Abstract
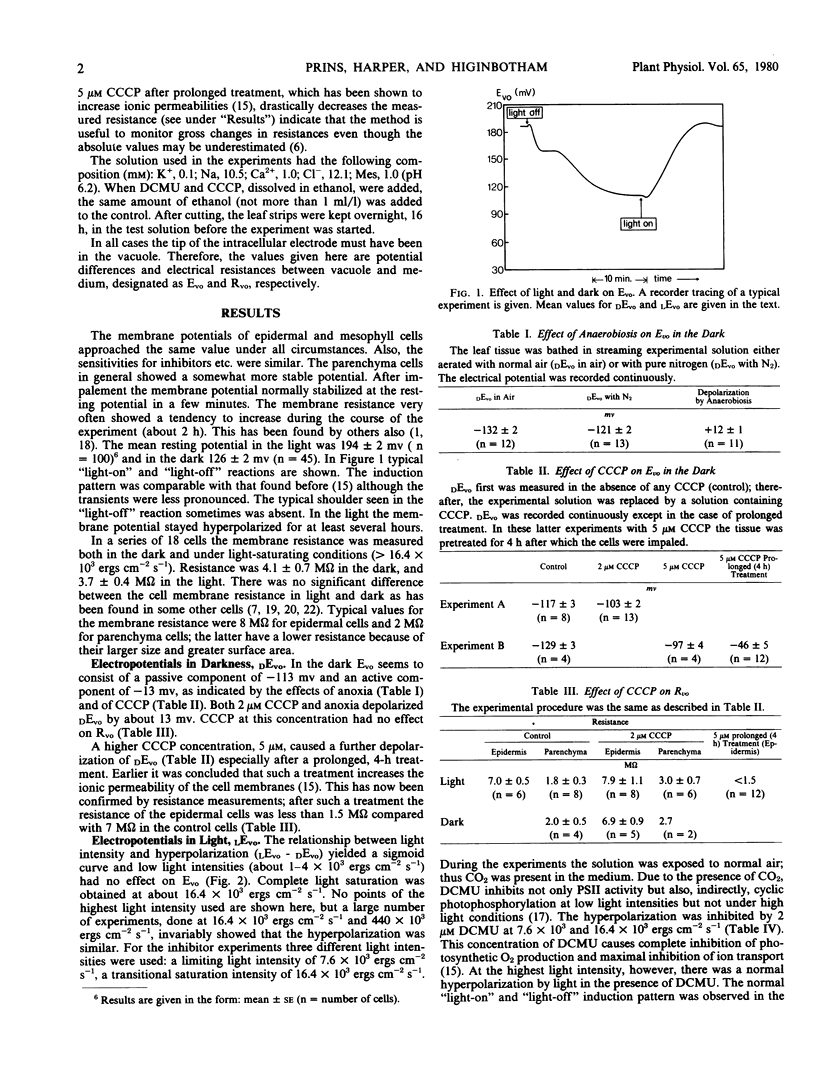
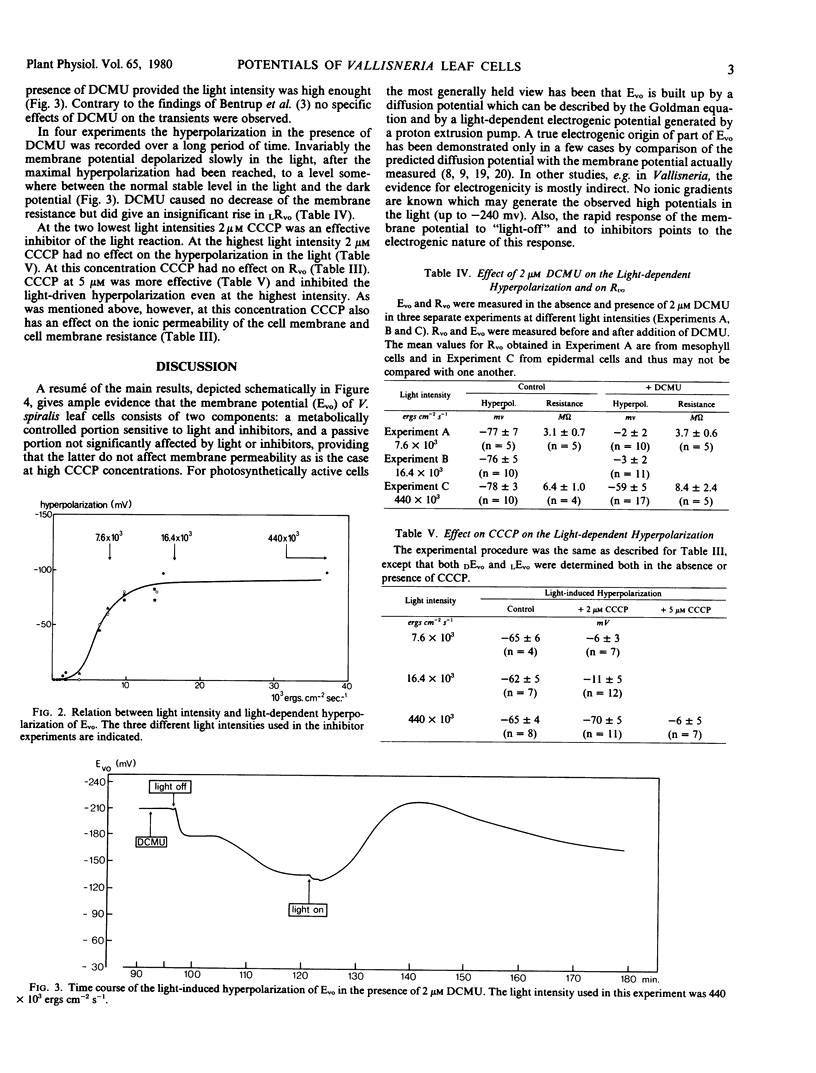
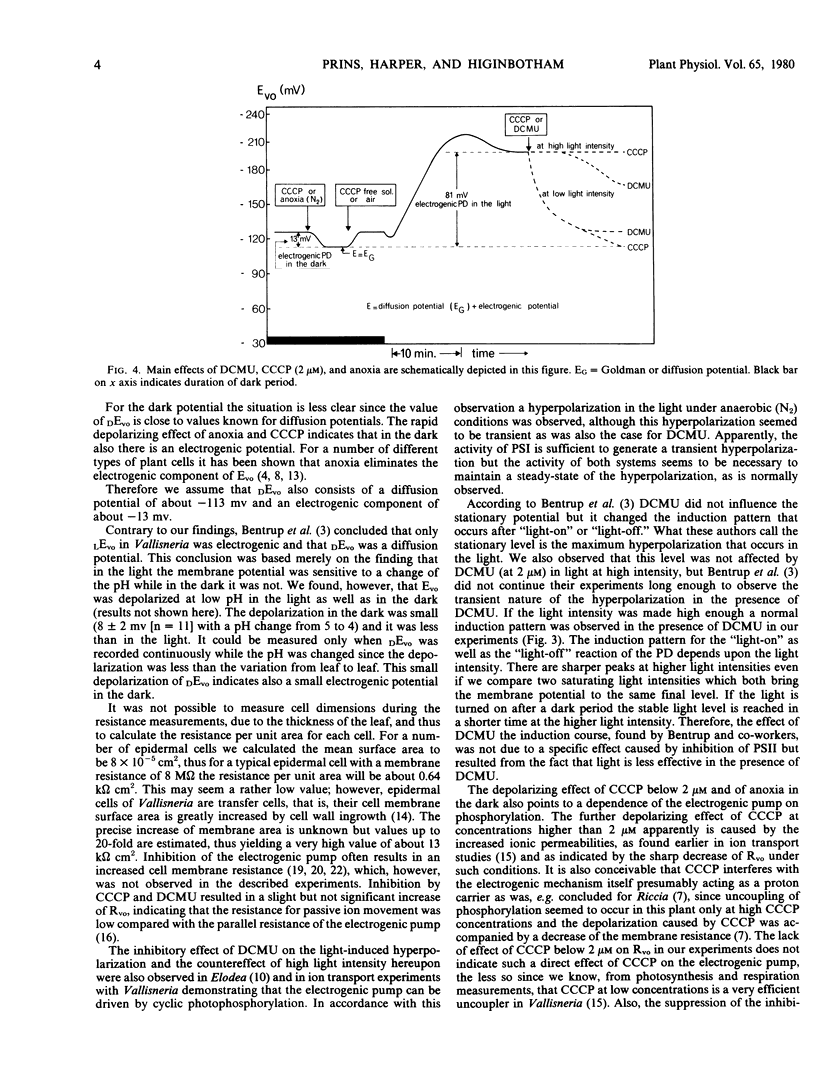
A study has been made of the effects of the inhibitors carbonylcyanide m-chlorophenylhydrazone (CCCP), 3-(3,4-dichlorophenyl)-1,1-dimethyl urea (DCMU), and of anoxia on the light-sensitive membrane potential of Vallisneria leaf cells. The present results are compared with the known effects of these inhibitors on ion transport and photosynthesis (Prins 1974 Ph.D thesis). The membrane potential is composed of a diffusion potential plus an electrogenic component. The electrogenic potential is about −13 millivolts in the dark and −80 millivolts in the light. The inhibitory effect of DCMU and CCCP on the electrogenic mechanisms strongly depends on the light intensity used, the inhibition being less at a higher light intensity. This is of significance in view of the often conflicting results obtained with these inhibitors. With ion transport in Vallisneria the electrogenic pump derives its energy from phosphorylation; however, the process which causes the initial light-induced hyperpolarization and the process that keeps the membrane potential at a steady hyperpolarized state in the light have different energy requirements. The action of photosystem I alone is sufficient to induce the initial hyperpolarization. For continuous operation in the light the activity of photosystem II also is needed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. P., Hendrix D. L., Higinbotham N. Higher plant cell membrane resistance by a single intracellular electrode method. Plant Physiol. 1974 Jan;53(1):122–124. doi: 10.1104/pp.53.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Shavit N. Inhibitors and uncouplers of photophosphorylation. Biochim Biophys Acta. 1965 Nov 29;109(2):317–331. doi: 10.1016/0926-6585(65)90160-3. [DOI] [PubMed] [Google Scholar]
- Contardi P. J., Davis R. F. Membrane Potential in Phaeoceros laevis: Effects of Anoxia, External Ions, Light, and Inhibitors. Plant Physiol. 1978 Feb;61(2):164–169. doi: 10.1104/pp.61.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kiewiet D. Y., Hall D. O., Jenner E. L. Effect of carbonylcyanide m-chlorophenylhydrazone on the photochemical reactions of isolated chloroplasts. Biochim Biophys Acta. 1965 Sep 27;109(1):284–292. doi: 10.1016/0926-6585(65)90112-3. [DOI] [PubMed] [Google Scholar]
- Degroote D., Kennedy R. A. Photosynthesis In Elodea canadensis Michx: Four-Carbon Acid Synthesis. Plant Physiol. 1977 Jun;59(6):1133–1135. doi: 10.1104/pp.59.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Comparison of three methods for measuring electrical resistances of plant cell membranes. Plant Physiol. 1977 Nov;60(5):684–688. doi: 10.1104/pp.60.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. L., Hope A. B. The role of protons in determining membrane electrical characteristics in Chara corallina. J Membr Biol. 1974;16(2):121–144. doi: 10.1007/BF01872410. [DOI] [PubMed] [Google Scholar]
- Tanner W., Loffler M., Kandler O. Cyclic Photophosphorylation in Vivo and its Relation to Photosynthetic CO(2)-Fixation. Plant Physiol. 1969 Mar;44(3):422–428. doi: 10.1104/pp.44.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]


