burst frequency did not depend on cell volume (Fig. 6B), showing that the mechanism is distinct from the volume-compensating mechanism described above.
Figure 6. A cis-acting factor decreases transcription frequency immediately upon DNA replication.
A. Number of transcription sites by cell cycle stage in primary fibroblast cells. We determined cell cycle stage by Cyclin A2 and Histone 1H4E mRNA counts. Dashed lines represent half the number of transcription sites in G1. We normalize all G1 data to two gene copies, and all G2 data to four gene copies. For EEF2, MYC, and UBC (early replicators), we normalize S phase data to four gene copies. For TUSC3 (late replicator), we normalize S phase data to two gene copies. B. Number of transcription sites per gene copy classified by volume in primary fibroblast cells. Each data point represents the mean number of transcription sites for a quartile of cells classified by volume. We calculated volume for EEF2 data using EEF2 as a guide, and volume for MYC data using GAPDH. We use GAPDH as a proxy for volume for UBC and TUSC3. For frequency measurements, data for EEF2, UBC, and TUSC3 are a combination of two biological replicates (EEF2: n = 516, UBC: n = 332, TUSC3: n = 255 transcription sites). Data for MYC is from one of two biological replicates (MYC: n = 103 transcription sites). C. Schematic depicting different potential mechanisms for changing gene expression with cell cycle. See also supplemental figs. 3-4.
We were surprised that the mechanism for cell cycle was different from the one compensating for volume. In principle, limiting factor models that may be responsible for volume conservation would also compensate for increased gene copy number due to DNA replication. However, these models predict an inappropriate boost in transcription for genes that replicate early in S phase because a limiting factor would distribute itself over all the DNA, essentially “double counting” the small percentage of genes that replicate considerably earlier than the majority of DNA (Fig. 6C). To see if this was the case, we measured transcriptional burst frequency for early replicating genes in S phase (EEF2, MYC, UBC; see Supplemental Fig. 4 for replication timing). These genes all showed the same transcription frequencies per gene copy in S and G2, ruling out the possibility that a factor simply gets diluted between copies of replicated DNA.
This leaves two alternatives for burst frequency reduction between G1 and G2. Transcription frequency could universally decrease by a factor of two upon the initiation of S phase, but this would potentially cause the opposite problem–genes that replicate late in S phase would be under-transcribed for the majority of S phase. Alternatively, transcription frequency could decrease on a gene-by-gene basis (i.e., in cis) immediately upon DNA replication. To test between these alternatives, we imaged transcription of a gene that replicates very late in S phase (TUSC3; Supplemental Fig. 4). If transcription frequency were universally reduced by a factor of two at the beginning of S phase, this gene would have the same transcription frequency in S and G2. However, we found that this late-replicating gene maintains G1 levels of transcription through S phase, and does not reduce transcription frequency until G2. Therefore we conclude that there exists a mechanism whereby transcription frequency is reduced by a factor of two immediately upon replication of that gene, with different timing for different genes. Candidates for a mechanism include the partitioning or modification of DNA-linked factors, such as histones with particular modifications, upon DNA replication, resulting in half the transcriptional burst frequency as before replication.
Together, our data demonstrate the existence of two separate transcriptional mechanisms that allow cells to maintain RNA concentration homeostasis despite changes in DNA content and cellular volume. Cells modulate transcriptional burst size through a trans mechanism to allow larger cells to produce more mRNA from the same amount of DNA, and modulate burst frequency over the cell cycle in cis to maintain RNA concentration despite changes in DNA content.
Single-cell RNA sequencing reveals that cell type specific genes are “noisier” than ubiquitously expressed genes
Our RNA FISH data revealed that while the expression of most genes was consistent with a volume-dependent transcription rate, many of the genes also showed strong variability in transcript concentration from cell to cell (Li and Xie, 2011; Raj and van Oudenaarden, 2009; Raj et al., 2008; Sanchez and Golding, 2013).
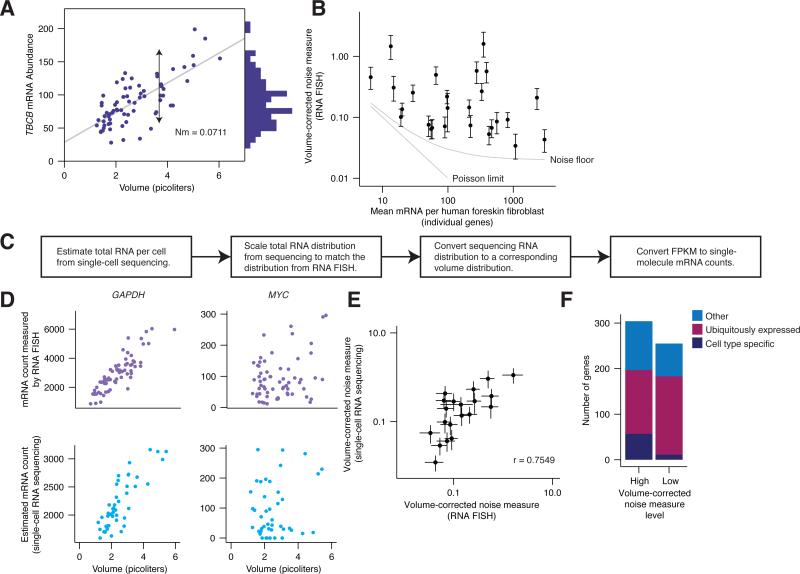
In order to accurately quantify gene expression noise while accounting for cell volume, we developed a metric we call volume-corrected noise measure (Nm, Fig. 7A), defined as (Bowsher and Swain, 2012; Johnston et al., 2012; Swain et al., 2002):
where
and a, b indicate the intercept and slope of a best-fit line for mRNA and volume,
Volume-corrected noise measure is in principle similar to the squared coefficient of variation of mRNA concentration, but accounts for volume-independent transcription (Supplemental Fig. 5).
Figure 7. Connection between single cell RNA-sequencing and RNA FISH reveals that cell-type specific genes exhibit higher noise levels.
A. RNA FISH data. TBCB mRNA abundance and volume in primary fibroblast cells. Each point represents a single-cell measurement. Histogram indicates mRNA distribution. Arrow indicates volume-corrected noise measure. Gray line is best linear fit. B. Volume-corrected noise measure values for different genes in primary fibroblast cells. Each data point represents a collection of single-cell measurements for one gene. The straight gray line represents the Poisson limit. The curved gray line is the Poisson limit plus our experimental noise limit, a combination of the Poisson limit and a 15% measurement error. Error bars represent SEM. Data for each gene is a combination of at least two biological replicates, with at least 30 cells per replicate. C. Pipeline for converting FPKM from single-cell sequencing to RNA FISH-equivalent counts and cellular volume in picoliters. D. Qualitative comparison of count vs. volume from RNA FISH and single-cell RNA sequencing. Example low-Nm (GAPDH) and high-Nm gene (MYC). E. Comparison between Nm calculated from RNA FISH data and single-cell RNA-seq data. Each point represents a single gene. Nm is calculated by bootstrapping; error bars represent 95% confidence interval, calculated by bootstrapping. F. Breakdown of high- and low-noise genes into ubiquitously expressing genes and genes that express in a cell-type-dependent manner. See also supplemental figs. 5-7.
We evaluated Nm for all of the genes we measured by RNA FISH. A standard measure of gene noise is the coefficient of variation (CV, standard deviation divided by mean), which only takes into account the spread and the mean of the expression data. Using such a measurement, most of the genes we measured by RNA FISH would be deemed “noisy”, or far from Poisson noise levels (Supplemental Fig. 5). However, when we instead measure Nm for each of our RNA FISH genes, we see that many of them display levels of variability near to or indistinguishable from Poisson (Fig. 7B) once we account for measurement error (upper bound of around 15% (Raj et al., 2006)).
To quantify this variability genome-wide, we performed single-cell RNA sequencing (Brennecke et al., 2013; Grün et al., 2014; Shalek et al., 2013) on human foreskin fibroblast cells, calibrating the sequencing data using our RNA FISH results (Fig. 7C, Supplemental Fig. 6) and adding synthetic RNA at known concentrations to estimate cell volume. Briefly, we used the ratio of RNA-sequencing reads mapping to the transcriptome to the reads mapping to spiked-in RNA from the External RNA Controls Consortium (ERCCs (Devonshire et al., 2010)) to estimate the total relative amount of RNA in the cell (Marinov et al., 2014; Wu et al., 2014), dropping a small number of cells that were qualitatively inconsistent with the rest (Supplemental Fig. 7). We used the measured relationship between GAPDH mRNA count and cellular volume from RNA FISH to convert total RNA to relative cellular volume, matching this to our measured volume distribution to obtain cellular volumes. We then used the correlation between FPKM (fragments per kilobase per million fragments mapped) and RNA FISH counts for our gene panel to provide estimates of absolute RNA counts for all genes in individual cells. (It is important to note, however, that there is substantial variance in this relationship, and that the relationship is nonlinear.) We found that both RNA FISH and single cell mRNA sequencing yielded similar results for noisy and non-noisy genes (Fig. 7D-E).
Upon quantifying Nm for the genes in our RNA FISH experiments in both human foreskin fibroblast and lung cancer cells, we noticed that 3 of the 4 genes (ICAM1, LUM, ACTA2) with the strongest degree of cell-type specificity were also the 3 genes with the highest noise measure of all the genes in our study (Supplemental Fig. 7). To see if this trend held more generally, we used our single cell RNA-sequencing data to explore noise measure across all genes. We selected genes with high or low noise measures and looked for enrichment in genes exhibiting cell-type specific expression between human lung cancer cell and foreskin fibroblast cell data. We found that the set of high noise measure genes contained a significantly higher proportion of cell-type specific genes than low-noise genes (Fig. 7F, see Supplemental Fig. 7 for classification details). Such findings mirror those showing that more ubiquitously expressed “housekeeping” genes typically exhibit lower levels of noise than other types of genes, although the notion of cell-type specificity is more difficult to relate to studies performed in single-celled organisms (Bar-Even et al., 2006; Newman et al., 2006; Taniguchi et al., 2010).
Discussion
We have shown that individual cells globally control transcription to compensate for variability in the ratio of DNA to cellular content. Our results point to two independent mechanisms: one that compensates for cell size fluctuations and one that compensates for DNA content changes during the cell cycle. These compensatory mechanisms help to maintain the concentration of mRNA in the cell, which is presumably useful from the perspective of the cell because the rates of most chemical reactions in the cell depend on concentration rather than absolute number. Importantly, the fact that these mechanisms seem to be global in nature and not gene-specific means that other specific forms of transcriptional regulation, for instance during development or in response to particular cues, can still function properly in both large and small cells without the need for a complex interplay between the specific regulation and the mechanism governing concentrational homeostasis; indeed, the expression of most genes is likely not specifically compensated to maintain concentration (Springer et al., 2010). This is not to say that the concentration of most gene expression products is arbitrary and unregulated. Rather, these global mechanisms provide a means by which any such regulation may operate to achieve said concentration without having to take into account differences in DNA content due to cellular volume or DNA content differences. This is important in a number of biological contexts such as development and embryogenesis, in which rapid cell divisions lead to an exponential decrease in individual cell volume, but the organism must maintain the concentration of most proteins while still enabling dynamic transcriptional programs to occur (Nair et al., 2013).
Our work also highlights the importance of taking cellular volume into account when interpreting gene expression data and points to the significance of global factors in studying single cell expression in general (Elowitz et al., 2002; Neves et al., 2010; Volfson et al., 2005). In particular, our cell fusion experiments show that changing the amount of cellular content in and of itself can lead to changes in total RNA abundance, whereas previous experiments largely relied on cell-size mutants that make inferences of cause and effect more difficult (Fraser and Nurse, 1979; Miettinen et al., 2014; Schmidt and Schibler, 1995; Zhurinsky et al., 2010). These cell fusion experiments directly establish that any perturbation that changes overall cellular volume may result in global changes in overall transcript abundance as a secondary rather than primary effect per the generic mechanism of a diffusible trans factor that senses an increased ratio of volume to DNA. Thus, we believe one must take care in interpreting experiments showing global changes in transcript abundance (Lin et al., 2012; Nie et al., 2012), both from the perspective of establishing causal relationships, given that cellular volume/content can by itself change transcription rates, and in the interpretation of the functional significance, given that the concentration of many transcripts will remain roughly the same despite these overall changes. Our study does not, however, address the question of why cells have different volumes and how expression plays a role in that heterogeneity–such questions necessarily involve the examination of mechanisms regulating cell growth and proliferation. Rather, our results show how cells may globally cope with such changes to maintain biomolecule concentration.
We do not yet know the factor (or factors) linking the ratio of cellular volume to DNA content to the amount of transcription. One potential candidate is some element of the general transcription machinery, such as the RNA polymerase II holoenzyme. We have shown that RNA polymerase II is almost completely nuclear, and it is required for transcription; indeed, its reduction changes burst size, much as reduced volume does. Its transcription also scales with volume (Supplemental Fig. 4). Other studies (Marguerat and Bähler, 2012; Zhurinsky et al., 2010) have speculated that RNA polymerase II holoenzyme may act as a limiting factor titrated by DNA, but various studies (Kimura et al., 1999; 2002) show that RNA polymerase II is only directly associated with DNA for short periods of time. Thus, we believe that the scenario in which the factor remains in the nucleus and nuclear volume scales only weakly with cell size is the most plausible given our current evidence (see supplemental note). Indeed, our model shows that the weak scaling of nuclear volume with cell size we observe can also explain why we observed higher mRNA concentrations in smaller cells, sometimes by a factor of two or more, further supporting this view, although more experiments will be required to establish this model.
Regardless of the origin of the effect, it is clear that mRNA concentration is typically higher in smaller cells. We do not know the consequences of this effect, in particular on cell growth, nor how it may vary in different cell types and contexts. One practical consequence of this finding is that the time-honored practice of normalizing transcript levels to GAPDH mRNA abundance, while largely sound, does not fully account for differences in mRNA concentration between small and large cells. This suggests that new strategies may be required for measuring cellular volume when interpreting single cell RNA-sequencing experiments.
We found it striking that the volume compensation mechanism is distinct from the one that compensates for changes in DNA content as the cell cycle progresses. We found that the burst frequency appears to decrease upon DNA replication for each gene rather than at a particular time in the cell cycle for all genes. One plausible explanation for this feature is to ensure proper transcriptional output regardless of whether a gene is replicated early or late in S-phase, which can proceed for many hours. The molecular underpinnings of this mechanism remain unclear, although our results demonstrate that it must be a factor that remains bound to DNA and changes in character during DNA replication. A likely candidate may be a DNA or histone modification that completely coats the DNA during G1 but is diluted by a factor of two upon DNA replication in S-phase.
Together, these findings provide a deeper quantitative understanding of single cell gene expression and its role in maintaining cellular homeostasis. Further work may elucidate how these homeostatic mechanisms for maintaining biomolecule concentration manifest themselves in biological contexts and whether they are an important point of dysregulation in disease processes.
Materials and Methods
Cell culture
We grew primary human foreskin fibroblast cells (CCD-1079Sk, ATCC CRL-2097™) and A549 cells (human lung carcinoma, A549, ATCC CCL-185™) in Dulbecco's Modified Eagle Medium supplemented with 10% FBS and 50U/mL penicillin and streptomycin (Pen/Strep). To create quiescent cells, we grew primary fibroblast cells in DMEM with Pen/Strep, without FBS for seven days. We cultured WM983b-GFP-NLS cells (melanoma cell line from the lab of Meenhard Herlyn) in Tu2% media. The WM983b-GFP-NLS contains EGFP fused to a NLS driven by a cytomegalovirus promoter that we stably transfected into the parental cell line.
RNA fluorescence in situ hybridization and imaging
We performed single molecule RNA FISH on the samples as described previously (Femino et al., 1998; Raj and Tyagi, 2010; Raj et al., 2008). We co-stained the actin cytoskeleton with Phalloidin-Alexa 488 (Life Technologies) to detect cell boundaries.
We used Cyclin A2 mRNA to specifically label cells in S, G2 and M phase (Eward et al., 2004). To distinguish cells in S phase from G2 (Robertson et al., 2000; Whitfield et al., 2002), we labeled Histone H4 mRNA (see Supplemental Fig. 3). Supplemental data file 1 lists all sequences of oligonucleotide probes.
We imaged the cells with a Nikon Ti-E equipped with appropriate filter sets. We took a series of optical z-sections, each 0.2-0.35 microns high, that spanned the vertical extent of the cell.
Image analysis and quantification
We manually identified cell boundaries and counted and localized RNA spots using custom software written in MATLAB (Raj and Tyagi, 2010; Raj et al., 2008). We estimate the technical error in our RNA count determination to be at most 15%.
To compute the volume of a cell, we used the 3D positions of a highly abundant mRNA found by RNA FISH to define the outline of the cell, applying corrections for bias in volume estimation. The volume computation did not depend on the number of spots identified nor on the choice of mRNA (Supplemental Fig. 1). We limited ourselves to the cytoplasmic volume by removing a vertical cylinder corresponding to the nuclear outline.
We identified transcription sites through intron/exon probe colocalization. We manually annotated transcription sites by visually inspecting images of intron and exon probes to determine instances of colocalized signal and computationally determined their intensity.
RNA degradation
We measured UBC and IER2 mRNA degradation by inhibiting transcription for four hours by applying actinomycin D at 1ug/ml. We interpreted the data using models of volume-dependent or independent degradation.
LMNA siRNA knockdown
We incubated primary fibroblast cells with an siRNA targeting LMNA for 72 hours, verifying protein knockdown via Western blot.
Heterokaryon formation
We created heterokaryons by pelleting cultures of primary fibroblast cells and WM983b-GFP-NLS cells and resuspending in PEG for 2 minutes. We added media over the course of five minutes to allow cells to fuse, then plated the cells onto two-well chambered coverglasses (Lab-Tek) and fixed the cells after 12 hours.
Fractionation and RNA polymerase II Western blot
We performed cell fractionation and blotting as described in (Bhatt et al., 2012) and based on (Wuarin and Schibler, 1994) with modifications.
Triptolide
We degraded RNA polymerase II in primary fibroblast cells by incubating cells in 100nM triptolide for one hour, then fixed cells in methanol.
Repli-seq analysis
We accessed Repli-seq data from Hansen et al. 2010 (Hansen et al., 2010) using the UW Repliseq track on the UCSC Genome Browser.
Bulk RNA Sequencing
We sequenced total RNA from primary fibroblast cells. We used the NEB Next Ultimate Library Preparation Kit for Illumina and the Ribo-Zero Magnetic Gold Kit. We used 50b single-end reads and sequenced each of two replicates at a depth of 10-15M reads. We aligned reads to hg19 using STAR's included annotation. We quantified reads per gene using HTSeq and a RefSeq hg19 annotation. We calculated FPKM for each gene using R. All sequencing data is available at GEO accession number GSE66053.
Single-cell RNA Sequencing
We prepared 96 cells for RNA sequencing on a Fluidigm C1 Single-Cell Auto Prep System using a large size chip. We added ERCC (External RNA Controls Consortium) RNA controls, Mix 1 (Ambion 4456740) at a concentration of 1:10,000 before loading and prepared cDNA libraries as per the Fluidigm instructions. We obtained 75b paired-end reads to a depth of ~1-2M reads per library. We quantified reads per gene using HTSeq and a RefSeq hg19 annotation and transformed the data to obtain an estimate of molecule count per cell. All sequencing data is available at GEO accession number GSE66053.
C. elegans growth and imaging
We grew N2 (wild type) and CB502 (sma-2 mutant) C. elegans at 20º C under standard conditions. We performed RNA FISH and analyzed the data as previously described (Raj et al. 2008), determining the volume of each analyzed region computationally.
Supplementary Material
Highlights.
Transcription scales with cell volume to maintain transcript concentration.
Cell fusion shows that increasing cellular content can increase transcription.
Transcriptional burst size changes with cell volume, burst frequency with cell cycle.
The burst frequency mechanism allows for proper transcription during early S phase.
Acknowledgments
We thank members of the Raj lab for many helpful suggestions. We thank Jeff Carey for suggesting the LMNA knockdown experiment and Sydney Shaffer for the WM983b-GFP-NLS cell line. We thank Jan Skotheim for useful discussions about mechanism and John I. Murray for pointing out that the simple trans factor model would lead to over-transcription in early S phase. We thank Hyun Youk and Uschi Symmons for a careful reading of the manuscript. We thank the Herlyn lab for providing the WM983b cell line. A.R. acknowledges support from an NSF CAREER award, NIH New Innovator Award 1DP2OD008514, and a Burroughs Wellcome Fund Career Award at the Scientific Interface. G.N. is a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation (www.lsrf.org). A.M. was supported by Long-Term Postdoctoral Fellowships of the Human Frontier Science Program (LT000314/2013-L) and EMBO (ALTF858-2012). L.S.C. acknowledges support from NIH grant (NHGRI R01HG007173), a Damon Runyon Cancer Research Foundation Frey Award, and a Burroughs Wellcome Fund Career Award at the Scientific Interface.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bar-Even A, Paulsson J, Maheshri N, Carmi M, O'Shea E, Pilpel Y, Barkai N. Noise in protein expression scales with natural protein abundance. Nat. Genet. 2006;38:636–643. doi: 10.1038/ng1807. [DOI] [PubMed] [Google Scholar]
- Bensaude O. Inhibiting eukaryotic transcription: Which compound to choose? How to evaluate its activity? Transcription. 2011;2:103–108. doi: 10.4161/trns.2.3.16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DM, Pandya-Jones A, Tong A-J, Barozzi I, Lissner MM, Natoli G, Black DL, Smale ST. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell. 2012;150:279–290. doi: 10.1016/j.cell.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher CG, Swain PS. Identifying sources of variation and the flow of information in biochemical networks. Proc Natl Acad Sci USA. 2012;109:E1320–E1328. doi: 10.1073/pnas.1119407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke P, Anders S, Kim JK, Kołodziejczyk AA, Zhang X, Proserpio V, Baying B, Benes V, Teichmann SA, Marioni JC, et al. Accounting for technical noise in single-cell RNA-seq experiments. Nature Methods. 2013;10:1093–1095. doi: 10.1038/nmeth.2645. [DOI] [PubMed] [Google Scholar]
- Bryan AK, Hecht VC, Shen W, Payer K, Grover WH, Manalis SR. Measuring single cell mass, volume, and density with dual suspended microchannel resonators. Lab on a Chip. 2014;14:569–576. doi: 10.1039/c3lc51022k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JR, Trcek T, Shenoy SM, Singer RH. Transcriptional pulsing of a developmental gene. Curr Biol. 2006;16:1018–1025. doi: 10.1016/j.cub.2006.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crissman HA, Steinkamp JA. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973;59:766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar RD, Razooky BS, Singh A, Trimeloni TV, McCollum JM, Cox CD, Simpson ML, Weinberger LS. Transcriptional burst frequency and burst size are equally modulated across the human genome. Proc Natl Acad Sci USA. 2012;109:17454–17459. doi: 10.1073/pnas.1213530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire AS, Elaswarapu R, Foy CA. Evaluation of external RNA controls for the standardisation of gene expression biomarker measurements. BMC Genomics. 2010;11:662. doi: 10.1186/1471-2164-11-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Eward KL, Van Ert MN, Thornton M, Helmstetter CE. Cyclin mRNA stability does not vary during the cell cycle. Cell Cycle. 2004;3:1057–1061. [PubMed] [Google Scholar]
- Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single RNA transcripts in situ. Science. 1998;280:585–590. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- Fraser RS, Nurse P. Altered patterns of ribonucleic acid synthesis during the cell cycle: a mechanism compensating for variation in gene concentration. J Cell Sci. 1979;35:25–40. doi: 10.1242/jcs.35.1.25. [DOI] [PubMed] [Google Scholar]
- Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Grün D, Kester L, van Oudenaarden A. Validation of noise models for single-cell transcriptomics. Nature Methods. 2014 doi: 10.1038/nmeth.2930. [DOI] [PubMed] [Google Scholar]
- Hansen RS, Thomas S, Sandstrom R, Canfield TK, Thurman RE, Weaver M, Dorschner MO, Gartler SM, Stamatoyannopoulos JA. Sequencing newly replicated DNA reveals widespread plasticity in human replication timing. Proc Natl Acad Sci USA. 2010;107:139–144. doi: 10.1073/pnas.0912402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao CY, Salic A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc Natl Acad Sci USA. 2008;105:15779–15784. doi: 10.1073/pnas.0808480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IG, Gaal B, Neves RPD, Enver T, Iborra FJ, Jones NS. Mitochondrial variability as a source of extrinsic cellular noise. PLoS Comput Biol. 2012;8:e1002416. doi: 10.1371/journal.pcbi.1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Tao Y, Roeder RG, Cook PR. Quantitation of RNA polymerase II and its transcription factors in an HeLa cell: little soluble holoenzyme but significant amounts of polymerases attached to the nuclear substructure. Mol Cell Biol. 1999;19:5383–5392. doi: 10.1128/mcb.19.8.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Sugaya K, Cook PR. The transcription cycle of RNA polymerase II in living cells. J Cell Biol. 2002;159:777–782. doi: 10.1083/jcb.200206019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque MJ, Raj A. Single-chromosome transcriptional profiling reveals chromosomal gene expression regulation. Nature Methods. 2013;10:246–248. doi: 10.1038/nmeth.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levsky JM, Shenoy SM, Pezo RC, Singer RH. Single-cell gene expression profiling. Science. 2002;297:836–840. doi: 10.1126/science.1072241. [DOI] [PubMed] [Google Scholar]
- Li G-W, Xie XS. Central dogma at the single-molecule level in living cells. Nature. 2011;475:308–315. doi: 10.1038/nature10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Lovén J, Rahl PB, Paranal RM, Burge CB, Bradner JE, Lee TI, Young RA. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maamar H, Cabili MN, Rinn J, Raj A. linc-HOXA1 is a noncoding RNA that represses Hoxa1 transcription in cis. Genes & Development. 2013;27:1260–1271. doi: 10.1101/gad.217018.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerat S, Bähler J. Coordinating genome expression with cell size. Trends in Genetics. 2012;28:560–565. doi: 10.1016/j.tig.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Marguerat S, Schmidt A, Codlin S, Chen W, Aebersold R, Bähler J. Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell. 2012;151:671–683. doi: 10.1016/j.cell.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinov GK, Williams BA, McCue K, Schroth GP, Gertz J, Myers RM, Wold BJ. From single-cell to cell-pool transcriptomes: Stochasticity in gene expression and RNA splicing. Genome Res. 2014;24:496–510. doi: 10.1101/gr.161034.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, di Iulio J, Maleri S, Eser U, Vierstra J, Reynolds A, Sandstrom R, Stamatoyannopoulos JA, Churchman LS. Native elongating transcript sequencing reveals human transcriptional activity at nucleotide resolution. Cell. 2015 doi: 10.1016/j.cell.2015.03.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen TP, Pessa HKJ, Caldez MJ, Fuhrer T, Diril MK, Sauer U, Kaldis P, Björklund M. Identification of transcriptional and metabolic programs related to mammalian cell size. Curr Biol. 2014;24:598–608. doi: 10.1016/j.cub.2014.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair G, Walton T, Murray JI, Raj A. Gene transcription is coordinated with, but not dependent on, cell divisions during C. elegans embryonic fate specification. Development. 2013;140:3385–3394. doi: 10.1242/dev.098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves, das RP, Jones NS, Andreu L, Gupta R, Enver T, Iborra FJ. Connecting variability in global transcription rate to mitochondrial variability. PLoS Biol. 2010;8:e1000560. doi: 10.1371/journal.pbio.1000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JRS, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, DeRisi JL, Weissman JS. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature. 2006;441:840–846. doi: 10.1038/nature04785. [DOI] [PubMed] [Google Scholar]
- Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz JH, Mukherjee S, Palermo AT, Blau HM. Reprogramming to a muscle fate by fusion recapitulates differentiation. J Cell Sci. 2009;122:1045–1053. doi: 10.1242/jcs.041376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Tyagi S. Detection of individual endogenous RNA transcripts in situ using multiple singly labeled probes. Meth Enzymol. 2010;472:365–386. doi: 10.1016/S0076-6879(10)72004-8. [DOI] [PubMed] [Google Scholar]
- Raj A, van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van Oudenaarden A. Single-molecule approaches to stochastic gene expression. Annual Review of Biophysics. 2009;38:255–270. doi: 10.1146/annurev.biophys.37.032807.125928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 2006;4:e309. doi: 10.1371/journal.pbio.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nature Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD, Keyomarsi K, Gonzales FA, Velicescu M, Jones PA. Differential mRNA expression of the human DNA methyltransferases (DNMTs) 1, 3a and 3b during the G(0)/G(1) to S phase transition in normal and tumor cells. Nucleic Acids Res. 2000;28:2108–2113. doi: 10.1093/nar/28.10.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Golding I. Genetic determinants and cellular constraints in noisy gene expression. Science. 2013;342:1188–1193. doi: 10.1126/science.1242975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EE, Schibler U. Cell size regulation, a mechanism that controls cellular RNA accumulation: consequences on regulation of the ubiquitous transcription factors Oct1 and NF-Y and the liver-enriched transcription factor DBP. J Cell Biol. 1995;128:467–483. doi: 10.1083/jcb.128.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senecal A, Munsky B, Proux F, Ly N, Braye FE, Zimmer C, Mueller F, Darzacq X. Transcription factors modulate c-Fos transcriptional bursts. CellReports. 2014;8:75–83. doi: 10.1016/j.celrep.2014.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalek AK, Satija R, Adiconis X, Gertner RS, Gaublomme JT, Raychowdhury R, Schwartz S, Yosef N, Malboeuf C, Lu D, et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature. 2013;498:236–240. doi: 10.1038/nature12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M, Weissman JS, Kirschner MW. A general lack of compensation for gene dosage in yeast. Molecular Systems Biology. 2010;6:368. doi: 10.1038/msb.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Molina N, Gatfield D, Schneider K, Schibler U, Naef F. Mammalian genes are transcribed with widely different bursting kinetics. Science. 2011;332:472–474. doi: 10.1126/science.1198817. [DOI] [PubMed] [Google Scholar]
- Swain PS, Elowitz MB, Siggia ED. Intrinsic and extrinsic contributions to stochasticity in gene expression. Proc Natl Acad Sci USA. 2002;99:12795–12800. doi: 10.1073/pnas.162041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104–1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Mizutani R, Salam KA, Tano K, Ijiri K, Wakamatsu A, Isogai T, Suzuki Y, Akimitsu N. Genome-wide determination of RNA stability reveals hundreds of short-lived noncoding transcripts in mammals. Genome Res. 2012;22:947–956. doi: 10.1101/gr.130559.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Choi PJ, Li G-W, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur A, Kafri R, LeBleu VS, Lahav G, Kirschner MW. Cell growth and size homeostasis in proliferating animal cells. Science. 2009;325:167–171. doi: 10.1126/science.1174294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DY, Raj A, Marras SAE, Kramer FR, Tyagi S. Mechanism of mRNA transport in the nucleus. Proc Natl Acad Sci USA. 2005;102:17008–17013. doi: 10.1073/pnas.0505580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volfson D, Marciniak J, Blake WJ, Ostroff N, Tsimring LS, Hasty J. Origins of extrinsic variability in eukaryotic gene expression. Nature. 2005;439:861–864. doi: 10.1038/nature04281. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Ishihara T, Ohshima Y. Mutants carrying two sma mutations are super small in the nematode C. elegans. Genes Cells. 2007;12:603–609. doi: 10.1111/j.1365-2443.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM, Brown PO, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AR, Neff NF, Kalisky T, Dalerba P, Treutlein B, Rothenberg ME, Mburu FM, Mantalas GL, Sim S, Clarke MF, et al. Quantitative assessment of single-cell RNA-sequencing methods. Nature Methods. 2014;11:41–46. doi: 10.1038/nmeth.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-Y, Rolfe PA, Gifford DK, Fink GR. Control of transcription by cell size. PLoS Biol. 2010;8:e1000523. doi: 10.1371/journal.pbio.1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin J, Schibler U. Physical isolation of nascent RNA chains transcribed by RNA polymerase II: evidence for cotranscriptional splicing. Mol Cell Biol. 1994 Nov. 1994;14(11):7219–25. doi: 10.1128/mcb.14.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen D, Larson DR, Singer RH. Single-RNA counting reveals alternative modes of gene expression in yeast. Nat Struct Mol Biol. 2008;15:1263–1271. doi: 10.1038/nsmb.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurinsky J, Leonhard K, Watt S, Marguerat S, Bähler J, Nurse P. A coordinated global control over cellular transcription. Curr Biol. 2010;20:2010–2015. doi: 10.1016/j.cub.2010.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.