Abstract
Localized provoked vulvodynia (LPVD) affects approximately 16% of the female population, but biological mechanisms underlying symptoms remain unknown. Like in other, often comorbid chronic pain disorders, altered sensory processing and modulation of pain, including central sensitization, dysregulation of endogenous pain modulatory systems, and attentional enhancement of pain perception have been implicated. The aim of this study was to test whether regions of interest showing differences in LPVD compared to healthy controls (HCs) in structural and evoked-pain neuroimaging studies, also show alterations in during rest compared to HCs and a chronic pain control group (irritable bowel syndrome, IBS). Functional magnetic resonance imaging was performed during resting state in 87 age-matched premenopausal females (29 LPVD, 29 HCs, 29 IBS). Group independent component analysis and general linear models were applied to investigate group differences in the intrinsic connectivity of regions comprising sensorimotor, salience, and default mode resting state networks. LPVD subjects showed substantial alterations in the intrinsic connectivity of these networks compared to HCs and IBS. The intrinsic connectivity of many of the regions showing group differences during rest were moderately associated with clinical symptom reports in LPVD. Findings were robust to controlling for affect and medication usage. The current findings indicate LPVD subjects have alterations in the intrinsic connectivity of regions comprising the sensorimotor, salience, and default mode networks. Although shared brain mechanisms between different chronic pain disorders have been postulated, the current findings suggest some alterations in functional connectivity may show disease specificity.
Keywords: localized provoked vulvodynia (LPVD), irritable bowel syndrome (IBS), resting state networks, salience, sensorimotor, default mode network, pelvic pain
INTRODUCTION
Localized provoked vulvodynia (LPVD) affects approximately 16% of women [3; 29; 68], with some studies reporting incidence rates as high as 27% [41]. LPVD is a chronic pain disorder characterized by localized hypersensitivity of the vulvar vestibule [4; 29]. Symptoms are described as burning, sharp pain limited to the vulvar vestibule during genital contact (e.g., intercourse and tampon use) [29]. Despite the high prevalence rates and reduced quality of life, most affected women are undiagnosed or fail to achieve a satisfactory response to treatment [65]. LPVD often coexists with other chronic somatic and visceral pain disorders such as irritable bowel syndrome (IBS) and fibromyalgia, suggesting a common etiology or shared mechanisms [57; 64; 66].
Both peripheral and central abnormalities and alterations in sensory processing have been reported in women with LPVD [2; 20; 24; 72; 77]. Localized inflammation [27; 46], altered vestibular innervation [35], and tonic, dysfunctional pelvic floor muscle contractions have been implicated as possible causes [55; 83].
A few neuroimaging studies examining responses to evoked vaginal pain, and gray-matter structure in the brain have provided initial evidence that vulvar pain sensitivity may be associated with central sensitization, dysregulation of endogenous pain modulatory systems, and attentional enhancement of pain perception [35; 36; 63; 70]. These studies have reported alterations in brain regions comprising the sensorimotor, salience, and default mode resting state networks. The sensorimotor network receives sensory input from the periphery and plays an important role in body sensation awareness and generation of appropriate motor responses [12]. The salience network monitors the homeostatic state of the body on a millisecond scale, and automatically adjusts to real or expected disturbances in homeostasis through autonomic nervous system responses and behavioral responses [13]. The default mode network comprises a set of regions that are engaged at rest, decreased during goal-oriented tasks, and associated with self-referential thinking [54]. It remains to be determined if the reported brain changes play a causal role in symptom generation, or are secondary responses to the chronic pain condition.
Task free resting-state magnetic resonance imaging (MRI) studies in other chronic pain populations have also demonstrated alterations in these same three networks [6; 16; 34; 38-40; 56; 59; 81], and these alterations have been suggested to contribute to the central pain amplification in these disorders [16; 23; 34; 38; 39; 56]. These resting state networks have not been examined in LPVD.
The aim of this study was to determine whether the brain regions reported to be altered in previous neuroimaging studies in LPVD also show altered intrinsic connectivity within three resting state networks compared to healthy controls (HCs) and a chronic visceral pain group. We compare LPVD to HCs to assess whether the resting state alterations are specific to pain. To determine whether the central alterations in LPVD represent distinct or shared mechanisms with a chronic visceral pain disorder, we compared LPVD to IBS subjects. Activity in the resting brain of 87 age-matched female subjects was measured to test the following hypotheses: 1) Intrinsic connectivity of regions comprising the sensorimotor, salience, and default mode networks are different in LPVD compared to HCs and IBS and 2) the intrinsic connectivity of regions showing group differences at rest are associated with key LPVD clinical symptoms, including vulvar pain and vaginal muscle tenderness.
MATERIALS AND METHODS
Participants
Subjects with LPVD were recruited through the University of California, Los Angeles (UCLA) Obstetrics and Gynecology Clinic. The diagnosis of LPVD was identified during a clinical examination by an OB/GYN. Inclusion criteria for participants with LPVD were ≥6 months of pain in the vulvar vestibule of at least 4 out of 10 in severity (0=no pain to 10= worst pain imaginable) during attempted intercourse or other activities involving vestibular pressure (e.g. tampon use) and findings on exam consistent with vestibulodynia. Examination confirmed LPVD if the cotton swab test was positive for pain ≥4 out of 10 (0=no pain to 10= worst pain imaginable) limited to the vulvar muscle (5, 6, 7, 10 or 2 o'clock) and absence of other pathology such as dermatitis, dermatoses, vulvar vaginal atrophy, or peripheral neuropathy. Infections such as candida, bacterial vaginosis or herpes simplex were ruled out by history, visual inspection, vaginal pH, and saline and potassium hydroxide slide prep. Speculum examination of the vagina and bimanual pelvic examination were preformed to exclude other pathology that could contribute to the pain. Age-matched data for female IBS and female healthy control (HC) subjects was obtained from past subjects enrolled in neuroimaging studies at the Center for Neurobiology of Stress between 2010 and 2013 and have been used in previous publications [34; 38; 39]. Healthy control subjects (HCs) were recruited by advertisement and screened via history and medical exam for absence of pain disorders, and were recruited from the UCLA and local Los Angeles community.
Exclusionary criteria for all subjects included pregnancy or lactation, substance abuse, tobacco dependence (smoked half a package of cigarettes or more daily), abdominal surgery other than appendectomy or cholecystectomy, current or past psychiatric illness, extreme strenuous exercise (exercise more than one hour per day), and major medical or neurological conditions. In addition, subjects with current regular use of analgesic drugs (including narcotics, opioids, and α2-δ ligands) were excluded. Use of medications such as antidepressants (low-dose tricyclic anti-depressants, selective serotonin uptake inhibitors, nonselective serotonin reuptake inhibitors) was only allowed if subjects had been on a stable dose for a minimum of 3 months (10.34%, N=9 subjects of the total sample were on the medications specified above, of which N=3 (3.44%) were LPVD subjects and N=6 (6.90%) were IBS subjects). We did not exclude subjects who used NSAIDS such as diclofenac. Instead we asked that subjects refrain from taking this medication 12 hours prior to their scanning visit. All subjects were right handed and premenopausal confirmed by self-report, and most (74%, N=64) were scanned during the follicular phase of the menstrual cycle. All subjects were naturally cycling and were excluded if they were on hormonal contraceptives in order to avoid confounds associated with more sensitive vulvar vestibules. To account for the comorbidity between LPVD and IBS, presence of IBS symptoms for the LPVD subjects and presence of LPVD symptoms for the IBS subjects were recorded during the medical history. However, potential LPVD subjects were excluded if IBS was distressing or was their most important pain complaint.
The study was approved by the University of California, Los Angeles Institutional Review Board, and was conducted in accordance with the institutional guidelines regulating research on human subjects. All subjects provided written informed consent to participate and were compensated for participating in the study.
Clinical Assessments and Questionnaires
Clinical Assessment of LPVD
During clinical examination, detailed information was obtained regarding vulvar pain for the LPVD patients. Patients with LPVD were asked to report their pain duration and level of pain intensity (scale 0-20, 0=neutral, 20=extremely intense), and level of pain unpleasentness (scale 0-20, 0=neutral, 20=very intolerable) in the past 24 hours using the Gracely Differential Descriptor Pain Scale [30]. Level of pain related to sex and not related to sex was also recorded (scale 0-100, 0= no pain, 100= most intense pain imaginable). A brief neurosensory examination was conducted [45; 83]. Pain testing of the vulva and vestibule was performed using a cotton swab, which is the main diagnostic instrument for LPVD [26]. To exclude specific neuropathy, sensory testing of the sensory dermatomes (T12, L1, S2, S3/4, S5, S1) of the mons pubis, vulva and the perineum were examined bilaterally for allodynia (pain with gentle touch with the cotton tip), hyperalgesia (pain with touch with the sharp wooden end of a broken cotton swab), or normal sensation.
Mapping of pain in the vulvar vestibule was then performed by touching the vestibule perpendicularly with the cotton end of swab (enough to indent the mucosa to a depth of less than 1/3 of the cotton end) for 1 second at 5, 6, 7 (posterior vestibule), 10, and 2 o'clock (periurethral, anterior vestibule). Subjects were asked to rate the pain severity (scale=0-10; 0=none, 10=, most severe pain imaginable) and describe quality (verbal descriptor e.g., sharp, burning). A vulvar pain total “score” was created by adding the pain scores at each of the 5 vestibule sites (0-50).
The vaginal muscle examination was then performed. Internal muscle tone and tenderness was assessed with a single lubricated digit, applying approximately 2 kg of pressure for 2 seconds. (The examiner's finger pressure was calibrated immediately before the exam with an algometer). The bulbocavernosis muscles at 5 and 7 o'clock, and the levator ani complex were assessed in the midline and laterally at 5 and 7 o'clock. Participants were asked to rate the pain at each site (scale=0-10, 0 =no pain, 10 =the most severe pain imaginable, almost unconscious). A vaginal muscle tenderness total “score” was created by adding the pain scores at each of the 5 muscle sites (0-50).
Clinical Assessment of IBS
Subjects with IBS met Rome III symptom criteria for a diagnosis of IBS [22]. A gastroenterologist or gastrointestinal nurse practitioner obtained histories and conducted physical examinations. Patients with IBS who had all types of predominant bowel habits were included. Questionnaires were completed before scanning to determine IBS symptom type, severity, duration of symptoms, and abdominal sensation [Bowel Symptom Questionnaire] [17]. Overall GI symptom severity and abdominal pain in the past week were assessed using a 21-point Numerical Rating Scale (scale=0 - 20, 0 = no pain and 20 =the most intense symptoms imaginable). Usual symptom severity was assessed on an ordinal scale where 1 = None, 2 = Mild, 3 = Moderate, 4 = Severe, and 5 = Very Severe.
For all subjects levels of anxiety and depression were also assessed using a self-report 14-item instrument (Hospital Anxiety and Depression Scale; HADS) [82]. The Early Traumatic Inventory [14] was used to access histories of childhood traumatic and adverse life events in all subjects. All subjects also completed the Pain Catastrophising Scale [75].
fMRI Data Acquisition
Whole brain functional resonance imaging (fMRI) data was acquired using a 3.0T MRI scanner (Siemens Trio; Siemens, Erlangen, Germany). A high resolution structural image was acquired from each subject for registration purposes with a magnetization-prepared rapid acquisition gradient-echo sequence, repetition time = 2200ms, echo time = 3.26ms, structural acquisition time =5m 12s, slice thickness = 1mm, 176 slices, 256*256 voxel matrix, 1mm voxel size. Resting state scans were acquired using the following parameters: 40-slice whole brain volumes, slice thickness = 4mm, repetition time = 2000ms, echo time= 28ms, resting acquisition time = 10m6s, flip angle = 77°, field of view = 220, 2×2×2 mm voxel size. Noise reducing headphones were used. Subjects rested with eyes closed while functional blood oxygen-level dependent images were acquired.
Data Analysis: Image Processing and Data Analysis
Preprocessing
Resting state processing was conducted using SPM8 software (Welcome Department of Cognitive Neurology, London, UK). The first two volumes were discarded to allow for stabilization of the magnetic field. Slice timing correction was performed first, followed by rigid six-degree motion-correction realignment. The motion correction parameters in each degree were examined for excessive motion. If any volume-to-volume motion correction parameter was above 2 mm translation or 2° rotation, it was excluded from the dataset. The resting state images were then co-registered to their respective anatomical T1 images. Each T1 image was then segmented and normalized to a smoothed template brain in Montreal Neurological Institute (MNI) template space. Each subject's T1 normalization parameters were then applied to that subject's resting state image, resulting in an MNI space normalized resting state image. The resulting images were smoothed with 5mm3 Gaussian kernel. For each subject, a sample of the 300 volumes was inspected for any artifacts and anomalies. Levels of signal dropout were also visually inspected for excessive dropout in a priori regions of interest.
Resting State Brain Network Identification
Group-specific independent component analysis was implemented with GIFT 2.0c (http://www.icatb.sourceforge.com) to identify components that represented the networks of interest (i.e., sensorimotor, salience, and default mode networks) in LPVD, HCs and IBS. Multiple runs (i.e., 20 iterations) of ICA were performed using ICASSO to ensure the reliability of the ICA algorithm and to increase the robustness of the results [37]. The minimum cluster size was set to 16 and the maximum cluster size to 20. The minimum was determined by using .8 times the number of ICASSO runs and the maximum is the number of ICASSO runs based on [49]. The minimum description length was used to objectively identify the number of independent components to be extracted [48]. For each group, 15 orthogonal components were extracted with independent components analysis (ICA) using the infomax algorithm [10]. All images were visually inspected.
Components representing the sensorimotor, salience, and default mode networks were identified by spatial correlation with templates provided by Smith et al. [9; 73]. Specifically, the sensorimotor network was identified by spatial correlation with the template entitled “sensorimotor” (Map 620) independent component provided by Smith et al. [73]. The correlations between the ICA derived sensorimotor network and the Map 620 canonical template for LPVD was r=.58, for IBS was r= .55 and for HC was r=.62. The core regions in this network include the primary somatosensory cortex (primary sensory cortex), primary motor cortex (primary motor cortex), secondary somatosensory cortex and supplemental motor area [12]. The salience network was identified by spatial correlation with the template entitled the “executive control” independent component (Map 820) [73]. The correlations between the ICA derived salience executive control network and the Map 820 canonical template for LPVD was r=.49, for IBS was r= .54 and for HC was r=.57. The “executive control network” template includes dorsal anterior cingulate and insula, the core regions of the salience network [71]. The default mode network was identified by spatial correlation with the template entitled “default mode network” (Map 420) independent component provided by Smith et al. [73]. The correlations between the ICA derived default mode network and the Map 420 canonical template for LPVD was r=.57, for IBS was r= .54 and for HC was r=.60. The core regions in this network include the precuneus, posterior cingulate cortex, bilateral inferior-lateral-parietal and ventromedial frontal cortex.
Individual subject maps were then back reconstructed and converted to z-score maps representing the degree of correlation between the voxel signal and the group averaged time-course of the component. These z values reflect the functional connectivity of the voxel and the resting state network. High z scores (i.e. intrinsic connectivity) indicate greater influence of that voxel on the network [31]. As a final noise reduction step, for each group separately, subject maps for each network were entered into one-sample t-tests in SPM8 and group results were extracted using a threshold at p<0.05 corrected for family-wise error rate as recommend by Calhoun et al. [15]. The resulting thresholded t-statistic maps were then binarized to form group-specific component masks.
Data analysis
The union of these group-specific masks for each network was then utilized as an explicit mask for analyses of hypothesized group differences. To test the specified hypotheses, linear contrast analysis within the framework of the general linear model was applied in SPM8 specifying group as a factor [1; 60; 67]. Linear contrast analyses specified to test the hypotheses were 1) LPVD compared to HC and 2) LPVD compared to IBS. Two-stage cluster-extent based thresholding was implemented to control for multiple comparisons. First, clusters were defined as groups of contiguous voxels lying above a primary threshold of p<.001, uncorrected, based on recommendations by [80]. Cluster significance was then considered at p <.05 corrected for family-wise error rate [60]. This test for statistical significance controls the estimated false positive probability of the cluster as a whole, and not each individual voxel in the contiguous cluster [80]. Results for this analysis were overlaid on the MNI template available in MRIcroN (http:// http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html) for presentation purposes. In addition covariate analyses were applied to determine the sensitivity of the results to anxiety and depression. The results of these analyses are described in the text and included in the result tables.
Data analysis of non-imaging data
Differences in clinical and demographics variables were examined using the general linear model in the Statistical Package for the Social Sciences (SPSS) software (version 19). Linear contrast analysis within the framework of the general linear model was also employed to examine differences in clinical variables between 1) LPVD compared to HC and 2) LPVD compared to IBS. Significance was considered at p<.05 corrected for false discovery rate correction tests [62]. Resting state activity of regions showing group differences were correlated with clinical (measures of vulvar pain and vaginal muscle tenderness, self-report of pain), and behavioral variables (anxiety, depression, pain catastrophizing [PCS] and pain duration) in LPVD patients only. Significance was considered at p<.05, uncorrected but we emphasized effect size in this small sample, where r=.30 (r2=.09) is considered a moderate effect and r=.50 (r2=.25) is considered a large effect.
RESULTS
Clinical and behavioral characteristics
Mean clinical and behavioral characteristics of LPVD, IBS, and HCs are summarized in Table 1. Mean age of subjects was 30 years old. Although within normal clinical ranges, LPVD subjects had significantly higher anxiety and depression symptom scores compared to HCs (F(1, 57)=19.74, p=.0008 and IBS (F(1, 57)=8.35, p=.006). Pain catastrophizing scores were significantly higher in LPVD subjects compared to HCs on two subscales: rumination: p=.008, and helplessness: p=.001. No significant differences were observed between LPVD compared to IBS, or between LPVD and HCs. No statistically significant differences were found on any of the other measures between LPVD and IBS.
Table 1.
Study Demographics and Clinical/Behavioral Measures
| Premenopausal Females | LPVD (N=29) | IBS (N=29) | HC (N=29) | LPVD vs. IBS | LPVD vs. HC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F(1, 57) | p-value | p-value | F(1, 57) | p-value | p-value | |
| Age (yrs) | 30.31 | 6.79 | 30.31 | 6.95 | 30.31 | 6.80 | - | - | - | - | ||
| HADS Anxiety | 7.14 | 4.18 | 7.38 | 4.53 | 2.86 | 3.01 | .04 | .84 | .84 | 19.74 | .0008 | .002 |
| HADS Depression | 2.80 | 3.12 | 3.21 | 2.53 | 0.86 | 1.79 | .30 | .59 | .84 | 8.35 | .006 | .003 |
| PCS Overall | 18.36 | 10.52 | 20.30 | 11.44 | 5.60 | 9.97 | .37 | .55 | .84 | 11.12 | .002 | .006 |
| ETI Total | 3.56 | 3.44 | 5.13 | 4.22 | 4.80 | 4.41 | 2.03 | .16 | .27 | 1.16 | .29 | .48 |
Group differences were tested using contrast analysis within the framework of the general linear model. The F statistic associated with each test is reported along with uncorrected p values and p values corrected for false discovery rate, i.e., q-values.
Abbreviations: ETI, Early Traumatic Inventory; HADS, Hospital Anxiety and Depression Scale; HC, Healthy Control; IBS, Irritable Bowel Syndrome; LPVD, Localized Provoked Vulvodynia; N, number of subjects; PCS, Pain Catastrophizing Scale; SD, standard deviation; p-value <.05 uncorrected, y=year
Values for vulvar vestibular pain as assessed by cotton swab and for vaginal muscle tenderness as assessed by finger pressure exam for the LPVD subjects are summarized in Table 2a. The average duration of LPVD pain was about 7.5 years (90 months). For the LPVD subjects the average level of pain intensity during the past 24 hours was 5.96 (SD=6.17), and average level of pain unpleasantness during the past 24 hours was 5.29 (SD=4.86). The values for abdominal pain and gastrointestinal symptoms for the IBS subjects are summarized in Table 2b. The average symptom duration for IBS subjects was about 11 years (132 months). Of the 29 LPVD subjects, 5 reported IBS symptoms in the past and 1 reported current symptoms within the last six months. None of the 29 IBS patients reported comorbid LPVD symptoms.
Table 2.
Clinical/Behavioral Characteristics for the A. Vulvodynia Subjects and B. for the Irritable Bowel Syndrome Subjects
| A. | |||
|---|---|---|---|
| LPVD (N=29) | Range | Mean | SD |
| Gracely Differential Descriptor Pain Scale | |||
| Pain Duration (months) | 15-360 | 90.32 | 84.10 |
| Level of Pain Intensity | 0-18 | 5.96 | 6.17 |
| Level of Pain Unpleasantness | 0-17 | 5.29 | 4.86 |
| Pain Related to Sex | |||
| Level of Pain related to Sex | 0-100 | 45.48 | 32.96 |
| Level of Pain not related to Sex | 0-80 | 8.96 | 17.62 |
| Vulvar Pain | Mean | SD | |
|---|---|---|---|
| @ 10 o'clock | 3.64 | 2.82 | |
| @ 7 o'clock | 4.96 | 2.52 | |
| @ 6 o'clock | 5.25 | 2.26 | |
| @ 5 o'clock | 5.07 | 2.46 | |
| @ 2 o'clock | 3.61 | 2.67 | |
| Total Vulvar Pain Score | 22.68 | 11.82 |
| Vaginal Muscle Tenderness | Mean | SD | |
|---|---|---|---|
| Bulba @ 5 o'clock | 2.07 | 2.07 | |
| Bulba @ 7 o'clock | 2.00 | 2.11 | |
| Pubococcygeous @ 6 o'clock (levator at midline) | 1.29 | 2.18 | |
| Levator @ 5 o'clock | 1.89 | 1.93 | |
| Levator @ 7 o'clock | 1.93 | 2.16 | |
| Total Vaginal Muscle Tenderness Score | 7.97 | 7.77 |
| B. | |||
|---|---|---|---|
| IBS (N=29) | Mean | SD | |
| Bowel Symptom Questionnaire | |||
| Range | Mean | SD | |
| Overall Symptoms in the past week | 0-20 | 6.88 | 5.11 |
| Abdominal Pain in the past week | 0-20 | 4.96 | 2.52 |
| Symptom severity | 1-5 | 2.87 | .97 |
| Symptom duration (years) | 2-31 | 11.83 | 8.20 |
Pain duration and level of pain intensity were assessed on a 21-point numeric rating scale (0=neutral, 20=extremely intense) using the Gracely Differential Descriptor Pain Scale. Level of pain unpleasentness was also assesed using a 21-point numerical rating scale (scale 0-20, 0=neutral, 20=very intolerable) in the past 24 hours using the Gracely Differential Descriptor Pain Scale. Level of pain related to sex and not related to sex were recorded on a scale of 0-100 (0 = no pain, 100 = most intense pain imaginable).
For vulvar pain, pain severity was measured on an 11-point Numerical Rating Scale where 0 = none and 10 = most severe pain imaginable and total vulvar pain was calculated by totaling the scores from the 5 sites (0-50). Vaginal muscle tenderness was measured on an 11-point Numerical Rating Scale, with 0 representing no pain and 10, representing the most severe pain imaginable, almost unconscious and total vaginal muscle tenderness score was calculated by totaling the scores from the 5 sites (0-50).
Abbreviations: LPVD, localized provoked vulvodynia; N, number of subjects; SD= standard deviation
Questionnaire: Overall symptoms in the past week were measured on a 0-20 scale (0=neutral, 20=extremely intense), Abdominal Pain in the past week was measured on a 0-20 scale (0=neutral, 20=very intolerable).
Abbreviations: IBS, irritable bowel syndrome; N, number of subjects; SD, standard deviation
Disease Related Differences in the Sensorimotor Network Connectivity
In LPVD subjects compared to HCs, bilateral supplementary motor area had greater connectivity within the sensorimotor network (Table 3, Figure 1a). In LPVD subjects compared to IBS subjects, anterior supplementary motor area, posterior supplementary motor area, and left primary motor cortex had greater connectivity within the sensorimotor network (Table 3, Figure 1b). However, compared with IBS subjects, the left and right primary sensory cortex and the right superior temporal cortex had less connectivity in LPVD subjects (Table 3, Figure 1c).
Table 3.
Significant disease related alterations in the intrinsic connectivity of the sensorimotor resting state network
| Sensorimotor Network | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Controlling for Depression | Controlling for Anxiety | |||||||||||||
| Contrast | Region | Hemisphere | BA | Z | p | k | X | Y | Z | Region | p | k | Region | p | k |
| LPVD > HC | |||||||||||||||
| SMA | Left/Right | 6 | 4.20 | .008 | 55 | 0 | 6 | 58 | SMA (Right) | .043 | 26 | ||||
| PSC (Left) | .011 | 46 | |||||||||||||
| HC > LPVD | |||||||||||||||
| PSC (Left) | .008 | 50 | PSC (Left) | .087 | 46 | ||||||||||
| LPVD > IBS | |||||||||||||||
| SMA | Left/Right | 6 | 5.84 | 4.07−7 | 305 | −2 | −24 | 52 | SMA (Left/Right) | 4.26−7 | 335 | SMA (Left/Right) | 4.65−7 | 319 | |
| PMC | Left | 6 | 5.41 | 1.84−6 | 259 | −30 | −22 | 62 | PMC (Left) | 1.74−6 | 287 | PMC (Left) | 1.23−6 | 291 | |
| SMA | Right | 6 | 4.25 | .022 | 39 | 2 | 6 | 60 | SMA (Right) | .022 | 39 | SMA (Right) | .022 | 39 | |
| IBS > LPVD | |||||||||||||||
| STC | Right | 41, 42 | 6.80 | .037 | 31 | 62 | −8 | 6 | STC (Right) | .037 | 31 | STC (Right) | .037 | 31 | |
| PSC | Left | 3 | 6.03 | 1.09−5 | 208 | −44 | −14 | 32 | PSC (Left) | 1.31−4 | 192 | PSC (Left) | 1.45−4 | 197 | |
| PSC | Right | 4 | 5.88 | .012 | 49 | 54 | −6 | 34 | PSC (Right) | .015 | 45 | PSC (Right) | .014 | 46 | |
Results from the whole brain contrast analyses and covariate analyses using the general linear model to test for group differences in intrinsic connectivity are presented here.We only report group differences significant at p<.05, corrected for family wise error using SPM8. We also report results for the analysis after controlling for depression and anxiety. Missing probability values for covariate analysis indicate the cluster did not achieved an uncorrected p value <.05 uncorrected. All regions are represented in Montreal Neurological Institute (MNI) space with X, Y, Z coordinates.
Abbreviations: BA, Brodmann's area; HC, healthy control; IBS, irritable bowel syndrome; k, number of voxels in the cluster; LPVD, localized provoked vulvodynia; PMC, primary motor cortex; p, probability value corrected for family wise error rate; PSC, primary somatosensory cortex; SMA, supplementary motor area; STC, superior temporal cortex
Figure 1. Brain regions showing significant disease related alterations in the intrinsic connectivity of the sensorimotor resting state network.
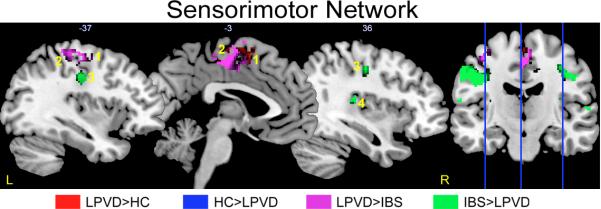
Regions showing group differences in intrinsic connectivity within the sensorimotor resting state network. Whole brain voxel-wise images are thresholded at p<.05 corrected for family wise error. Contrasts are color-coded. Voxels showing overlap by contrast have dark hues. Each sagittal slice is depicted by vertical blue line on the brain on the right and the X slice is identified in white as depicted in Montreal Neurological Institute (MNI) space.
Abbreviations: IBS: irritable bowel syndrome; HC: healthy control; L, left; LPVD, localized provoked vulvodynia, R, right.
1: left/right supplemental motor area (SMA)
2: left primary motor cortex (PMC)
3: left/right primary somatosensory cortex (PSC)
4: right superior temporal cortex (STC)
To determine the potential influence of anxiety and depression on the observed results, additional covariate analyses were performed (Table 3). When controlling for depression, all differences were maintained. However, the spatial extent and probability associated with greater connectivity of the supplementary motor area in LPVD compared to HC was reduced but remained significant at p<.05 corrected. Also when controlling for depression, it was observed that LPVD compared to controls showed weaker connectivity of the left primary sensory cortex, consistent with what was observed in LPVD compared to IBS. When controlling for anxiety, all initial results remained except differences between LPVD and HC in supplementary motor area connectivity were no longer significant. Instead greater connectivity between the left primary sensory cortex and the sensorimotor network was observed in LPVD compared to HCs.
Disease Related Differences in the Salience Network Connectivity
In LPVD subjects compared to HC subjects, left globus pallidus, left anterior midcingulate cortex, and left putamen had greater connectivity within the salience network (Table 4, Figure 2a). Compared to HCs, bilateral orbital medial prefrontal cortex (PFC) had less connectivity within the salience network in LPVD patients (Table 4, Figure 2b). In LPVD patients compared to IBS patients, the left anterior mid-cingulate cortex, left globus pallidus, and right caudate nucleus had greater connectivity within the salience network (Table 4, Figure 2c). Compared to IBS, bilateral dorsal medial PFC had less connectivity within the salience network in LPVD patients (Table 4, Figure 2d).
Table 4.
Significant disease related alterations in the intrinsic connectivity of the salience resting state network
| Salience Network | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Controlling for Depression | Controlling for Anxiety | |||||||||||||
| Contrast | Region | Hemisphere | BA | Z | Cluster FWE p | Cluster k | X | Y | Z | Region | Cluster FWE p | Cluster k | Region | Cluster FWE p | Cluster k |
|
LPVD > HC |
|||||||||||||||
| Globus Pallidus |
Left | 5.60 | 2.69−6 | 229 | − 10 |
2 | 6 | Globus Pallidus (Left) |
.002 | 81 | Globus Pallidus (Left) |
1.2=5 | 129 | ||
| aMCC | Left | 24 | 5.41 | 2.79−5 | 173 | −4 | 20 | 32 | aMCC (Left) |
2.61−5 | 169 | ||||
| Putamen | Left | 4.31 | .018 | 49 | − 14 |
10 | 4 | Putamen (Left) |
.003 | 74 | |||||
|
HC > LPVD |
|||||||||||||||
| omPFC | Left/Right | 10 | 4.90 | 4.93−6 | 214 | 0 | 60 | − 12 |
omPFC (Right) |
4.98−6 | 281 | omPFC (Right) |
4.8−6 | 239 | |
|
LPVD > IBS |
|||||||||||||||
| aMCC | Left | 24 | 6.18 | 1.49−14 | 843 | −4 | 20 | 32 | aMCC (Left) |
1.61−14 | 864 | aMCC (Left) |
1.51−14 | 825 | |
| Globus Pallidus |
Left | 5.89 | 3.19−5 | 170 | − 10 |
0 | 6 | Globus Pallidus (Left) |
2.49−5 | 159 | Globus Pallidus (Left) |
2.45−5 | 149 | ||
| Caudate Nucleus |
Right | 4.70 | .008 | 61 | 12 | 18 | 0 | Caudate Nucleus (Right) |
.004 | 69 | Caudate Nucleus (Right) |
.007 | 51 | ||
| IBS>LPVD | |||||||||||||||
| dmPFC | Left | 10 | 6.25 | 1.02−9 | 452 | 2 | 52 | 36 | dmPFC (Left) |
1.32−8 | 435 | dmPFC (Left) |
1.12−9 | 458 | |
| dmPFC | Right | 9 | 4.84 | .001 | 101 | 14 | 52 | 36 | dmPFC (Right) |
.002 | 85 | dmPFC (Right) |
.001 | 90 | |
Results from the whole brain contrast analyses and covariate analyses using the general linear model to test for group differences in intrinsic connectivity are presented here. We only report group differences significant at p<.05, corrected for family wise error using SPM8. We also report results for the analysis after controlling for depression and anxiety. Missing probability values for covariate analysis indicate the cluster did not achieved an uncorrected p value <.05 uncorrected. All regions are represented in Montreal Neurological Institute (MNI) space with X, Y, Z coordinates
Abbreviations: aMCC, anterior mid-cingulate cortex; BA, Brodmann's area; dmPFC, dorsal medial prefrontal cortex; HC, healthy control; IBS, irritable bowel syndrome; k, number of voxels in the cluster; LPVD, localized provoked vulvodynia; omPFC, orbital medial prefrontal cortex; p, probability value corrected for family wise error rate.
Figure 2. Brain regions showing significant disease related alterations in the intrinsic connectivity of the salience resting state network.
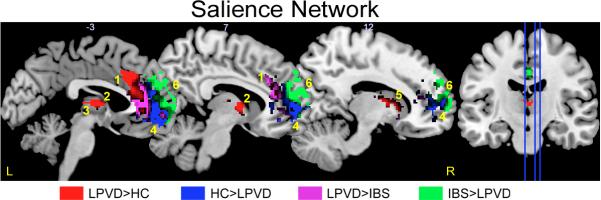
Regions showing group differences in intrinsic connectivity within the salience resting state network. Whole brain voxel-wise images are thresholded at p<.05 corrected for family wise error. Contrasts are color-coded. Voxels showing overlap by contrast have dark hues. Each sagittal slice is depicted by vertical blue line on the brain on the right and the X slice is identified in white as depicted in Montreal Neurological Institute (MNI) space.
Abbreviations: IBS: irritable bowel syndrome; HC: healthy control; L, left; LPVD, localized provoked vulvodynia, R, right.
1: left anterior mid-cingulate cortex (aMCC)
2: left putamen
3: left globus pallidus
4: left/right orbital medial prefrontal cortex (omPFC)
5: right caudate nucleus
6: left/right dorsal medial prefrontal cortex (dmPFC)
As a sensitivity analysis, analyses were rerun controlling for anxiety and depression. All but two of the initial findings were maintained. Specifically, differences between LPVD and HCs were no longer observed for anterior mid-cingulate cortex when controlling for depression and left putamen when controlling for anxiety.
Disease Related Differences in the Default Model Network Connectivity
Within the default mode network, LPVD (compared to HCs) had greater bilateral angular gyrus and right anterior precuneus connectivity (Table 5, Figure 3a), and less connectivity of the right posterior precuneus, left dorsal and ventral posterior cingulate cortex (Table 5, Figure 3b). Furthermore compared to IBS, LPVD had greater connectivity of bilateral angular gyrus and bilateral precuneus within the default mode network (Table 5, Figure 3c) but less connectivity of right precuneus and right dorsal/ventral posterior cingulate cortex within the default mode network (Table 5, Figure 3d).
Table 5.
Significant disease related alterations in the intrinsic connectivity of the default mode resting state network
| Default Mode Network (DMN) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | Controlling for Depression | Controlling for Anxiety | |||||||||||||
| Contrast | Region | Hemisphere | BA | Z | Cluster FWE p | Cluster k | X | Y | Z | Region | Cluster FWE p | Cluster k | Region | Cluster FWE p | Cluster k |
|
LPVD > HC |
|||||||||||||||
| Angular Gyrus |
Left | 7.65 | 7.67−11 | 542 | − 48 |
− 64 |
32 | Angular Gyrus (Left) |
3.45−9 | 496 | Angular Gyrus (Left) |
2.45−9 | 326 | ||
| Angular Gyrus |
Right | 5.66 | 4.71−5 | 183 | 50 | − 58 |
26 | Angular Gyrus (Right) |
2.21−4 | 163 | Angular Gyrus (Right) |
.004 | 77 | ||
| Anterior Precuneus |
Right | 6.76 | 2.22−16 | 1203 | 6 | − 52 |
12 | Precuneus (Right) |
4.21−17 | 1244 | Precuneus (Right) |
1.98−14 | 792 | ||
|
HC > LPVD |
|||||||||||||||
| Posterior Precuneus |
Right | 7.01 | 8.19−7 | 300 | 14 | − 64 |
36 | Precuneus (Right) |
2.98−6 | 306 | Precuneus (Right) |
4.98−6 | 250 | ||
| vPCC | Left | 23, 31 |
5.67 | .019 | 50 | −4 | − 38 |
22 | vPCC (Left) | .023 | 45 | ||||
| dPCC | Right | 24 | 5.25 | .024 | 46 | 4 | − 24 |
28 | dPCC (Left) | .021 | 47 | dPCC (Left) | .057 | 28 | |
|
LPVD > IBS |
|||||||||||||||
| Angular Gyrus |
Left | 39 | 7.48 | 2.62−9 | 496 | − 48 |
− 64 |
32 | Angular Gyrus (Left) |
2.61−9 | 432 | Angular Gyrus (Left) |
1.51−9 | 443 | |
| Angular Gyrus |
Right | 39 | 6.06 | 4.71−5 | 183 | 50 | − 64 |
34 | Angular Gyrus (Right) |
3.49−5 | 144 | Angular Gyrus (Right) |
2.45−4 | 141 | |
| Precuneus | Left | 4.53 | .012 | 58 | −4 | − 56 |
18 | Precuneus (Left) |
.046 | 35 | Precuneus (Left) |
.022 | 46 | ||
| Precuneus | Right | 3.66 | .037 | 39 | 16 | − 54 |
16 | Precuneus (Right) |
.048 | 22 | Precuneus (Right) |
.042 | 23 | ||
|
IBS > LPVD |
|||||||||||||||
| dPCC/vPCC | Right | 23, 31 |
6.33 | 9.15−6 | 228 | 4 | − 36 |
26 | dPCC/vPCC (Right) |
8.32−7 | 251 | dPCC/vPCC (Right) |
1.12−7 | 249 | |
| Precuneus | Right | 4.93 | 1.06−6 | 292 | 6 | − 72 |
50 | Precuneus (Right) |
1.32−6 | 312 | Precuneus (Right) |
4.42−6 | 315 | ||
Results from the whole brain contrast analyses and covariate analyses using the general linear model to test for group differences in intrinsic connectivity are presented here. We only report group differences significant at p<.05, corrected for family wise error using SPM8. We also report results for the analysis after controlling for depression and anxiety. Missing probability values for covariate analysis indicate the cluster did not achieved an uncorrected p value <.05 uncorrected. All regions are represented in Montreal Neurological Institute (MNI) space with X, Y, Z coordinates
Abbreviations: BA, Brodmann's area; dPCC, dorsal posterior cingulate cortex; HC, healthy control IBS, irritable bowel syndrome; k, number of voxels in the cluster; LPVD, localized provoked vulvodynia; p, probability value corrected for family wise error rate; vPCC, ventral posterior cingulate cortex.
Figure 3. Brain regions showing significant disease related alterations in the intrinsic connectivity of the default mode resting state network.
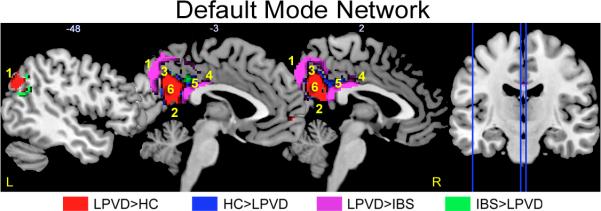
Regions showing group differences in intrinsic connectivity within the default mode resting state network. Whole brain voxel-wise images are thresholded at p<.05 corrected for family wise error. Contrasts are color-coded. Voxels showing overlap by contrast have dark hues. Each sagittal slice is depicted by vertical blue line on the brain on the right and the X slice is identified in white as depicted in Montreal Neurological Institute (MNI) space.
Abbreviations: IBS, irritable bowel syndrome; HC: healthy control; L, left; LPVD, localized provoked vulvodynia, R, right.
1: left/right angular gyrus
2: right anterior precuneus
3: right posterior precuneus
4: right dorsal posterior cingulate cortex (dPCC)
5: left/right ventral posterior cingulate cortex (vPCC)
6: left/right precuneus
When statistically controlling for anxiety and depression, all but two of the initial findings were maintained. Specifically, differences between HCs compared to LPVD were no longer observed for the left ventral posterior cingulate cortex and left dorsal posterior cingulate cortex when controlling for anxiety.
Sensitivity analysis for medication usage
We also reran all analyses after removing the 9 subjects who were on antidepressants (3 of the LPVD subjects and 6 of the IBS subjects). All disease related group differences were maintained.
Correlations between Resting State Activity and Clinical and Behavioral Measures
In LPVD subjects only, exploratory correlations were performed to examine the relationship between the intrinsic connectivity of regions showing significant group differences and clinical and behavioral measures (see Table 6). Sensorimotor Network: 1) For the contrast LPVD compared to HCs, resting state activity in the bilateral supplementary motor area showed moderate correlation with pain not related to intercourse (r=.46, p=.01). 2) For the contrast LPVD compared to IBS, bilateral supplementary motor area showed moderate correlation with total muscle tenderness scores (r=.45, p=.01), and with pain not related to intercourse (r=.40, p=.04). The left primary motor cortex correlated moderately with several behavioral variables including anxiety (r=.46, p=.02), depression (r=.43, p=.02), and pain catastrophizing (r=.45, p=.02). Salience Network: For the contrast LPVD compared to HCs, resting state activity in the left globus pallidus was negatively correlated with total vulvar pain (r=−0.38, p=0.04), and the left putamen was negatively correlated with highest daily pain (r=-.39, p=.04). For the contrast LPVD compared to IBS, the right caudate nucleus was negatively correlated with highest daily pain (r=-.42, p=.03). For the contrast IBS compared to LPVD, the left dorsal medial PFC was positively correlated with anxiety (r=.39, p=.04), but negatively correlated with pain not related to sex (r=-.41, p=.03). Default Mode Network: For the contrast LPVD compared to HCs, resting state activity in the right angular gyrus was positively correlated with pain duration (r=.46, p=.01), and the right precuneus was correlated with depression (r=.43, p=.02). For the contrast IBS compared to LPVD, the right dorsal/ventral posterior cingulate cortex was negatively correlated with total vagina muscle tenderness (r=-.39, p=.04).
Table 6.
Correlations between the intrinsic connectivity of regions showing group differences and LVPD subjective symptom reports
| Sensorimotor Network | |||
|---|---|---|---|
| Region | Measure | Correlation | p-value |
| Contrast LPVD >HC | |||
| SMA (Bilateral) | Pain not related to intercourse | .46 | .01 |
| Contrast LPVD> IBS | |||
| SMA (Bilateral) | Total Vaginal Muscle Tenderness | .45 | .01 |
| Pain not related to intercourse | .40 | .04 | |
| PMC (left) | Anxiety | .46 | .02 |
| Depression | .43 | .02 | |
| Pain Catastrophizing Scale | .45 | .02 | |
| Salience Network | |||
|---|---|---|---|
| Region | Measure | Correlation | p-value |
| Contrast LPVD>HC | |||
| Globus Pallidus (Left) | Total Vulvar Pain | −.38 | .04 |
| Putamen (Left) | Highest Daily Pain | −.39 | .04 |
| Contrast LPVD> IBS | |||
| Caudate Nucleus (Right) | Highest Daily Pain | −.42 | .03 |
| Contrast IBS>LPVD | |||
| dmPFC (Left) | Anxiety | .39 | .04 |
| Pain not related to intercourse | −.41 | .03 | |
| Default Mode Network | |||
|---|---|---|---|
| Region | Measure | Correlation | p-value |
| Contrast LPVD>HC | |||
| Angular Gyrus (Right) | Pain Duration | .46 | .01 |
| Precuneus (Right) | Depression | .43 | .02 |
| Contrast IBS>LPVD | |||
| dPCC/vPCC (Right) | Total Vaginal Muscle Tenderness | −.39 | .04 |
Abbreviations: HC, Healthy Control; dPCC/vPCC, dorsal posterior cingulate cortex/ventral posterior cingulate cortex; IBS, irritable bowel syndrome; LPVD, localized provoked vulvodynia; PMC, primary motor cortex; p-value, uncorrected probability; SMA, supplementary motor area.
DISCUSSION
The aim of the study was to identify disease-related differences in the resting state connectivity of brain regions within the sensorimotor, salience and default mode networks, in localized provoked vulvodynia, compared to HCs and a disease control group, IBS. The findings demonstrate that many of the regions reported as altered in previously reported task-based and structural MRI studies in LPVD also show altered connectivity during rest. Furthermore, although shared mechanisms have been suggested between LPVD and IBS, differences in the intrinsic connectivity of sensorimotor, salience, and default mode networks were observed. The intrinsic connectivity of many of the regions showing group differences during rest was moderately correlated with subjective reports of vulvar pain and vaginal muscle tenderness. These findings were robust and not affected when controlling for affect, state anxiety and depression, and medication usage, suggesting that the observed differences are specific to disease status rather than other confounding factors.
Sensorimotor Network Connectivity
Compared to IBS, LPVD patients showed greater connectivity of the bilateral supplementary motor area and primary motor cortex and less connectivity of primary sensory cortex with the sensorimotor network even after controlling for affect. Supplementary motor area connectivity was moderately correlated with the total muscle tenderness scores and pain not related to intercourse but not with vulvar vestibular pain scores in the LPVD.
Even though pelvic floor muscle contractions were not quantified in the current study several pieces of evidence support a possible role of the motor cortex underlying tonic contractions of the pelvic floor muscles often noted during clinical examination of women with vestibulodynia [55]. The supplementary motor area and bilateral primary sensory cortex and left primary motor cortex are reported to be activated during pelvic floor muscle contractions in female subjects [43; 44; 69]. Various clinical observations support the importance of the pelvic floor muscles in the pathophysiology and management of LPVD [2; 55]. Pelvic floor muscle biofeedback [28] or physical therapy [11; 19] show reasonable therapeutic efficacy for up to 50% of the women evaluated in published studies with small samples and physical therapy is generally included in the multidisciplinary approach [2]. Tentative support for a primary role of tonic motor contractions comes from studies in women with IC/PBS who show tenderness and impaired muscular control of the pelvic floor, and also respond to pelvic floor physical therapy [8; 25]. Resting fMRI intrinsic brain oscillations in women with painful bladder syndrome/interstitial cystitis, a syndrome often comorbid with LPVD also showed altered frequency distributions in motor and somatosensory regions comparable to those seen in the women with LPVD [40]. Despite these indirect pieces of evidence supporting a pathophysiological role of altered connectivity of the supplementary motor area and the primary motor and sensory cortices in pelvic floor or vaginal muscle contractions, these peripheral correlates were not evaluated in this study and alternative interpretations are possible.
Salience Network Connectivity
The salience network is involved in evaluating the subjective salience of internal and external stimuli in order to generate appropriate motor and autonomic outputs [71]. We found greater connectivity within the salience network in LPVD subjects for basal ganglia (left globus pallidus, left putamen, right caudate nucleus) and anterior mid-cingulate cortex compared to both HCs and IBS. Furthermore the intrinsic connectivity of the regions of the basal ganglia was associated with greater LPVD reports of total vulvar pain and highest daily pain ratings. IBS patients compared to HCs have shown increased connectivity within the salience network specifically the anterior insula, anterior cingulate, and putamen regions [34]. Alterations within the salience network have also been implicated in the pathophysiology of other chronic pain conditions [5; 13; 56; 59]. The salience network has strong connections to medial prefrontal, basal ganglia, and temporal regions, which likely provide contextual and emotional modulation of sensory stimuli [13; 33]. One may speculate that the more pronounced alterations within the salience network in LPVD compared to IBS suggest greater impairments in LPVD patients’ ability to appraise, process and respond to sensory information from the pelvis. Mechanistic studies are required to test this hypothesis.
Even after controlling for affective levels, subregions of bilateral dorsal medial PFC showed less connectivity with the salience network when LPVD was compared to the two other groups. Lower connectivity of the dorsal medial PFC was moderately correlated with pain not related to intercourse in LPVD. The medial PFC plays a prominent role in cortico-limbic inhibition providing inhibitory input to the amygdala and anterior insula[50; 79] and may also play a role in the cortical input to descending pain modulation [42]. One may speculate that LPVD subjects have a compromised ability to engage prefrontal modulatory influences on the affective dimension of the pain experience [32; 53], and on descending pain modulation systems [51; 76].
Default Mode Network Connectivity
In general, greater connectivity of known attentional regions, medial and lateral parietal regions (angular gyrus and anterior precuneus)[18; 61], with the default mode network was observed for LPVD compare to the HC and IBS with less connectivity observed for the dorsal/ventral posterior cingulate cortex. The greater connectivity of the right angular gyrus in LPVD compared to HCs was moderately correlated with increased pain duration in LPVD. The reduced connectivity of the right dorsal/ventral posterior cingulate cortex that was observed in LPVD compared to IBS correlated with increased total vaginal muscle tenderness scores, suggesting specificity of this finding to LPVD. Finally, reduced connectivity of the left ventral posterior cingulate and right dorsal posterior cingulate cortices with the default mode network was observed in LPVD patients compared to HCs but covariate analysis indicated that these effects were dependent on anxiety levels.
Our results are similar to some other studies that have demonstrated alterations of default mode network connectivity in patients with chronic pain disorders [6; 7; 56; 58; 81]. Similar to our study, increased connectivity within the default mode network has been observed in fibromyalgia patients but the activity was mainly observed in regions different to those found in this study (such as in the anterior, middle, and posterior insula) [56]. However, in a recent study, decreased connectivity was observed from the anterior insula to the bilateral precuneus and the angular gyrus in IBS subjects [39], suggesting that LPVD subjects show different patterns of resting state activity in the default mode network compared to IBS patients. The functional and pathophysiological correlates of the observed altered intrinsic connectivity within default mode network remain unknown. However, recent evidence suggests that the intrinsic connectivity of the precuneus and angular gyrus within the default mode network is strongly associated with greater efficiency in performing an executive control attention task[78].
The interplay of the default mode network with other networks such as the salience network has also been suggested in other studies [52; 74]. An important function of the salience network is to switch from the default network to other action related networks[13] For example, it is possible that increased connectivity of the attentional regions with the other regions of the default mode network are caused by dysfunction in this switching function. It remains to be determined to what degree the varying results reported in different studies reflect methodological differences, or reflect true differences in underlying brain mechanisms.
Limitations
Even though we studied only premenopausal women predominantly during the follicular phase of the menstrual cycle, we did not measure female sex hormones and therefore could not address a possible influence of sex hormones on the current findings. Additionally, since a psychiatric diagnosis was an exclusion criterion, subjects with severe LPVD and IBS with comorbid pathological levels of anxiety were excluded from the study sample. However, we did not find any significant correlations with anxiety and depression for the sensorimotor or the salience networks. Finally, interpretation of the pathophysiological relevance of the data is limited by the fact that pelvic floor or vaginal muscle contractions were not assessed during the study.
Summary and conclusions
LPVD subjects show alterations in the intrinsic connectivity of sensorimotor, salience, and default mode networks. These impairments are substantial and exceeded those seen in IBS. Although shared brain mechanisms between different chronic pain disorders have been postulated [13; 21; 47], the current findings suggest alterations in functional connectivity may also show some disease specificity. Future research is required to determine similarities and difference in the central mechanisms associated with LPVD and other chronic pain disorders. Furthermore, longitudinal studies are necessary to determine if these alterations are primary or a secondary abnormality due to chronic nociceptive input to the brain, abnormal pelvic motor function (muscle relaxation, pelvic floor physiotherapy), or cortical control mechanisms (cognitive behavioral therapy).
ACKNOWLEDGEMENTS
Funding Sources: This research was supported in part by grants from the National Vulvodynia Association and National Institutes of Health: P30 DK041301, R01 DK048351, R01 HD076756, P50DK64539. Pilot scans were provided by the Ahmanson-Lovelace Brain Mapping Center, UCLA
Footnotes
Author Contributions:
- Funding (AR, JSL, EAM, KT)
- Study Conceptualization and Design (AG, AR, JSL, EAM, KT)
- Data Acquisition (JS, SM)
- Data Analysis (AG, ZG, CF, JSL,
- Data Interpretation (AG, AR, LK, JSL)
- Manuscript preparation and Critical Revisions (AG, AR, CF, ZG, LK, JSL)
Disclosures: No conflicts of interest exist.
REFERENCES
- 1.Abdi H, Williams LJ. Contrast Analysis. In: Salkind NJ, editor. Encyclopedia of research design. Sage; Thousand Oaks, CA, USA: 2010. [Google Scholar]
- 2.Andrews JC. Vulvodynia interventions--systematic review and evidence grading. Obstetrical & gynecological survey. 2011;66(5):299–315. doi: 10.1097/OGX.0b013e3182277fb7. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstetrics and gynecology. 2006;107(3):617–624. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann GA, Rosen R, Pinn VW, Utian WH, Ayers C, Basson R, Binik YM, Brown C, Foster DC, Gibbons JM, Jr., Goldstein I, Graziottin A, Haefner HK, Harlow BL, Spadt SK, Leiblum SR, Masheb RM, Reed BD, Sobel JD, Veasley C, Wesselmann U, Witkin SS. Vulvodynia: a state-of-the-art consensus on definitions, diagnosis and management. The Journal of reproductive medicine. 2006;51(6):447–456. [PubMed] [Google Scholar]
- 5.Baliki MN, Baria AT, Apkarian AV. The Cortical Rhythms of Chronic Back Pain. J Neurosci. 2011;31(39):13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Mansour AR, Baria AT, Apkarian AV. Functional reorganization of the default mode network across chronic pain conditions. Plos One. 2014;9(9):e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassaly R, Tidwell N, Bertolino S, Hoyte L, Downes K, Hart S. Myofascial pain and pelvic floor dysfunction in patients with interstitial cystitis. International urogynecology journal. 2011;22(4):413–418. doi: 10.1007/s00192-010-1301-3. [DOI] [PubMed] [Google Scholar]
- 9.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell AJ, Sejnowski TJ. An Information Maximization Approach to Blind Separation and Blind Deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 11.Bergeron S, Brown C, Lord MJ, Oala M, Binik YM, Khalife S. Physical therapy for vulvar vestibulitis syndrome: a retrospective study. J Sex Marital Ther. 2002;28(3):183–192. doi: 10.1080/009262302760328226. [DOI] [PubMed] [Google Scholar]
- 12.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 13.Borsook D, Edwards R, Elman I, Becerra L, Levine J. Pain and analgesia: The value of salience circuits. Prog Neurobiol. 2013;104:93–105. doi: 10.1016/j.pneurobio.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bremner JD, Bolus R, Mayer EA. The early trauma inventory self report (ETI-SR). Gastroenterology. 2005;128(4):A340–A340. [Google Scholar]
- 15.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human brain mapping. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cauda F, Sacco K, D'Agata F, Duca S, Cocito D, Geminiani G, Migliorati F, Isoardo G. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in diabetic neuropathic pain. Bmc Neurosci. 2009:10. doi: 10.1186/1471-2202-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96(12):3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 18.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis SN, Bergeron S, Binik YM, Lambert B. Women with provoked vestibulodynia experience clinically significant reductions in pain regardless of treatment: results from a 2-year follow-up study. J Sex Med. 2013;10(12):3080–3087. doi: 10.1111/jsm.12309. [DOI] [PubMed] [Google Scholar]
- 20.de Belilovsky C. [2013 vulvodynia update]. Gynecologie, obstetrique & fertilite. 2013;41(9):505–510. doi: 10.1016/j.gyobfe.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Dohnel K, Sommer M, Ibach B, Rothmayr C, Meinhardt J, Hajak G. Neural correlates of emotional working memory in patients with mild cognitive impairment. Neuropsychologia. 2008;46(1):37–48. doi: 10.1016/j.neuropsychologia.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130(5):1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett. 2012;520(2):197–203. doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farmer MA, Maykut CA, Huberman JS, Huang L, Khalife S, Binik YM, Apkarian AV, Schweinhardt P. Psychophysical properties of female genital sensation. Pain. 2013;154(11):2277–2286. doi: 10.1016/j.pain.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 25.FitzGerald MP, Payne CK, Lukacz ES, Yang CC, Peters KM, Chai TC, Nickel JC, Hanno PM, Kreder KJ, Burks DA, Mayer R, Kotarinos R, Fortman C, Allen TM, Fraser L, Mason-Cover M, Furey C, Odabachian L, Sanfield A, Chu J, Huestis K, Tata GE, Dugan N, Sheth H, Bewyer K, Anaeme A, Newton K, Featherstone W, Halle-Podell R, Cen L, Landis JR, Propert KJ, Foster HE, Jr., Kusek JW, Nyberg LM, Interstitial Cystitis Collaborative Research N Randomized multicenter clinical trial of myofascial physical therapy in women with interstitial cystitis/painful bladder syndrome and pelvic floor tenderness. The Journal of urology. 2012;187(6):2113–2118. doi: 10.1016/j.juro.2012.01.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich EG., Jr Vulvar vestibulitis syndrome. The Journal of reproductive medicine. 1987;32(2):110–114. [PubMed] [Google Scholar]
- 27.Gerber S, Witkin SS, Stucki D. Immunological and genetic characterization of women with vulvodynia. Journal of medicine and life. 2008;1(4):432–438. [PMC free article] [PubMed] [Google Scholar]
- 28.Glazer HI, Laine CD. Pelvic floor muscle biofeedback in the treatment of urinary incontinence: a literature review. Appl Psychophysiol Biofeedback. 2006;31(3):187–201. doi: 10.1007/s10484-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein AT, Burrows L. Vulvodynia. The journal of sexual medicine. 2008;5(1):5–14. doi: 10.1111/j.1743-6109.2007.00679.x. quiz 15. [DOI] [PubMed] [Google Scholar]
- 30.Gracely RH, Kwilosz DM. The Descriptor Differential Scale: applying psychophysical principles to clinical pain assessment. Pain. 1988;35(3):279–288. doi: 10.1016/0304-3959(88)90138-8. [DOI] [PubMed] [Google Scholar]
- 31.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 32.Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature reviews Neuroscience. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guitart-Masip M, Bunzeck N, Stephan KE, Dolan RJ, Duzel E. Contextual novelty changes reward representations in the striatum. J Neurosci. 2010;30(5):1721–1726. doi: 10.1523/JNEUROSCI.5331-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta A, Kilpatrick L, Labus JS, Tillisch K, Braun A, Hong JY, Ashe-McNalley C, Naliboff B, Mayer EA. Early Adverse Life Events and Resting State Neural Networks in Patients with Chronic Abdominal Pain: Evidence for Sex Differences. Psychosomatic Medicine. 2014;76(6):404–412. doi: 10.1097/PSY.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halperin R, Zehavi S, Vaknin Z, Ben-Ami I, Pansky M, Schneider D. The major histopathologic characteristics in the vulvar vestibulitis syndrome. Gynecologic and obstetric investigation. 2005;59(2):75–79. doi: 10.1159/000082112. [DOI] [PubMed] [Google Scholar]
- 36.Hampson JP, Reed BD, Clauw DJ, Bhavsar R, Gracely RH, Haefner HK, Harris RE. Augmented central pain processing in vulvodynia. The journal of pain : official journal of the American Pain Society. 2013;14(6):579–589. doi: 10.1016/j.jpain.2013.01.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22(3):1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang ZG, Ashe-McNalley C, Stains J, Heendeniya N, Ebrat B, Smith S, Tillisch K, Naliboff B, Mayer EA. Patients with Chronic Visceral Pain Show Sex-Related Alterations in Intrinsic Oscillations of the Resting Brain. J Neurosci. 2013;33(29):11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katiban D, Ashe-McNalley C, Stains J, Heendeniya n, Smith S, Tillisch K, Naliboff B, Mayer EA. Sex and Disease-Related Alterations of Anterior Insula Functional Connectivity in Chronic Abdominal Pain. J Neurosci. 2014 doi: 10.1523/JNEUROSCI.1683-14.2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff B, Labus J, Jiang Z, Farmer M, Apkarian AV, Mackey S, Martucci KT, Clauw D, Harris RE, Deutsch G, Ness T, Yang CC, Maravilla K, Mullins C, Mayer EA. Alterations in resting state oscillations and connectivity within sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. The Journal of urology. 2014 doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingdon J. Vulvodynia: a comprehensive review. Nursing for women's health. 2009;13(1):48–57. doi: 10.1111/j.1751-486X.2009.01373.x. quiz 58. [DOI] [PubMed] [Google Scholar]
- 42.Kong J, Jensen K, Loiotile R, Cheetham A, Wey HY, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 2013;154(3):459–467. doi: 10.1016/j.pain.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krhut J, Holy P, Tintera J, Zachoval R, Zvara P. Brain activity during bladder filling and pelvic floor muscle contractions: a study using functional magnetic resonance imaging and synchronous urodynamics. International journal of urology : official journal of the Japanese Urological Association. 2014;21(2):169–174. doi: 10.1111/iju.12211. [DOI] [PubMed] [Google Scholar]
- 44.Kuhtz-Buschbeck JP, van der Horst C, Wolff S, Filippow N, Nabavi A, Jansen O, Braun PM. Activation of the supplementary motor area (SMA) during voluntary pelvic floor muscle contractions--an fMRI study. NeuroImage. 2007;35(2):449–457. doi: 10.1016/j.neuroimage.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 45.Lamvu G, Nguyen RHN, Burrows LJ, Rapkin A, Witzeman K, Marvel RP, Hutchins D, Witkin SS, Veasely C, Fillingim R, Zoulnoun D. The EVA (Evidence-Based Vulvodynia Assessment) Project: A National Registry for the Study of Vulvodynia. Journal of Reproductive Medicine. In Press. [PubMed] [Google Scholar]
- 46.Leclair CM, Leeborg NJ, Jacobson-Dunlop E, Goetsch MF, Morgan TK. CD4-positive T-cell recruitment in primary-provoked localized vulvodynia: potential insights into disease triggers. Journal of lower genital tract disease. 2014;18(2):195–201. doi: 10.1097/LGT.0b013e3182a55591. [DOI] [PubMed] [Google Scholar]
- 47.Lee MC, Tracey I. Unravelling the mystery of pain, suffering, and relief with brain imaging. Current pain and headache reports. 2010;14(2):124–131. doi: 10.1007/s11916-010-0103-0. [DOI] [PubMed] [Google Scholar]
- 48.Li YA,T, Calhoun VD. Sample Dependence correction for order selection in fMRI analysis. Proc ISBI.; Washington, D.C.: 2006. [Google Scholar]
- 49.Ma S, Correa NM, Li XL, Eichele T, Calhoun VD, Adali T. Automatic identification of functional clusters in FMRI data using spatial dependence. IEEE transactions on bio-medical engineering. 2011;58(12):3406–3417. doi: 10.1109/TBME.2011.2167149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115(3):398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131(6):1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual review of psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molnar-Szakacs I, Uddin LQ. Self-processing and the default mode network: interactions with the mirror neuron system. Frontiers in human neuroscience. 2013;7:571. doi: 10.3389/fnhum.2013.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morin M, Bergeron S, Khalife S, Mayrand MH, Binik YM. Morphometry of the pelvic floor muscles in women with and without provoked vestibulodynia using 4D ultrasound. The journal of sexual medicine. 2014;11(3):776–785. doi: 10.1111/jsm.12367. [DOI] [PubMed] [Google Scholar]
- 56.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic Brain Connectivity in Fibromyalgia Is Associated With Chronic Pain Intensity. Arthritis Rheum-Us. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen RH, Veasley C, Smolenski D. Latent class analysis of comorbidity patterns among women with generalized and localized vulvodynia: preliminary findings. Journal of pain research. 2013;6:303–309. doi: 10.2147/JPR.S42940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otti A, Guendel H, Henningsen P, Zimmer C, Wohlschlaeger AM, Noll-Hussong M. Functional network connectivity of pain-related resting state networks in somatoform pain disorder: an exploratory fMRI study. J Psychiatry Neurosci. 2013;38(1):57–65. doi: 10.1503/jpn.110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otti A, Guendel H, Wohlschlager A, Zimmer C, Noll-Hussong M. Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC Psychiatry. 2013;13:84. doi: 10.1186/1471-244X-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical Parametric Mapping: The Analysis of Functional Brain Images. 2nd Edition Academic Press; London, UK: 2007. [Google Scholar]
- 61.Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annual review of neuroscience. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pike N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol. 2011;2(3):278–282. [Google Scholar]
- 63.Pukall CF, Strigo IA, Binik YM, Amsel R, Khalife S, Bushnell MC. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115(1-2):118–127. doi: 10.1016/j.pain.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 64.Reed BD, Harlow SD, Sen A, Edwards RM, Chen D, Haefner HK. Relationship between vulvodynia and chronic comorbid pain conditions. Obstetrics and gynecology. 2012;120(1):145–151. doi: 10.1097/AOG.0b013e31825957cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reed BD, Harlow SD, Sen A, Legocki LJ, Edwards RM, Arato N, Haefner HK. Prevalence and demographic characteristics of vulvodynia in a population-based sample. American journal of obstetrics and gynecology. 2012;206(2):170, e171–179. doi: 10.1016/j.ajog.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reed BD, Legocki LJ, Plegue MA, Sen A, Haefner HK, Harlow SD. Factors associated with vulvodynia incidence. Obstetrics and gynecology. 2014;123(2 Pt 1):225–231. doi: 10.1097/AOG.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenthal RJ, Rosnow LJ. Contrasts and effect sizes in behavioral research: A correlational approach. Cambridge University Press; Boston, MA, USA: 2003. [Google Scholar]
- 68.Sadownik LA. Etiology, diagnosis, and clinical management of vulvodynia. International journal of women's health. 2014;6:437–449. doi: 10.2147/IJWH.S37660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schrum A, Wolff S, van der Horst C, Kuhtz-Buschbeck JP. Motor cortical representation of the pelvic floor muscles. J Urol. 2011;186(1):185–190. doi: 10.1016/j.juro.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140(3):411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 71.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smart OC, MacLean AB. Vulvodynia. Current opinion in obstetrics & gynecology. 2003;15(6):497–500. doi: 10.1097/00001703-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. P Natl Acad Sci USA. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assessment. 1995;7(4):524–532. [Google Scholar]
- 76.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. The British journal of dermatology. 2003;148(5):1021–1027. doi: 10.1046/j.1365-2133.2003.05308.x. [DOI] [PubMed] [Google Scholar]
- 78.Visintin E, De Panfilis C, Antonucci C, Capecci C, Marchesi C, Sambataro F. Parsing The Intrinsic Networks Underlying Attention: A Resting State Study. Behavioural brain research. 2014 doi: 10.1016/j.bbr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Wiech K, Jbabdi S, Lin CS, Andersson J, Tracey I. Differential structural and resting state connectivity between insular subdivisions and other pain-related brain regions. Pain. 2014 doi: 10.1016/j.pain.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xue T, Yuan K, Zhao L, Yu DH, Zhao LM, Dong T, Cheng P, von Deneen KM, Qin W, Tian J. Intrinsic Brain Network Abnormalities in Migraines without Aura Revealed in Resting-State fMRI. Plos One. 2012;7(12) doi: 10.1371/journal.pone.0052927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 83.Zolnoun D, Bair E, Essick G, Gracely R, Goyal V, Maixner W. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. The journal of pain : official journal of the American Pain Society. 2012;13(9):910–920. doi: 10.1016/j.jpain.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


