Abstract
Improving QOL for HIV-infected individuals is an important objective of HIV care, given the considerable physical and emotional burden associated with living with HIV. Although worse QOL has been associated with depression, no research has quantified the potential of improvement in depression to prospectively improve QOL among HIV-infected adults. We analyzed data from 115 HIV-infected adults with depression enrolled in a randomized controlled trial to evaluate the effectiveness of improved depression care on antiretroviral drug adherence. Improvement in depression, the exposure of interest, was defined as the relative change in depression at 6 months compared to baseline and categorized as full response (≥50% improvement), partial response (25%–49% improvement) and no response (<25% improvement). Multivariable linear regression was used to investigate the relationship between improvement in depression and four continuous measures of QOL at 6 months: physical QOL, mental QOL, HIV symptoms, and fatigue intensity. In multivariable analyses, physical QOL was higher among partial responders (MD=2.51, 95% CI −1.51, 6.54) and full responders (MD=3.68, 95% CI −0.36, 7.72) compared to individuals who did not respond. Mental QOL was an average of 4.01 points higher (95% CI −1.01, 9.03) among partial responders and 14.34 points higher (95% CI 9.42, 19.25) among full responders. HIV symptoms were lower for partial responders (MD=−0.69; 95% CI −1.69, 0.30) and full responders (MD=−1.51; 95% CI −2.50, −0.53). Fatigue intensity was also lower for partial responders (MD=−0.94; 95% CI −1.94, 0.07) and full responders (MD=−3.00; 95% CI −3.98, −2.02). Among HIV-infected adults with depression, improving access to high-quality depression treatment may also improve important QOL outcomes.
Keywords: HIV, depression, quality of life, patient-centered outcomes
Introduction
The scale-up of antiretroviral (ARV) treatment to >8 million individuals (Joint United Nations Programme on HIV/AIDS (UNAIDS) & World Health Organization, 2012) has transformed HIV from an acute illness to a chronic condition. With early diagnosis and access to treatment, the average life expectancy for HIV-infected adults is now ~75 years. (Nakagawa et al., 2012) As individuals continue to live longer with HIV, quality of life (QOL) becomes an increasingly important patient-centered outcome for this population.(Sherbourne et al., 2000)
HIV-infected individuals face a range of issues impacting QOL, including HIV-related stigma, loss of social support, HIV symptom burden, and physical and mental health comorbidities.(Charles et al., 2012; Herrmann et al., 2013; Rao et al., 2012) When assessed, aspects which have the greatest impact on HIV-infected individuals’ daily lives, such as fatigue (Barroso, Harmon, Madison, & Pence, 2013) and HIV symptoms,(Webb & Norton, 2004a) are rarely included.
For people living with HIV, depression may play an important role in influencing QOL. Depression affects 20–30% of people living with HIV and is the most common mental health disorder among patients engaged in HIV medical care.(Bing et al., 2001) Although depression has been associated with lower QOL among HIV-infected individuals in cross-sectional studies,(Reis et al., 2011; Zimpel & Fleck, 2013) little research has focused on whether changes in depression have the potential to improve QOL.
Accordingly, we report on the relationship between improvement in depression and QOL over six months of prospective follow-up among HIV-infected individuals.
Methods
Data come from an ongoing randomized controlled trial to evaluate the effect of depression treatment on ARV adherence (the SLAM DUNC Study), described elsewhere.(Pence et al., 2012) Briefly, HIV-infected patients receiving medical care were eligible if they were English speaking, ages 18–65, screened positive for depression (score ≥10) on the Patient Health Questionnaire-9 (Spitzer, Kroenke, & Williams, 1999), and had a confirmed current major depressive disorder on the Mini International Neuropsychiatric Interview.(Sheehan et al., 1998) Exclusion criteria included history of bipolar or psychotic disorder, failure of ≥2 adequate antidepressant trials, or psychiatric presentation requiring acute intervention.(Pence et al., 2012) Participants were randomized to receive either enhanced usual care or a depression treatment model called Measurement-Based Care.(Adams et al., 2012) All participants provided written informed consent, and ethical approval was provided by Duke University and all study sites.
Measures
Change in depression, the exposure of interest, was defined as the relative change in depressive symptom severity at 6 months compared to baseline on the Hamilton Depression Rating Scale (HAM-D),(Hamilton, 1967; Leucht et al., 2013) which was administered by trained assessors blinded to study arm. The change between baseline and six months was categorized into clinically meaningful response categories (Nierenberg & DeCecco, 2001): full response (≥50% improvement), partial response (25%–49% improvement) and no response (<25% improvement).
As outcomes, we considered four continuous measures of QOL at six months: physical QOL, mental QOL, HIV symptoms, and fatigue intensity. Physical and mental QOL were measured using the Short Form-12 and scored following standard methodology to yield scores on a 0–100 scale (higher numbers indicate greater functionality; mean=50 and one standard deviation=10 in the normative US population).(J. Ware, Kosinski, Turner-Bowker, & Gandek, 2002; J. Ware Jr, Kosinski, & Keller, 1996) The number of HIV symptoms was measured by asking about the presence of 12 symptoms (headaches; fever, sweats or chills; pain in the mouth; white patches in the mouth; painful rashes or sores; nausea or loss of appetite; eye trouble; sinus infection; numbness in hands or feet; persistent cough; diarrhea) in the past six months.(Bing et al., 2001) Fatigue was measured with the fatigue intensity subscale of the HIV-Related Fatigue Scale (range: 1–10).(Barroso & Lynn, 2002; Pence, Barroso, Leserman, Harmon, & Salahuddin, 2008)
Analysis sample
Participants included here had passed the study’s six-month time point, scored ≥8 on the baseline HAM-D (indicating depressive symptoms not in remission), and completed the six-month research assessment. Of 176 study participants with a baseline HAM-D at the time of analysis, 169 (96%) had a baseline HAM-D score ≥8 and 115 (68%) of those had completed the six-month research interview.
Statistical analysis
We used linear regression to estimate the mean difference (MD; improvement or decline) on each outcome at six months comparing those demonstrating full, partial, or no depression response. Model assumptions were assessed. Final models were adjusted for age (≤45 years or >45 years)(Balderson et al., 2013), current gender (Mrus, Williams, Tsevat, Cohn, & Wu, 2005) and the baseline value of the outcome measurement. Based on literature review and sample size considerations, a parsimonious set of additional included confounders were baseline HIV symptoms (Hays et al., 2000) (physical QOL model), baseline primary partner status (Peter, Kamath, Andrews, & Hegde, 2014) (yes or no; mental QOL model), and baseline CD4 count (Jia, Uphold, Wu, Chen, & Duncan, 2005) (>350/≤350; HIV symptoms model). The functional form of each confounder (i.e. baseline age) with the outcome was assessed to determine variable coding choices. Statistical analyses were performed using Stata 11 (StataCorp, College Station, TX).
Results
The 115 participants included in this analysis were predominately of older age (median age 44), male (69%) and African American, non-Hispanic (63%). Less than a quarter of study participants were employed (24%) and the mean income in the past month was under $1,500. Participants reported being HIV-infected for a mean of 11.6 years and exhibited good clinical HIV indicators at baseline (mean CD4 count 616; 71% virally suppressed (<48 c/mL) (Table 1).
Table 1.
Baseline characteristics of 115 HIV-infected adults with depression.
| Characteristic | Mean (SD) or n (%) | Missing |
|---|---|---|
| Age, years (range: 20–61) | 43.5 (9.8) | |
| Current gender | ||
| Male | 79 (69.3) | |
| Female | 32 (28.1) | |
| Transgender / Other | 3 (2.6) | |
| Race / Ethnicity | ||
| Caucasian non-Hispanic | 35 (30.4) | |
| African American non-Hispanic | 73 (63.4) | |
| Hispanic | 2 (1.7) | |
| Other | 5 (4.3) | |
| Education, years (range: 7–28) | 13.5 (2.9) | 1 |
| Income, past month (range:0–10,000) | 1,435.30 (1,676.73) | 10 |
| Employment status | ||
| Employed full-time | 17 (14.8) | |
| Employed part-time | 10 (8.7) | |
| Unemployed | 88 (76.5) | |
| Time since infection, years (range: 0–40) | 11.6 (8.6) | 3 |
| Sexual orientation | 2 | |
| Male heterosexual | 17 (15.0) | |
| Male gay or bisexual | 58 (51.3) | |
| Female heterosexual | 31 (27.4) | |
| Female lesbian or bisexual | 2 (1.8) | |
| Other | 5 (4.4) | |
| CD4 count, cells/mm3 (range: 5–1,963) | 615.8 (354.9) | 3 |
| HIV RNA viral load suppressed (<48 c/mL) (range: 0– 415,000) | 79 (71.2) | 4 |
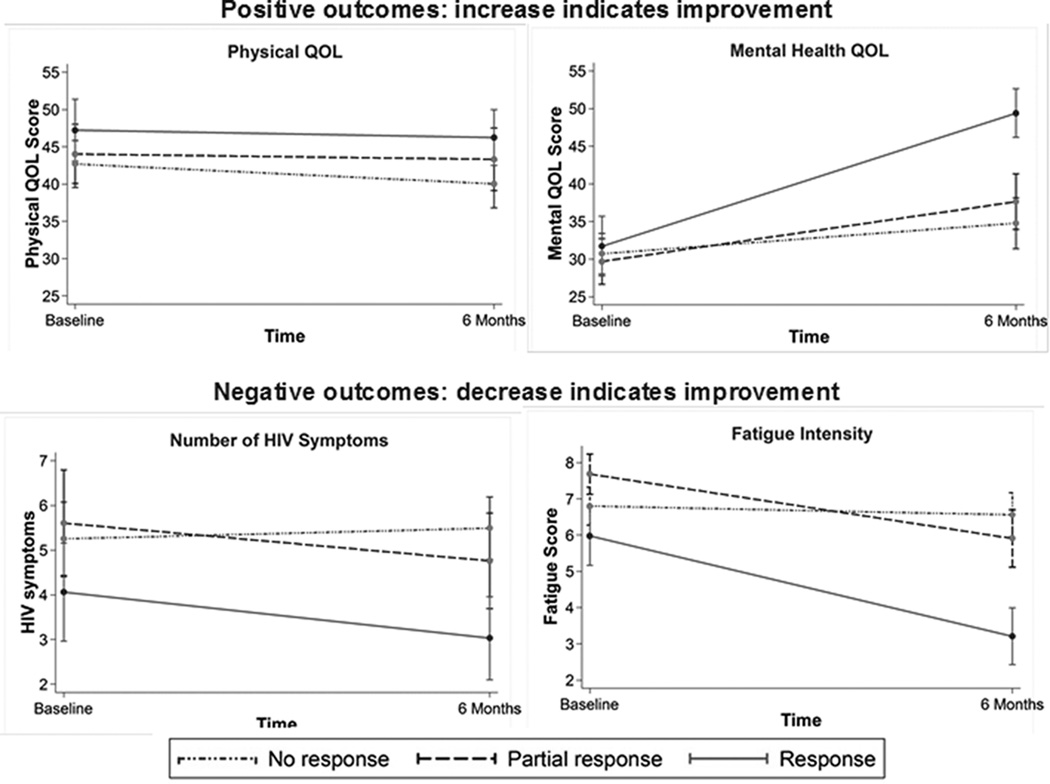
Baseline HAM-D measurements indicated severe or very severe depression (mean HAM-D 20.6; range 9–36). Just under half (47%) of participants experienced either partial depression response or full depression response (Table 2). At six months, mental QOL improved on average 8.1 points, the number of HIV symptoms decreased by an average 0.2 symptoms and fatigue improved on average 1.2 points. Physical QOL declined by an average of 1.6 points (Table 2). Those demonstrating partial or full depression response by six months showed greater improvements in mental QOL, HIV symptoms, and fatigue intensity than those who demonstrated no response (Figure 1).
Table 2.
Depression and QOL outcomes between baseline and 6 months.
| Baseline | 6 months | Change | ||||
|---|---|---|---|---|---|---|
| Characteristic | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) or n(%) | Range |
| Depressive severity (H0041M-D) | 20.6 (6.2) | 9–36 | 15.9 (8.1) | 0–33 | −4.7 (8.1) | −22 – 16 |
| No response (<25% improvement) | --- | --- | --- | --- | 61 (53.0) | --- |
| Partial response (25–49% improvement) | --- | --- | --- | --- | 26 (22.6) | --- |
| Full response (≥50% improvement) | --- | --- | --- | --- | 28 (24.4) | --- |
| Positive outcomes (increase indicates improvement) | ||||||
| Physical health-related quality of life (SF-12) | 44.1 (11.7) | 18.9–69.8 | 42.4 (11.7) | 13.3–63.6 | −1.6 (9.4) | −22.0 – 20.0 |
| Mental health-related quality of life (SF-12) | 30.7 (10.0) | 5.1–54.7 | 38.8 (13.0) | 9.7–71.1 | 8.1 (13.0) | −22.7 – 38.5 |
| Negative outcomes (decrease indicates improvement) | ||||||
| Number of HIV symptoms | 5.1 (3.1) | 0–11 | 4.8 (2.8) | 0–11 | −0.2 (2.6) | −5 – 8 |
| Fatigue intensity (HRFS) | 6.8 (2.0) | 1–10 | 5.6 (2.6) | 1–10 | −1.2 (2.6) | −9 – 5.4 |
Figure 1.
In multivariable analyses, improvement in depression was generally associated with better six-month QOL, with full responders faring better than partial responders. Despite the overall decline in physical QOL, physical QOL was higher among partial responders (MD=2.51, 95% CI −1.51, 6.54) and full responders (MD=3.68, 95% CI −0.36, 7.72) compared to individuals who did not respond. Mental QOL was an average of 4.01 points higher (95% CI −1.01, 9.03) among partial responders and 14.34 points higher (95% CI 9.42, 19.25) among full responders. HIV symptoms were lower for partial responders (MD=−0.69; 95% CI −1.69, 0.30) and full responders (MD=−1.51; 95% CI −2.50, −0.53). Fatigue intensity was also lower for partial responders (MD=−0.94; 95% CI −1.94, 0.07) and full responders (MD=−3.00; 95% CI −3.98, −2.02)(Table 3).
Table 3.
Multivariable associations between depression response and QOL outcomes at 6 months, adjusted for baseline measures.
| Increase indicates improvement | Decrease indicates improvement | |||||||
|---|---|---|---|---|---|---|---|---|
| Physical QOL* | Mental QOL** | HIV symptoms† | Fatigue intensity | |||||
| Depression response at 6 months | Mean difference | 95% CI | Mean difference | 95% CI | Mean difference | 95% CI | Mean difference | 95% CI |
| No response (ref) | - | - | - | - | - | - | - | - |
| Partial response | 2.51 | (−1.51, 6.54) | 4.01 | (−1.01, 9.03) | −0.69 | (−1.69, 0.30) | −0.94 | (−1.94, 0.07) |
| Full response | 3.68 | (−0.36, 7.72) | 14.34 | (9.42, 19.25) | −1.51 | (−2.50, −0.53) | −3.00 | (−3.98, −2.02) |
All mean differences adjusted for age (≤45/>45), gender (male/female) and baseline outcome measure.
Further ajusted for baseline HIV symptoms (continuous).
Further adjusted for baseline primary partner status (no/yes).
Further adjusted for baseline CD4 count (>350/≤350).
Discussion
In our analysis of HIV-infected adults with depression, improvement in depression over six months was associated with better QOL on a range of measures, including mental health-related functional impairment, fatigue, and HIV symptoms. Physical QOL showed the least improvement associated with changes in depression, possibly due to the overall decline in physical QOL among non-responders and the fact that over half of study participants were above age 45. For each outcome evaluated, greater improvements in depression were associated with better QOL. Overall, our results suggest that improvement in depression over time can lead to meaningful improvements in a range of important QOL measures for people living with HIV.
Improving QOL for HIV-infected individuals is an important objective of HIV care. HIV-infected individuals consistently report lower QOL compared to the general public (Hays et al., 2000). In our sample, the mean physical health QOL score at baseline was over half a standard deviation below the US population and the mean mental health QOL score was nearly two standard deviations below.(J. Ware et al., 2002) For people living with HIV, fatigue and HIV symptom burden also greatly affect daily functioning.(Barroso et al., 2013; Webb & Norton, 2004b) Participants reported a mean of 5 out of 12 HIV symptoms at baseline and a fatigue intensity of nearly 7 on a 1–10 scale. The frequency and intensity of fatigue and HIV symptoms among participants suggests that these factors should be considered, along with traditional measures such as mental and physical QOL, when assessing QOL in HIV-infected patients.
Depression is an essential determinant of QOL (Brettschneider et al., 2013), and may be particularly important for HIV-infected individuals. The prevalence of depression among people living with HIV is as much as 15% higher than in the general public.(Bing et al., 2001; Kessler et al., 2003) and is often unrecognized, untreated or inadequately treated (Asch et al., 2003; Kessler et al., 2003; Weaver et al., 2008). In our analysis, both partial and full depression response were associated with improvements in QOL. The overall trend of improvements in depression associated with better QOL suggests an opportunity to enhance QOL for HIV-infected individuals by treating depression more effectively.
Strengths of this study include prospective measures of depression with the widely used and validated HAM-D and a broad range of QOL measures. Limitations include the inability to control for potential confounding by comorbid chronic conditions, which were not measured in the study. Chronic conditions such as diabetes are associated with both depression (Egede, Zheng, & Simpson, 2002; Katon & Sullivan, 1990; Massie, 2004; Ormel et al., 2007) and lower QOL.(Alonso et al., 2004; Bayliss et al., 2012) Information on study arm and antidepressant prescription was also not available at this stage of the study. However, these variables would affect QOL by affecting depression response, thus the present analysis remains clinically relevant.
Quality of life is an important and multifaceted patient-centered outcome for people living with HIV. In our analysis, both partial depression response and full depression response led to improvements in mental health, fatigue and HIV symptom burden aspects of QOL. Further, the trend towards greater improvements in depression leading to larger improvements in QOL suggests that improving access to effective depression treatment for HIV-infected individuals may provide an opportunity to improve QOL as well.
Acknowledgements
The authors wish to thank Kristen Shirey for her comments on an earlier version of this manuscript.
Funding: This work was supported by the National Institute of Mental Health and the National Institute of Nursing Research [grant number R01-MH086362].
Footnotes
None of the authors declare any potential conflicts of interest.
Contributor Information
Angela Bengtson, Department of Epidemiology, University of North Carolina-Chapel Hill, Chapel Hill, USA.
Brian W. Pence, Department of Epidemiology, University of North Carolina-Chapel Hill, Chapel Hill, USA
Julie O’Donnell, Department of Epidemiology, University of North Carolina-Chapel Hill, Chapel Hill, USA.
Nathan Thielman, Department of Medicine, Duke University, Durham, USA.
Amy Heine, Department of Medicine, University of North Carolina-Chapel Hill, Chapel Hill, USA.
Anne Zinski, Department of Medicine, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, USA.
Riddhi Modi, Department of Medicine, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, USA.
Teena McGuinness, Department of Medicine, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, USA.
Bradley Gaynes, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, USA.
References
- Adams JL, Gaynes BN, McGuinness T, Modi R, Willig J, Pence BW. Treating depression within the HIV "medical home": A guided algorithm for antidepressant management by HIV clinicians. AIDS Patient Care and STDs. 2012;26(11):647–654. doi: 10.1089/apc.2012.0113. 10.1089/apc.2012.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J, Ferrer M, Gandek B, Ware JE, Jr, Aaronson NK, Mosconi P IQOLA Project Group. Health-related quality of life associated with chronic conditions in eight countries: Results from the international quality of life assessment (IQOLA) project. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2004;13(2):283–298. doi: 10.1023/b:qure.0000018472.46236.05. [DOI] [PubMed] [Google Scholar]
- Asch SM, Kilbourne AM, Gifford AL, Burnam MA, Turner B, Shapiro MF HCSUS Consortium. Underdiagnosis of depression in HIV: Who are we missing? Journal of General Internal Medicine. 2003;18(6):450–460. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care. 2013;25(4):451–458. doi: 10.1080/09540121.2012.712669. 10.1080/09540121.2012.712669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso J, Harmon JL, Madison JL, Pence BW. Intensity, chronicity, circumstances, and consequences of HIV-related fatigue: A longitudinal study. Clinical Nursing Research. 2013 doi: 10.1177/1054773813492998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso J, Lynn MR. Psychometric properties of the HIV-related fatigue scale. The Journal of the Association of Nurses in AIDS Care : JANAC. 2002;13(1):66–75. doi: 10.1016/S1055-3290(06)60242-2. [DOI] [PubMed] [Google Scholar]
- Bayliss M, Rendas-Baum R, White MK, Maruish M, Bjorner J, Tunis SL. Health-related quality of life (HRQL) for individuals with self-reported chronic physical and/or mental health conditions: Panel survey of an adult sample in the united states. Health and Quality of Life Outcomes. 2012;10 doi: 10.1186/1477-7525-10-154. 154-7525-10-154. 10.1186/1477-7525-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the united states. Archives of General Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Brettschneider C, Leicht H, Bickel H, Dahlhaus A, Fuchs A, Gensichen J, Zieger M. Relative impact of multimorbid chronic conditions on health-related quality of life - results from the MultiCare cohort study. PloS One. 2013;8(6):e66742. doi: 10.1371/journal.pone.0066742. 10.1371/journal.pone.0066742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles B, Jeyaseelan L, Pandian AK, Sam AE, Thenmozhi M, Jayaseelan V. Association between stigma, depression and quality of life of people living with HIV/AIDS (PLHA) in south India - a community based cross sectional study. BMC Public Health. 2012;12 doi: 10.1186/1471-2458-12-463. 463-2458-12-463. 10.1186/1471-2458-12-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25(3):464–470. doi: 10.2337/diacare.25.3.464. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. The British Journal of Social and Clinical Psychology. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hays RD, Cunningham WE, Sherbourne CD, Wilson IB, Wu AW, Cleary PD, Bozzette SA. Health-related quality of life in patients with human immunodeficiency virus infection in the united states: Results from the HIV cost and services utilization study. The American Journal of Medicine. 2000;108(9):714–722. doi: 10.1016/s0002-9343(00)00387-9. [DOI] [PubMed] [Google Scholar]
- Herrmann S, McKinnon E, Hyland NB, Lalanne C, Mallal S, Nolan D, Duracinsky M. HIV-related stigma and physical symptoms have a persistent influence on health-related quality of life in Australians with HIV infection. Health and Quality of Life Outcomes. 2013;11 doi: 10.1186/1477-7525-11-56. 56-7525-11-56. 10.1186/1477-7525-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Uphold CR, Wu S, Chen GJ, Duncan PW. Predictors of changes in health-related quality of life among men with HIV infection in the HAART era. AIDS Patient Care and STDs. 2005;19(6):395–405. doi: 10.1089/apc.2005.19.395. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS), & World Health Organization. UNAIDS world AIDS day report. 2012
- Katon W, Sullivan MD. Depression and chronic medical illness. The Journal of Clinical Psychiatry. 1990;51(Suppl):3–11. discussion 12-4. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR National Comorbidity Survey Replication. The epidemiology of major depressive disorder: Results from the national comorbidity survey replication (NCS-R) JAMA : The Journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Leucht S, Fennema H, Engel R, Kaspers-Janssen M, Lepping P, Szegedi A. What does the HAMD mean? Journal of Affective Disorders. 2013;148(2–3):243–248. doi: 10.1016/j.jad.2012.12.001. 10.1016/j.jad.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. Journal of the National Cancer Institute. Monographs. 2004;32(32):57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- Mrus JM, Williams PL, Tsevat J, Cohn SE, Wu AW. Gender differences in health-related quality of life in patients with HIV/AIDS. Quality of Life Research : An International Journal of Quality of Life Aspects of Treatment, Care and Rehabilitation. 2005;14(2):479–491. doi: 10.1007/s11136-004-4693-z. [DOI] [PubMed] [Google Scholar]
- Nakagawa F, Lodwick RK, Smith CJ, Smith R, Cambiano V, Lundgren JD, Phillips AN. Projected life expectancy of people with HIV according to timing of diagnosis. AIDS (London, England) 2012;26(3):335–343. doi: 10.1097/QAD.0b013e32834dcec9. 10.1097/QAD.0b013e32834dcec9. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: A focus on treatment-resistant depression. The Journal of Clinical Psychiatry. 2001;6216(Suppl):5–9. [PubMed] [Google Scholar]
- Ormel J, Von Korff M, Burger H, Scott K, Demyttenaere K, Huang YQ, Kessler R. Mental disorders among persons with heart disease - results from world mental health surveys. General Hospital Psychiatry. 2007;29(4):325–334. doi: 10.1016/j.genhosppsych.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Barroso J, Leserman J, Harmon JL, Salahuddin N. Measuring fatigue in people living with HIV/AIDS: Psychometric characteristics of the HIV-related fatigue scale. AIDS Care. 2008;20(7):829–837. doi: 10.1080/09540120701694063. 10.1080/09540120701694063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, Gaynes BN, Williams Q, Modi R, Adams J, Quinlivan EB, Mugavero MJ. Assessing the effect of measurement-based care depression treatment on HIV medication adherence and health outcomes: Rationale and design of the SLAM DUNC study. Contemporary Clinical Trials. 2012;33(4):828–838. doi: 10.1016/j.cct.2012.04.002. 10.1016/j.cct.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter E, Kamath R, Andrews T, Hegde BM. Psychosocial determinants of health-related quality of life of people living with HIV/AIDS on antiretroviral therapy at Udupi district, southern India. International Journal of Preventive Medicine. 2014;5(2):203–209. [PMC free article] [PubMed] [Google Scholar]
- Rao D, Chen WT, Pearson CR, Simoni JM, Fredriksen-Goldsen K, Nelson K, Zhang F. Social support mediates the relationship between HIV stigma and depression/quality of life among people living with HIV in Beijing, China. International Journal of STD & AIDS. 2012;23(7):481–484. doi: 10.1258/ijsa.2009.009428. 10.1258/ijsa.2009.009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis RK, Haas VJ, Santos CB, Teles SA, Galvao MT, Gir E. Symptoms of depression and quality of life of people living with HIV/AIDS. Revista Latino-Americana De Enfermagem. 2011;19(4):874–881. doi: 10.1590/s0104-11692011000400004. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry. 1998;5920(Suppl):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sherbourne CD, Hays RD, Fleishman JA, Vitiello B, Magruder KM, Bing EG, Shapiro MF. Impact of psychiatric conditions on health-related quality of life in persons with HIV infection. The American Journal of Psychiatry. 2000;157(2):248–254. doi: 10.1176/appi.ajp.157.2.248. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. primary care evaluation of mental disorders. patient health questionnaire. JAMA : The Journal of the American Medical Association. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Ware J, Kosinski M, Turner-Bowker D, Gandek B. How to score version 2 of the SF-12 health survey. Lincoln, Rhode Island: Quality Metric Incorporated and Health Assessment Lab; 2002. [Google Scholar]
- Ware J, Jr, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Weaver MR, Conover CJ, Proescholdbell RJ, Arno PS, Ang A, Ettner SL Cost Subcommittee of the HIV/AIDS Treatment Adherence, Health Outcomes, and Cost Study Group. Utilization of mental health and substance abuse care for people living with HIV/AIDS, chronic mental illness, and substance abuse disorders. Journal of Acquired Immune Deficiency Syndromes (1999) 2008;47(4):449–458. doi: 10.1097/QAI.0b013e3181642244. 10.1097/QAI.0b013e3181642244. [DOI] [PubMed] [Google Scholar]
- Webb A, Norton M. Clinical assessment of symptom-focused health-related quality of life in HIV/AIDS. The Journal of the Association of Nurses in AIDS Care : JANAC. 2004a;15(2):67–78. quiz 79–81. [PubMed] [Google Scholar]
- Webb A, Norton M. Clinical assessment of symptom-focused health-related quality of life in HIV/AIDS. The Journal of the Association of Nurses in AIDS Care : JANAC. 2004b;15(2):67–78. quiz 79–81. [PubMed] [Google Scholar]
- Zimpel RR, Fleck MP. Depression as a major impact on the quality of life of HIV-positive Brazilians. Psychology, Health & Medicine. 2013 doi: 10.1080/13548506.2013.772302. [DOI] [PubMed] [Google Scholar]