…the residual risk of renal disease progression in patients with diabetic nephropathy is still extremely high despite current optimal treatment; innate immunity, MCP-1 and macrophage tissue infiltration seem very promising targets for future treatment of diabetic nephropathy on top of the standard of care with RAAS inhibition.
Keywords: biomarkers, diabetic nephropathy, hepcidin, interstitial fibrosis, monocyte chemoattractant protein-1
Abstract
Background
Urinary monocyte chemoattractant protein-1 (MCP-1) and hepcidin are potential biomarkers of renal inflammation. We examined their association with development of diabetic nephropathy (DN) lesions in normotensive normoalbuminuric subjects with type 1 diabetes (T1D) from the Renin-Angiotensin System Study.
Methods
Biomarker concentrations were measured in baseline urine samples from 224 subjects who underwent kidney biopsies at baseline and after 5 years. Fifty-eight urine samples below the limit of quantitation (LOQ, 28.8 pg/mL) of the MCP-1 assay were assigned concentrations of LOQ/√2 for analysis. Relationships between ln(MCP-1/Cr) or ln(hepcidin/Cr) and morphometric variables were assessed by sex using multiple linear regression after adjustment for age, T1D duration, HbA1c, mean arterial pressure, albumin excretion rate (AER) and glomerular filtration rate (GFR). In models that examined changes in morphometric variables, the baseline morphometric value was also included.
Results
Baseline mean age was 24.6 years, mean duration of T1D 11.2 years, median AER 6.4 µg/min and mean iohexol GFR 129 mL/min/1.73 m2. No associations were found between hepcidin/Cr and morphometric variables. Higher MCP-1/Cr was associated with higher interstitial fractional volume at baseline and after 5 years in women (baseline partial r = 0.244, P = 0.024; 5-year partial r = 0.299, P = 0.005), but not in men (baseline partial r = −0.049, P = 0.678; 5-year partial r = 0.026, P = 0.830). MCP-1 was not associated with glomerular lesions in either sex.
Conclusions
Elevated urinary MCP-1 concentration measured before clinical findings of DN in women with T1D was associated with changes in kidney interstitial volume, suggesting that inflammatory processes may be involved in the pathogenesis of early interstitial changes in DN.
INTRODUCTION
Diabetes is the leading cause of chronic kidney disease and end-stage renal disease (ESRD) in the United States [1]. Early identification of persons with diabetes who are at increased risk for diabetic nephropathy (DN) may reduce this disease burden. Currently, increased urinary albumin excretion is considered the best indicator of future loss of kidney function attributable to diabetes, but elevated levels of urinary albumin typically appear only after significant structural changes have already occurred in the kidneys or not at all even in relatively advanced disease [2, 3]. Thus, biomarkers that provide additional prognostic information beyond that provided by albuminuria are needed. Such biomarkers may also provide insights into disease mechanisms of DN.
Kidney biopsy tissue studied by quantitative morphometric methods predict clinical outcomes, even in early DN. Glomerular basement membrane (GBM) width in normoalbuminuric persons with type 1 diabetes (T1D), for example, forecasts development of microalbuminuria, proteinuria, ESRD and cardiovascular death [4–6]. Moreover, glomerular structural parameters correlate highly with renal functional changes throughout much of the natural history of DN, and renal interstitial changes are responsible for the loss of kidney function in the later stages of the disease [7–9]. These observations suggest that biomarkers associated with changes in kidney structure may improve risk prediction for the loss of kidney function associated with DN.
Several lines of evidence implicate inflammatory processes in the early pathogenesis of DN [10–12]. Molecules participating in inflammation may therefore be good candidates to study as potential biomarkers of DN. Two such molecules are hepcidin and monocyte chemoattractant protein-1 (MCP-1). Urinary hepcidin concentration correlates with interstitial inflammation in lupus nephritis [13], but the relationship between hepcidin and DN has not been previously examined. On the other hand, elevated mRNA levels for MCP-1 in the renal cortex of 5/6 nephrectomized rats correlate strongly with progression of kidney injury [14], and MCP-1 is differentially expressed in association with advanced tubulointerstitial lesions in patients with type 2 diabetes (T2D) and diabetic kidney disease [15]. It is one of several urinary cytokines that predict early decline in glomerular filtration rate (GFR) in patients with T1D and microalbuminuria [16, 17]. Higher urinary MCP-1 concentrations are also associated with increased risk of renal function decline, doubling of serum creatinine concentration and progression to dialysis or death at more advanced stages of DN [18, 19]. Neither urinary hepcidin nor MCP-1 has been investigated as a potential biomarker of early DN, before the onset of microalbuminuria.
In the Renin-Angiotensin System Study (RASS) [20], two research kidney biopsies were performed 5 years apart in a cohort of normotensive T1D subjects who had normoalbuminuria at enrollment. In the present study, we examined the association between normalized concentrations of hepcidin and MCP-1 measured in urine collected at enrollment and changes in renal structural variables over the 5 years of RASS. The choice of markers was made after considering amount and availability of stored specimens, availability and reliability of the assays and novelty and biological plausibility of the markers for diabetic kidney disease.
MATERIALS AND METHODS
The RASS was a multicenter clinical trial that tested whether treatment with either the angiotensin-converting enzyme inhibitor enalapril or the angiotensin receptor blocker losartan slowed progression of DN lesions over 5 years, relative to placebo, in T1D normotensive, normoalbuminuric patients with normal or increased GFR at baseline [20]. The trial demonstrated progression of structural lesions over 5 years, but found that early blockade of the renin-angiotensin system provided no benefit on progression of any lesions, compared with placebo, although it did slow the progression of retinopathy. Of the 285 subjects aged 16–65 years with T1D for 2–20 years who were enrolled in RASS, 254 underwent kidney biopsies at baseline and again after 5 years of follow-up. Among the 254 persons with paired kidney biopsies, 224 had baseline urine samples available for measurement of hepcidin and MCP-1 and are reported here. Height, weight, blood pressure, HbA1c, albumin excretion rate (AER) from timed overnight urine collections, and GFR by the plasma disappearance of iohexol (iGFR) were measured [21].
Morphometric measurements
Masked unbiased random sampling morphometric methods were used to measure DN renal structural parameters [22, 23]. Electron microscopic (EM) point counting was used to measure the fraction of the glomerular volume occupied by the mesangium [Vv(Mes/glom)]. Filtration surface estimated as peripheral glomerular basement membrane surface density [Sv(PGBM/glom)] was measured by an EM line intercept method, and GBM width was measured by the EM orthogonal intercept method. The fraction of renal cortical volume occupied by interstitium [Vv(Int/cortex)] was estimated by point counting on periodic Schiff-stained light microscopic (LM) slides of Zenker's fixed material. Measurement of Vv(Int/cortex) required substantial amounts of tissue for LM, since valid estimates required at least 65 coarse grid points to fall on renal cortex [24]. Of the 224 subjects with dual kidney biopsies and baseline measurement of hepcidin and MCP-1, 172 had sufficient tissue for measurement of Vv(Int/cortex) at both the baseline and 5-year biopsies.
Measurement of urinary hepcidin, MCP-1 and creatinine
Baseline spot urine specimens stored at −80°C were assayed for hepcidin with the human hepcidin-25 competitive ELISA Kit (Bachem) [13] and for MCP-1 with the human MCP-1 Quantikine ELISA Kit (Research & Diagnostic Systems) [25]. MCP-1 concentrations in 58 urine samples (25.9%) were below the limit of quantitation (LOQ, 28.8 pg/mL) of the assay. For analysis, these samples were assigned concentrations equal to the LOQ/√2 [26]. Hepcidin concentrations were all within the measurement range of the assay. Hepcidin and MCP-1 concentrations were divided by urinary creatinine concentration and expressed as hepcidin/creatinine (Cr) in µg/g and MCP-1/Cr in ng/g. A natural logarithmic transformation was used to achieve approximate normality. Urinary creatinine was measured with a modified Jaffé method [27].
Statistical analyses
Clinical features at baseline were described using numbers and percents for categorical variables, means and standard deviations (SDs) for normally distributed variables, and medians and inter-quartile ranges (IQRs) for those not normally distributed. Categorical variables were compared using chi-square tests, means using independent two-sample t-tests and medians using the Wilcoxon two-sample test. Paired differences in morphometric variables over 5 years were assessed by the Wilcoxon signed rank-sum test. Spearman's correlations between baseline urinary hepcidin/Cr, MCP-1/Cr, functional parameters, morphometric variables and changes in morphometric variables were calculated along with their P-values. Correlations between hepcidin/Cr and MCP-1/Cr were partialled for urine creatinine concentration. Changes in morphometric variables were computed as the logarithm of the 5-year structural measure minus the logarithm of the baseline measure (equal to the logarithm of the ratio of 5-year biopsy measurement to the baseline measurement). Changes in iGFR were computed as the 5-year measure minus the baseline measure. Individual iGFR slopes were also computed as time-averaged rates of change by simple linear regression on all measurements obtained during the study and were expressed as the annual change in mL/min/1.73 m2. Cross-sectional relationships between baseline hepcidin/Cr or MCP-1/Cr and baseline morphometric variables were further assessed after adjustment for age, sex, duration of T1D, HbA1c, mean arterial pressure (MAP), ln(AER) and iGFR by multiple linear regression. For longitudinal relationships with baseline hepcidin/Cr and MCP-1/Cr, the baseline value of the morphometric variable was included in the linear regression model. The effect of MCP-1/Cr on the ratio (5-year/baseline) of Vv(Int/cortex) was assessed visually using a correlation plot adjusted for the covariates described above. Partial correlations were reported for the association of relevant biomarkers with morphometric variables after adjustment for covariates.
Three sensitivity analyses were performed. The first analysis replaced the change in each morphometric variable with the 5-year measurement value alone. The second added treatment assignment from the clinical trial to the linear regression model. Because 58 urine samples had MCP-1 values below the LOQ of the assay, and MCP-1 values for these samples were assigned arbitrarily for the linear regression analyses, we performed a third analysis in which we compared this analytical approach with a standard Tobit censored-regression model fit using the LIFEREG procedure in SAS [28]. In this model, the logarithm of the MCP-1 values was assumed to have a normal distribution, and values below the LOQ were truncated and assigned expected values integrated over all possibilities at or below the LOQ. The Tobit model gave equivalent results to the linear regression model, and for ease of description and interpretation only results of the analysis in which MCP-1 concentrations below the LOQ were assigned the arbitrary value of LOQ/√2 are presented.
RESULTS
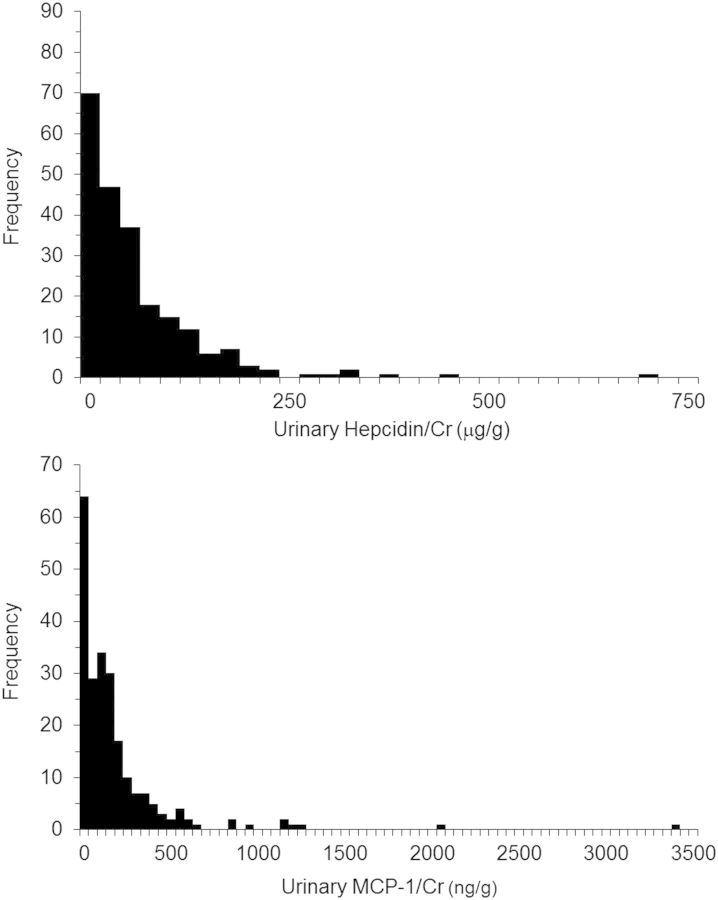
Clinical and demographic characteristics of the 224 subjects included in the present study are shown in Table 1. Characteristics of the 172 subjects in whom sufficient LM tissue was available for measurement of Vv(Int/cortex) at both kidney biopsies were virtually identical with those in the 52 subjects without adequate tissue for this measurement. Distributions of urinary hepcidin/Cr and MCP-1/Cr were highly skewed (Figure 1). Median hepcidin/Cr was higher in men (54.7 µg/g in men versus 40.7 µg/g in women, P = 0.008), whereas median MCP-1/Cr was higher in women (136.3 ng/g in women versus 108.3 ng/g in men, P = 0.018). Urinary hepcidin/Cr and MCP-1/Cr were both numerically higher in the subset of individuals in whom Vv(Int/cortex) was not measured, but the difference was statistically significant only for hepcidin/Cr (Table 1). Median urinary hepcidin/Cr and MCP-1/Cr were higher in subjects assigned to receive blockade of the renin-angiotensin system, but the differences were only marginally significant for hepcidin/Cr (51.8 µg/g versus 38.4 µg/g for hepcidin/Cr, P = 0.049; 139.2 ng/g versus 107.8 ng/g for MCP-1/Cr, P = 0.230).
Table 1.
Clinical characteristics at baseline of the 224 T1D subjects in RASS with two kidney biopsies and measurements of urinary hepcidin/Cr and MCP-1/Cr and the subset of 172 subjects in whom sufficient kidney tissue was available for measurement of Vv(Int/cortex) at both biopsies
| Entire cohort (N = 224) | Subset with ΔVv(Int/cortex) measurements (N = 172) | Subset without ΔVv(Int/cortex) measurements (N = 52) | P-value | |
|---|---|---|---|---|
| RASS treatment assignment (placebo, enalapril, losartan) | 72, 76, 76 | 59, 59, 54 | 13, 17, 22 | 0.208 |
| Age (years) | 24.6 ± 9.7 | 24.8 ± 9.7 | 24.1 ± 10.0 | 0.638 |
| Number male (%) | 103 (46.0%) | 80 (46.5%) | 23 (44.2%) | 0.772 |
| Number Caucasian (%) | 219 (97.8%) | 167 (97.1%) | 52 (100%) | 0.213 |
| Diabetes duration (years) | 11.2 ± 4.7 | 11.3 ± 4.7 | 10.8 ± 4.9 | 0.565 |
| HbA1c (%) | 8.5 ± 1.5 | 8.4 ± 1.4 | 8.8 ± 1.8 | 0.230 |
| Systolic BP (mm Hg) | 119 ± 12 | 119 ± 12 | 120 ± 12 | 0.901 |
| Diastolic BP (mm Hg) | 70 ± 8 | 70 ± 9 | 69 ± 7 | 0.271 |
| AER (µg/min)a | 5.0 (3.2–7.6) | 5.1 (3.3–7.5) | 4.8 (3.1–7.7) | 0.821 |
| iGFR (mL/min/1.73 m2) | 129 ± 19 | 129 ± 20 | 130 ± 17 | 0.628 |
| Hepcidin/Cr (µg/g)a | 46.4 (20.3–89.8) | 42.0 (18.1–81.9) | 63.2 (33.6–129.0) | 0.013 |
| MCP-1/Cr (ng/g)a | 127.4 (52.3–229.0) | 122.0 (54.5–225.6) | 152.0 (79.4–254.8) | 0.178 |
Data are numbers, numbers and percents, means and SDs, or medians and IQRs. P-values reflect differences in clinical characteristics between subjects with Vv(Int/cortex) measurements and those without.
AER, albumin excretion rate; BP, blood pressure; HbA1c, glycosylated hemoglobin; iGFR, iohexol glomerular filtration rate; MCP-1/Cr, urinary monocyte chemoattractant protein-1/creatinine ratio; RASS, Renin-Angiotensin System Study; Vv(Int/cortex), interstitial cortical fractional volume.
aMedian and IQR.
FIGURE 1:
Frequency distribution of urinary hepcidin/Cr (upper panel) and MCP-1/Cr (lower panel). Fifty-eight of the 224 specimens were below the level of detection of the urinary MCP-1 assay and they were included in the first column.
The iGFR averaged 129 mL/min/1.73 m2 at baseline and declined on average by 7.8 mL/min/1.73 m2 at the end of follow-up. The iGFR slope declined, on average, by 1.6 mL/min/1.73 m2 per year. Only two participants had their iGFR decline to <60 mL/min/1.73 m2 by the end of the study, losing more than half of their GFR during follow-up. Although baseline Vv(Int/cortex) correlated negatively with change in iGFR (r = −0.179, P = 0.013), none of the baseline morphometric measures nor their change over 5 years was associated with either the change in iGFR or its slope during follow-up, after adjustment for baseline age, sex, T1D duration, HbA1c, MAP, ln(AER) and iGFR.
Baseline and 5-year follow-up morphometric characteristics of the 224 subjects are shown in Table 2. No statistically significant increase in GBM width was observed during follow-up. Significant increases of about 10% were found for Vv(Mes/glom) and significant decreases of about 15% were found for Sv(PGBM/glom). Vv(Int/cortex) increased by >50% over baseline in the 172 subjects in whom it was measured. Baseline GBM width was higher in men than women (486 ± 88 nm in men versus 463 ± 95 nm in women, P = 0.047). Percent changes in each morphometric variable over 5 years were equivalent in men and women.
Table 2.
Renal structural characteristics of 224 subjects with T1D who had urinary hepcidin/Cr and MCP-1/Cr measured at baseline
| Baseline | 5 Years | P-value | |
|---|---|---|---|
| Vv(Mes/glom) (%) | 19 ± 5 | 21 ± 5 | 0.001 |
| GBM width (nm) | 474 ± 93 | 484 ± 94 | 0.075 |
| Sv(PGBM/glom) (µ2 µ3) | 13 ± 2 | 11 ± 2 | <0.001 |
| Vv(Int/cortex) (%)a | 11 ± 4 | 17 ± 5 | <0.001 |
Data are means and SDs.
GBM, glomerular basement membrane; MCP-1/Cr, urinary monocyte chemoattractant protein-1/creatinine ratio; Sv(PGBM/glom), surface density of the peripheral glomerular basement membrane; Vv(Int/cortex), interstitial cortical fractional volume; Vv(Mes/glom), mesangial fractional volume per glomerulus.
aN = 172.
Urinary hepcidin/Cr correlated negatively with baseline Vv(Mes/glom) (r = −0.164, P = 0.014) and urinary MCP-1/Cr correlated positively with baseline Vv(Int/cortex) (r = 0.149, P = 0.039). Spearman's correlations between hepcidin/Cr and MCP-1/Cr, baseline functional parameters and changes in structural parameters are shown in Table 3. Urinary hepcidin/Cr correlated positively with MCP-1/Cr and negatively with HbA1c. MCP-1/Cr correlated positively with AER, and neither marker correlated with changes in morphometric variables. HbA1c correlated with changes in all morphometric variables. Baseline GFR correlated negatively with change in iGFR or its slope, and the relationship was stronger in the women (r = −0.408, P < 0.001 for ΔiGFR; r = −0.325, P < 0.001 for iGFR slope) than in the men (r = −0.300, P = 0.002 for ΔiGFR; r = −0.193, P = 0.050 for iGFR slope).
Table 3.
Spearman's correlations (P-values) between hepcidin/Cr and MCP-1/Cr, functional parameters and changes in morphometric variables in 224 subjects with T1D mellitus.
| Hepcidin/Cr | MCP-1/Cr | iGFR | AER | HbA1c | ΔiGFR | iGFR slope | ΔVv(Mes/glom) | ΔGBM width | ΔSv(PGBM/glom) | ΔVv(Int/cortex)a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepcidin/Cr | 1.0 |
0.372b (<0.001) |
0.125 (0.062) |
0.116 (0.085) |
−0.135 (0.043) |
0.023 (0.733) |
0.104 (0.123) |
0.089 (0.186) |
0.001 (0.989) |
−0.066 (0.326) |
0.070 (0.363) |
| MCP-1/Cr | 1.0 | 0.056(0.401) | 0.191(0.004) | 0.026(0.697) | 0.039 (0.562) |
0.090 (0.178) |
0.048 (0.471) | 0.066 (0.327) | −0.125 (0.061) | −0.016 (0.830) | |
| iGFR | 1.0 | 0.186 (0.005) | 0.135 (0.044) | −0.336 (<0.001) |
−0.222 (<0.001) |
−0.002 (0.980) | 0.152 (0.023) | −0.082 (0.222) | −0.004 (0.962) | ||
| AER | 1.0 | 0.196 (0.003) | −0.054 (0.422) |
0.018 (0.785) |
0.054 (0.424) | 0.072 (0.283) | −0.229 (0.001) | −0.102 (0.184) | |||
| HbA1c | 1.0 | −0.123 (0.068) |
−0.112 (0.097) |
0.153 (0.022) | 0.197 (0.003) | −0.213 (0.001) | 0.175 (0.022) | ||||
| ΔGFR | 1.0 |
0.688 (<0.001) |
0.018 (0.785) |
0.044 (0.511) |
−0.041 (0.540) |
0.110 (0.153) |
|||||
| GFR slope | 1.0 | 0.024 (0.721) |
0.088 (0.190) |
−0.084 (0.210) |
0.047 (0.543) |
||||||
| ΔVv(Mes/glom) | 1.0 | 0.223 (0.001) | −0.467 (<0.001) | −0.160 (0.036) | |||||||
| ΔGBM width | 1.0 | −0.264 (<0.001) | 0.047 (0.539) | ||||||||
| ΔSv(PGBM/glom) | 1.0 | −0.092 (0.229) | |||||||||
| ΔVv(Int/cortex)a | 1.0 |
P-values less than 0.05 are shown in bold.
AER, albumin excretion rate; HbA1c, glycosylated hemoglobin; GBM, glomerular basement membrane; iGFR, iohexol glomerular filtration rate; MCP-1/Cr, urinary monocyte chemoattractant protein-1/creatinine ratio; Sv(PGBM/glom), surface density of the peripheral glomerular basement membrane; Vv(Int/cortex), interstitial cortical fractional volume; Vv(Mes/glom), mesangial fractional volume per glomerulus.
aN = 172.
bPartialled for urine creatinine concentration. The correlation not partialled for urine creatinine concentration is r = 0.373, P < 0.001.
After adjustment for baseline age, sex, T1D duration, HbA1c, MAP, ln(AER) and iGFR, baseline hepcidin/Cr was not significantly associated with any baseline morphometric variables, whereas MCP-1/Cr remained associated with baseline Vv(Int/cortex) (P = 0.040). Because of a statistically significant interaction between MCP-1/Cr and sex (P = 0.039), we examined the relationship between MCP-1/Cr and baseline Vv(Int/cortex) separately in men and women and found the association was stronger in women (partial r = 0.244, P = 0.024) than in men (partial r = −0.049, P = 0.678).
After additional adjustment for baseline Vv(Int/cortex), MCP-1/Cr was significantly associated with ‘change’ in Vv(Int/cortex) over 5 years, assessed by the 5 year/baseline Vv(Int/cortex) ratio (P = 0.034, Table 4). The longitudinal relationship was also stronger in women than in men, despite the absence of a statistically significant interaction between MCP-1/Cr and sex (P = 0.141) in this model. In separate models for men and women, baseline MCP-1/Cr was strongly associated with the Vv(Int/cortex) ratio in women (partial r = 0.299, P = 0.005) but not in men (partial r = 0.026, P = 0.830) after adjustment for age, T1D duration, HbA1c, MAP, ln(AER) and iGFR(Figure 2). Neither hepcidin/Cr nor MCP-1/Cr was associated with the 5-year/baseline ratio for any of the other structural variables (Table 4). Similar findings were observed when the 5-year/baseline ratio for each morphometric variable was replaced by the 5-year measurement value alone and when treatment assignment in the RASS subjects was added to the multivariate linear regression models (data not shown).
Table 4.
Parameter estimates from univariate and multivariate regression models for the association between baseline urinary hepcidin/Cr and MCP-1/Cr and the standardized log(5-year)-log baseline morphometric variable
| Variable | GBM width |
Vv(Mes/glom) |
Sv(PGBM/glom) |
Vv(Int/cortex) |
||||
|---|---|---|---|---|---|---|---|---|
| Δ | P-value | Δ | P-value | Δ | P-value | Δ | P-value | |
| Univariate Log (Hepcidin/Cr) | ||||||||
| Low tertile | Reference | Reference | Reference | Reference | ||||
| Middle tertile | 0.001 | 0.956 | −0.01 | 0.890 | −0.03 | 0.479 | 0.01 | 0.891 |
| High tertile | 0.001 | 0.981 | 0.04 | 0.293 | −0.03 | 0.327 | 0.08 | 0.248 |
| Log hepcidin/Cr (per SD) | 0.03 | 0.675 | 0.07 | 0.302 | −0.06 | 0.383 | −0.02 | 0.750 |
| Univariate Log (MCP-1/Cr) | ||||||||
| Low tertile | Reference | Reference | Reference | Reference | ||||
| Middle tertile | −0.01 | 0.767 | −0.05 | 0.252 | −0.03 | 0.394 | −0.13 | 0.072 |
| High tertile | 0.02 | 0.438 | 0.03 | 0.434 | −0.07 | 0.066 | −0.04 | 0.618 |
| Log MCP-1/Cr (per SD) | 0.14 | 0.041 | 0.08 | 0.264 | −0.11 | 0.094 | −0.03 | 0.735 |
| Multivariate Log(Hepcidin/Cr)a | ||||||||
| Low tertile | Reference | Reference | Reference | Reference | ||||
| Middle tertile | −0.0003 | 0.986 | −0.01 | 0.708 | −0.02 | 0.446 | −0.04 | 0.452 |
| High tertile | −0.01 | 0.550 | 0.003 | 0.919 | −0.01 | 0.834 | 0.052 | 0.350 |
| Log hepcidin/Cr (per SD) | −0.03 | 0.632 | −0.01 | 0.863 | 0.03 | 0.515 | −0.01 | 0.839 |
| Multivariate Log(MCP-1/Cr)a | ||||||||
| Low tertile | Reference | Reference | Reference | Reference | ||||
| Middle tertile | 0.001 | 0.963 | −0.04 | 0.202 | −0.003 | 0.903 | −0.07 | 0.185 |
| High tertile | 0.02 | 0.433 | 0.02 | 0.519 | −0.03 | 0.331 | 0.12 | 0.029 |
| Log MCP-1/Cr (per SD) | 0.09 | 0.095 | 0.05 | 0.362 | −0.04 | 0.471 | 0.12 | 0.034 |
P-values less than 0.05 are shown in bold.
AER, albumin excretion rate; GBM, glomerular basement membrane; HbA1c, glycosylated hemoglobin; iGFR, iohexol glomerular filtration rate; MAP, mean arterial pressure; MCP-1/Cr, urinary monocyte chemoattractant protein-1/creatinine ratio; Sv(PGBM/glom), surface density of the peripheral glomerular basement membrane; Vv(Int/cortex), interstitial cortical fractional volume; Vv(Mes/glom), mesangial fractional volume per glomerulus.
aAdjusted for age, sex, duration of diabetes, HbA1c, MAP, iGFR, ln(AER) and baseline structure.
FIGURE 2:
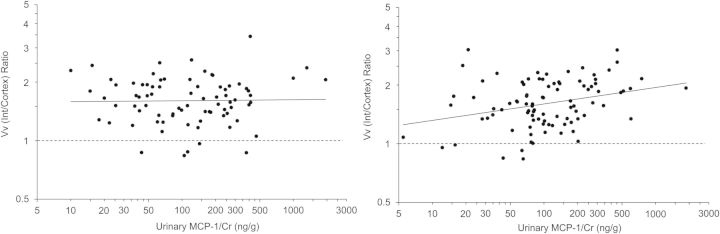
Correlation plots of the relationship between normalized urinary MCP-1 concentration and the ratio (5-year/baseline) of fractional cortical interstitial volume in the 79 men (left panel) and the 92 women (right panel) with data for all variables at baseline and 5 years (one man was missing HbA1c at baseline). Both the ratio of Vv(Int/cortex) and MCP-1/Cr were adjusted for baseline age, duration of diabetes, HbA1c, mean arterial pressure, ln(AER), iGFR,and Vv(Int/cortex) and plotted on logarithmic scales. The solid lines are the regression lines (partial r = 0.026, P = 0.830 in the men; partial r = 0.299, P = 0.005 in the women). Values above the dashed lines reflect an increase in Vv(Int/cortex) and those below the lines a decrease over 5 years.
DISCUSSION
We present the first biopsy study in patients with no clinical evidence of DN, in whom there is an association, albeit a modest one, of an inflammatory marker with the development of an early DN lesion before the onset of any significant decline in renal function. This association was seen only for the interstitial lesion Vv(Int/cortex) and not for glomerular lesions, and it was found with MCP-1/Cr and not with hepcidin/Cr. It is possible that development and progression of early glomerular DN lesions are unrelated to urinary MCP-1 levels. However, glomerular lesions, including GBM width, Vv(Mes/glom) and Sv(PGBM/glom), changed by less than 15% in paired kidney biopsies separated by 5 years in RASS, whereas Vv(Int/cortex) increased by more than 50% during the same period. Thus, the statistical power of RASS to detect the influence of urinary MCP-1 on progression of early DN lesions may have been substantially greater for the interstitial than for the glomerular changes. Higher baseline levels of urinary MCP-1 in the females in this study may have contributed to the ability to detect an association with Vv(Int/cortex). Alternatively, there may be sex differences in the pathophysiologic mechanisms of diabetic renal injury, since MCP-1 was associated with interstitial expansion in women but not in men. In the Cohen diabetic rat, ovariectomy resulted in decreased kidney injury, whereas estradiol treatment of the ovariectomized diabetic animals removed this protection [29]. On the other hand, castrated male streptozotocin diabetic Wistar–Kyoto rats had greater increases in mesangial volume than intact diabetic animals [30]. Interestingly, the male excess of T1D kidney disease observed in an earlier birth cohort from the Pittsburgh Epidemiology of Diabetes Complications Study was not found in a more recent cohort, emphasizing the potential complexity of sex as a risk variable in DN [31]. The lack of association between urinary hepcidin/Cr and morphometric changes in the present study suggests that elevation of urinary hepcidin in response to inflammation may occur later in the course of DN when inflammation, manifesting as renal invasion of inflammatory cells, is more pronounced. On the other hand, MCP-1/Cr may increase, at least in women, in response to lower levels of inflammatory processes in the absence of detectable increases in renal inflammatory cells.
The greater than 50% increase in Vv(Int/cortex) observed over 5 years in the RASS was nearly identical in all three treatment groups. In the present study, changes in the classical DN glomerular parameters of GBM width, Vv(Mes/glom), and Sv(PGBM/glom) and the interstitial parameter Vv(Int/cortex) correlated directly with the baseline values for these parameters and with HbA1c. The change in GBM width was also associated with age, sex and MAP. However, only Vv(Int/cortex) was associated with MCP-1/Cr, both cross-sectionally and longitudinally, after adjustment for traditional risk factors for DN.
The Natural History Study (NHS) [32] was an observational study that examined early renal structural changes in relatively young subjects with T1D averaging 8.0 years duration. Vv(Int/cortex) at baseline in the NHS was actually lower than the normal range (0.13 ± 0.04) [32]. Since Vv(Int/cortex) represents the fraction of cortex which is interstitium, this observation is probably related to diabetic renal enlargement, largely attributable to expansion of the tubular compartment. At the end of the 5-year follow-up in NHS, renal enlargement adjusted for body size had not increased further, but Vv(Int/cortex) increased into the normal range, suggesting early interstitial expansion. Consistent with this observation, in RASS, baseline Vv(Int/cortex) was in the normal range and increased by more than 50% during follow-up, so the mean values for Vv(Int/cortex) were clearly increased at the end of the study. We have shown previously that early increase in AER and decrease in GFR in T1D patients are primarily related to classical DN glomerular lesions [4–6]. However, tubulointerstitial lesions are also critically important in the progression of DN from moderately reduced GFR to ESRD [4, 7–9, 20, 32]. Therefore, a better understanding of the pathogenesis of early interstitial expansion in diabetes may provide better tools for identifying persons at greater risk of early DN progression and novel treatment targets that could substantially influence the course of this disease.
The present study adds to the growing evidence that inflammatory processes are active in the pathogenesis of early DN. MCP-1 is a monomeric polypeptide of the chemokine family secreted by mononuclear leukocytes and by cortical tubular epithelial cells and podocytes under stimulation by nuclear factor-κB (NF-κB) [15, 33]. Its receptors are expressed on monocyte and macrophage cell surfaces. MCP-1 is linked to a pathway that may be important in DN pathogenesis [34]. In patients with more advanced DN, urinary MCP-1/Cr was a stronger correlate of estimated GFR decline than urinary protein excretion in patients with T1D and T2D [35] and was related to the severity of tubulointerstitial injury and inflammation [15]. Activated NF-κB expression in kidney biopsies correlated strongly with proteinuria and with kidney biopsy staining for MCP-1 and RANTES/CCL5 (regulated upon activation, normal T cell expressed and secreted) in a small cohort of T2D patients with reduced GFR and overt proteinuria [36]. Wada et al. reported increased urinary MCP-1 in DN patients with T2D, primarily among those with nephrotic range proteinuria [15]. Urinary MCP-1 levels correlated with interstitial fibrosis and tubular atrophy and were higher in patients with more severe diabetic glomerulopathy and vasculopathy and greater interstitial inflammation.
One quarter of the urine samples in the present study had MCP-1 concentrations below the LOQ of the assay. Nonetheless, two different analytical approaches to address the data below the LOQ yielded the same conclusions. Also, because we examined associations between the biomarkers and change in kidney structure at a very early stage of DN, when renal function is relatively stable, we were unable to determine if the biomarkers studied here predict GFR decline, the critical functional change that ultimately leads to ESRD. Much longer follow-up than was available in this cohort would be required to examine this relationship. However, early changes in renal structure that may ultimately drive important clinical end points deserve further investigation. Strengths of the study include its longitudinal design, detailed characterization of the study cohort, use of paired kidney biopsies to provide unbiased morphometric measures of early DN structural rather than functional end points, and storage of the urine samples at −80°C from collection until measurement of hepcidin and MCP-1. Unpublished data from the laboratory in which these analytes were measured indicate that they are stable over multiple freeze-thaw cycles and that MCP-1 is stable over at least 2 years of storage; the effect of long-term storage on hepcidin concentration has not been evaluated.
In summary, elevated urinary MCP-1 concentrations before the onset of any clinical evidence of DN in women but not men with T1D is associated with early cortical interstitial expansion, a component of diabetic kidney injury, which later in the disease process is an important determinant of progression to ESRD. Thus, inflammatory processes may be involved in the pathogenesis of early interstitial DN changes and this relationship may be stronger in women than in men.
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or part, except in abstract form. Parts of this study were presented in abstract form at the American Society of Nephrology annual meeting and scientific exposition in Atlanta, Georgia, 7–10 November 2013. No conflicts of interest declared.
ACKNOWLEDGEMENTS
This work was supported by the Chronic Kidney Disease Biomarker Consortium funded by NIDDK U01DK85649, U01DK085673, U01DK085660, U01DK085688, U01DK085651 and U01DK085689, and by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The RASS Investigators include Bernard Zinman, MD and Sandra Donnelly, MDCM, MSc, University of Toronto, Toronto Canada; Robert Gardiner, MD, Samy Suissa, PhD, Keith Drummond, MD and Paul Goodyer, MD, McGill University, Montreal, Canada; Alan Sinaiko, MD and Trudy Strand, RN, University of Minnesota, Minneapolis, MN; Marie Claire Gubler, MD and Ronald Klein, MD, MPH, University of Wisconsin, Madison, WI.
REFERENCES
- 1.U.S. Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013 [Google Scholar]
- 2.Krolewski AS, Niewczas MA, Skupien J, et al. Early progressive renal decline precedes the onset of microalbuminuria and its progression to macroalbuminuria. Diabetes Care 2014; 37: 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes 2003; 52: 1036–1040 [DOI] [PubMed] [Google Scholar]
- 4.Steinke JM, Sinaiko AR, Kramer MS, et al. International Diabetic Nephropathy Study Group. The early natural history of nephropathy in type 1 diabetes. III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 2005; 54: 2164–2171 [DOI] [PubMed] [Google Scholar]
- 5.Perrin NE, Torbjörnsdotter T, Jaremko GA, et al. Risk markers of future microalbuminuria and hypertension based on clinical and morphological parameters in young type 1 diabetes patients. Pediatr Diabetes 2010; 11: 305–313 [DOI] [PubMed] [Google Scholar]
- 6.Caramori ML, Parks A, Mauer M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 2013; 24: 1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bader R, Bader H, Grund KE, et al. Structure and function of the kidney in diabetic glomerulosclerosis. Correlations between morphological and functional parameters. Pathol Res Pract 1980; 167: 204–216 [DOI] [PubMed] [Google Scholar]
- 8.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 1999; 56: 1627–1637 [DOI] [PubMed] [Google Scholar]
- 9.Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstital injury. Histol Histopathol 2002; 177: 632–643 [DOI] [PubMed] [Google Scholar]
- 10.Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes? Semin Nephrol 2012; 32: 452–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morii T, Fujita H, Narita T, et al. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications 2003; 17: 11–15 [DOI] [PubMed] [Google Scholar]
- 12.Lai KN, Leung JC, Chan LY, et al. Interaction between proximal tubular epithelial cells and infiltrating monncytes/T cells in the proteinuric state. Kidney Int 2007; 71: 526–538 [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Nagaraja HN, Nadasdy T, et al. A composite urine biomarker reflects interstitial inflammation in lupus nephritis kidney biopsies. Kidney Int 2012; 81: 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taal MW, Chertow GM, Rennke HG, et al. Mechanisms underlying renoprotection during renin-angiotensin system blockade. Am J Physiol Renal Physiol 2001; 280: F343–F355 [DOI] [PubMed] [Google Scholar]
- 15.Wada T, Furuichi K, Sakai N, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int 2000; 58: 1492–1499 [DOI] [PubMed] [Google Scholar]
- 16.Wolkow PP, Niewczas MA, Perkins B, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol 2008; 19: 789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gohda T, Niewczas MA, Ficociello LH, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol 2012; 23: 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Titan SM, Vieira JM, Jr, Dominguez WV, et al. Urinary MCP-1 and RBP: independent predictors of renal outcome in macroalbuminuric diabetic nephropathy. J Diabetes Complications 2012; 26: 546–553 [DOI] [PubMed] [Google Scholar]
- 19.Verhave JC, Bouchard J, Goupil R, et al. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract 2013; 101: 333–340 [DOI] [PubMed] [Google Scholar]
- 20.Mauer M, Zinman B, Gardiner R, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 2009; 361: 40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauer M, Zinman B, Gardiner R, et al. ACE-I and ARBs in early diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 2002; 3: 262–269 [DOI] [PubMed] [Google Scholar]
- 22.Caramori ML, Kim Y, Huang C, et al. Cellular basis of diabetic nephropathy. 1. Study design and renal structural functional relationships in patients with long-standing type 1 diabetes. Diabetes 2002; 51: 506–513 [DOI] [PubMed] [Google Scholar]
- 23.Klein R, Zinman B, Gardiner R, et al. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesionsin type 1 diabetic patients: the Renin Angiotensin System Study. Diabetes 2005; 54: 527–533 [DOI] [PubMed] [Google Scholar]
- 24.Katz A, Caramori ML, Sisson-Ross S, et al. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int 2002; 61: 2058–2066 [DOI] [PubMed] [Google Scholar]
- 25.Rovin BH, Song H, Birmingham DJ, et al. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol 2005; 16: 467–473 [DOI] [PubMed] [Google Scholar]
- 26.Glass DC, Gray CN. Estimating mean exposures from censored data: exposure to benzene in the Australian petroleum industry. Ann Occup Hyg 2001; 45: 275–282 [PubMed] [Google Scholar]
- 27.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol 1960; 30: 207–212 [PubMed] [Google Scholar]
- 28.Hughes MD. Analysis and design issues for studies using censored biomarker measurements with an example of viral load measurements in HIV clinical trials. Stat Med 2000; 19: 3171–3191 [DOI] [PubMed] [Google Scholar]
- 29.Rosenmann E, Yanko L, Cohen AM. Female sex hormone and nephropathy in Cohen diabetic rat (genetically selected sucrose-fed). Horm Metab Res 1984; 16: 11–16 [DOI] [PubMed] [Google Scholar]
- 30.Bach LA, Cooper ME, Vranes D, et al. Disparate effects of castration on renal structure and function in the streptozotocin diabetic rat. Diabetes Res 1994; 27: 27–38 [PubMed] [Google Scholar]
- 31.Costacou T, Fried L, Ellis D, et al. Sex differences in the development of kidney disease in individuals with type 1 diabetes mellitus: a contemporary analysis. Am J Kidney Dis 2011; 58: 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drummond K, Mauer M. The early natural history of nephropathy in type1 diabetes. II. Early renal structural changes in type 1 diabetes . Diabetes 2002; 51: 1580–1587 [DOI] [PubMed] [Google Scholar]
- 33.Tesch GH. MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol 2008; 294: F697–F701 [DOI] [PubMed] [Google Scholar]
- 34.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, et al. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 2011; 7: 327–340 [DOI] [PubMed] [Google Scholar]
- 35.Tam FW, Riser BL, Meeran K, et al. Urinary monocyte chemoattractant protein-1 (MCP-1) and connective tissue growth factor (CCN2) as prognostic markers for progression of diabetic nephropathy. Cytokine 2009; 47: 37–42 [DOI] [PubMed] [Google Scholar]
- 36.Mezzano S, Aros C, Droguett A, et al. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant 2004; 19: 2505–2512 [DOI] [PubMed] [Google Scholar]