Abstract
Study Objectives:
To better understand the sometimes catastrophic effects of sleep loss on naturalistic decision making, we investigated effects of sleep deprivation on decision making in a reversal learning paradigm requiring acquisition and updating of information based on outcome feedback.
Design:
Subjects were randomized to a sleep deprivation or control condition, with performance testing at baseline, after 2 nights of total sleep deprivation (or rested control), and following 2 nights of recovery sleep. Subjects performed a decision task involving initial learning of go and no go response sets followed by unannounced reversal of contingencies, requiring use of outcome feedback for decisions. A working memory scanning task and psychomotor vigilance test were also administered.
Setting:
Six consecutive days and nights in a controlled laboratory environment with continuous behavioral monitoring.
Subjects:
Twenty-six subjects (22–40 y of age; 10 women).
Interventions:
Thirteen subjects were randomized to a 62-h total sleep deprivation condition; the others were controls.
Results:
Unlike controls, sleep deprived subjects had difficulty with initial learning of go and no go stimuli sets and had profound impairment adapting to reversal. Skin conductance responses to outcome feedback were diminished, indicating blunted affective reactions to feedback accompanying sleep deprivation. Working memory scanning performance was not significantly affected by sleep deprivation. And although sleep deprived subjects showed expected attentional lapses, these could not account for impairments in reversal learning decision making.
Conclusions:
Sleep deprivation is particularly problematic for decision making involving uncertainty and unexpected change. Blunted reactions to feedback while sleep deprived underlie failures to adapt to uncertainty and changing contingencies. Thus, an error may register, but with diminished effect because of reduced affective valence of the feedback or because the feedback is not cognitively bound with the choice. This has important implications for understanding and managing sleep loss-induced cognitive impairment in emergency response, disaster management, military operations, and other dynamic real-world settings with uncertain outcomes and imperfect information.
Citation:
Whitney P, Hinson JM, Jackson ML, Van Dongen HPA. Feedback blunting: total sleep deprivation impairs decision making that requires updating based on feedback. SLEEP 2015;38(5):745–754.
Keywords: affective valence, attentional lapses, feedback blunting, hot cognition, naturalistic decision making, reversal learning, salience, skin conductance response, total sleep deprivation, working memory
INTRODUCTION
The effect of sleep deprivation on cognitive performance is not uniform.1 In laboratory studies, sleep deprivation has consistently been shown to substantially degrade vigilance and sustained attention, whereas its effects on demanding tests of complex cognition such as decision making appear to be inconsistent and relatively small.2,3 Paradoxically, in the natural environment there are well-documented deficits in decision making due to sleep deprivation.4,5 In emergency response, disaster management, military encounters, and other fast-paced situations with uncertain outcomes and imperfect information, good decision making is significantly hampered by sleep deprivation.6–8
Although the lapses of sustained attention that are characteristic of sleep deprivation contribute to errors and accidents,3,8 attentional lapses are not the whole story of sleep deprivation effects on naturalistic decision making. The laboratory tasks often used to examine sleep deprivation effects on decisions typically do not include elements of updating information over time or revising strategies based on changing circumstances, which are important factors in sleep deprivation-related failures in operational environments.9–11 In addition, evidence from simulated gambling tasks, which are predictive of naturalistic decision making,12 shows that sleep deprivation affects decisions requiring the ability to weigh the risks and benefits of possible gains and losses.13 Though reduced activity in frontal lobe circuits involved in the executive control of attention may be involved in these effects, the specific mechanisms that produce sleep deprivation effects on risky decision making are not yet understood.14
The key to understanding the apparent gap between the relatively small, inconsistent effects of sleep deprivation in laboratory tests of decision making and the apparently considerable, costly effects of sleep deprivation on decisions in many natural contexts may lie in differences in the types of decisions required in each environment. Decision tasks used in laboratory settings typically involve a series of independent decisions based on well-specified outcomes, whereas in the dynamic real-world environments where sleep deprivation is known to produce errors, decision making often requires that information be acquired over time and updated based on outcome feedback.
Outside the context of sleep deprivation, the ability to adjust behavioral choices in dynamic environments has been studied using reversal learning tasks, in which an optimal choice pattern learned from rewards must be updated based on a reversal of contingencies.15,16 In the current study, we administered a reversal learning decision task, along with two complementary cognitive measures of stimulus processing within a 62-h laboratory sleep deprivation design (Figure 1A).
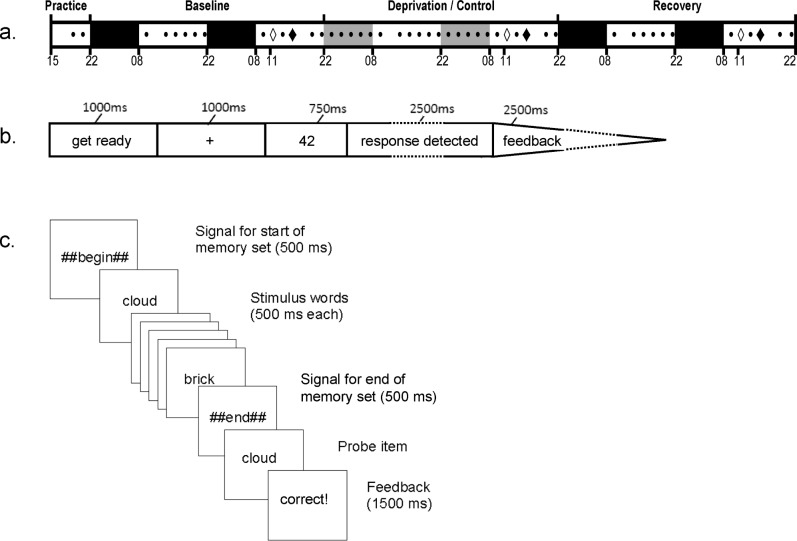
Figure 1.
Study design and performance task trial examples. (A) Subjects stayed in the laboratory continuously from 15:00 on day 1 until 22:00 on day 7. Black areas represent 10 h nocturnal periods in bed for sleep (22:00–08:00). Gray areas represent 10 h nocturnal periods in bed for sleep (22:00–08:00) for the control group only; the sleep deprivation group was kept awake continuously for a total of 62 h. Black diamonds indicate the three administrations of the reversal learning decision task (at 15:00): after 7 h of scheduled wakefulness during baseline; after 55 h of continuous wakefulness in the sleep deprivation group or 7 h of scheduled wakefulness in the control group; and after 7 h of scheduled wakefulness following 2 nights of recovery sleep. White diamonds indicate the three administrations of the WM scanning tasks (at 11:00): after 3 h of scheduled wakefulness during baseline; after 51 h of continuous wakefulness in the sleep deprivation group or 3 h of scheduled wakefulness in the control group; and after 3 h of scheduled wakefulness following 2 nights of recovery sleep. Black dots indicate PVT test bouts, which were administered at 2-h intervals throughout most of scheduled wakefulness. Tick marks denote time of day. (B) Trial schematic for the reversal learning decision task. Feedback included accuracy of the response or nonresponse and hypothetical monetary reward or punishment based on accuracy. (C) Trial schematic for the working memory scanning task.
The reversal learning decision task in our study was built on a go/no go task platform that required speeded responses to particular stimuli and withholding responses to other stimuli (Figure 1B). The task captured three key elements of decisions affected by sleep deprivation in the natural environment: choices are made under time pressure; choices produce good or bad outcomes; and feedback from outcomes must be used to guide future choices. In our reversal learning decision task, the ability to use feedback to guide choice was especially prominent. Subjects needed to initially learn the mapping of arbitrary stimuli to go and no go sets based on feedback, and at unpredictable points the mapping of stimuli to response sets was reversed, requiring subjects to use feedback to quickly learn the new contingencies. Skin conductance was recorded to assess affective reactions to feedback during the task.
Because making appropriate decisions is predicated on attending to stimuli and maintaining decision-relevant information in the focus of attention, or working memory (WM), we included two additional measures in order to independently assess the ability of sleep deprived subjects to sustain attention and to maintain information in WM. First, we administered the psychomotor vigilance test (PVT)17 as a standard measure of sustained attention. The PVT is known to be very sensitive to sustained attention deficits due to sleep deprivation.18,19 Second, to examine the encoding and maintenance of information in WM, we administered a WM scanning task in which memory set items were presented serially (Figure 1C). Using this procedure, it has previously been shown that the most recent two to three words presented before a probe are accessed more quickly and accurately because they remain in the focus of attention, whereas speed and accuracy of probe responses decline at later serial positions as items lose attentional activation.20 This task permitted us to assess whether sleep deprived subjects were encoding stimuli into WM and the degree to which information, once encoded, was maintained in a heightened state of activation. Memory scanning tasks of this type have strong test-retest reliability21 and show convergent validity with other WM tasks both in terms of behavioral performance and neurological substrates.22
Based on a previously used, highly controlled study design,23 we administered our reversal learning decision task, the PVT, and the WM scanning task at baseline, after sleep deprivation, and following recovery sleep (at fixed time of day), and compared sleep deprivation results to a control group, to systematically investigate the effects of sleep deprivation on decision making when information must be acquired over time and updated based on outcome feedback.
METHODS
Subjects and Study Design
Twenty-six subjects (22 to 40 y of age; 10 females) completed the study. Each group had 13 subjects, with seven women in the sleep deprivation group and three in the control group. Subjects were carefully screened to be healthy and free of drugs. They had normal or corrected to normal vision and hearing, and were native English speakers. Female subjects were not pregnant. Subjects reported having good habitual sleep, between 6 h and 10 h daily; regular bedtimes, getting up between 06:00 and 09:00; and no shift work or travel across time zones within 1 mo of entering the study. Polysomnography during the first night in the laboratory revealed no sleep disorders.
Subjects were in the laboratory for 7 days, 6 nights consecutively. The first 2 days and nights were baseline days, each with 10 h time in bed (TIB; 22:00–08:00) for polysomnographically recorded sleep. This was followed by 62 h of continuous wakefulness in the sleep deprivation group, or two nights with 10 h TIB (22:00–08:00) for polysomnographically recorded sleep in the control group. The last 2 days were recovery days, each with 10 h TIB (22:00–08:00) for polysomnographically recorded sleep.
Three versions of the reversal learning decision task and the WM scanning task were created, each with unique stimulus sets. The different versions were administered in randomized, counterbalanced order across the three task administrations for each individual, which occurred at 48-h intervals (Figure 1A)—at baseline, after sleep deprivation (or at equivalent time in the control group), and after recovery. The WM scanning task was administered at 11:00 each time (i.e., after 3 h of wakefulness, or 51 h of wakefulness in the sleep deprivation condition). The reversal learning task was administered at 15:00 each time (i.e., after 7 h of wakefulness, or 55 h of wakefulness in the sleep deprivation condition). The PVT, which required only one version as it is free of practice effects,17,24 was administered approximately every 2 h during scheduled wakefulness (Figure 1A).
The experiment was conducted under controlled laboratory conditions at the Washington State University Sleep and Performance Research Center. The laboratory was temperature controlled (21 ± 1°C) with fixed light levels (< 100 lux) during scheduled wakefulness and lights off during scheduled sleep periods. Up to four subjects were in the laboratory at a time. Each person had an isolated room for sleep and performance testing. Meals were provided every 4 waking hours. Between test bouts and meals, subjects were permitted only nonvigorous activities. Subjects were monitored throughout the experiment, and no visitors or phone calls were allowed.
During the experiment, as well as in the 7 days leading up to the experiment, subjects were not allowed to use caffeine, alcohol, or tobacco products. During the 7 days before the study they were required to keep their regular bedtimes and to refrain from daytime napping. Compliance was assessed by wrist actigraphy, sleep diary, and a time-stamped voice recorder that subjects called at bedtime and upon awakening.
The study was approved by the Institutional Review Board of Washington State University, and all subjects gave written informed consent.
Reversal Learning Decision Task
In this task, which was based on a go/no go paradigm, initially four two-digit numeric stimuli were assigned to the go (response) set and another four two-digit numeric stimuli were assigned to the no go (no response) set. Subjects had a 750-ms window after a number was presented to respond or decide to withhold their response (Figure 1B). Accuracy feedback, which included hypothetical monetary rewards and punishments, allowed subjects to determine which numbers were in the go set and which were in the no go set. After subjects had 56, 60, or 64 trials to acquire the go and no go sets, depending on the task version, the mapping of stimuli to response sets was reversed without warning. Postreversal, there were 40 trials during which subjects had to use accuracy feedback to update their response set.
We used a signal detection framework25 to convert responses to go stimuli (hits) and no go stimuli (false alarms) into d' discriminability values, which reflect overall ability to discriminate the go and no go stimulus sets, and c values, which reflect bias toward responding independent of d'.26,27
WM Scanning Task
In the WM scanning task, experimental stimuli consisted of 156 nouns drawn from the MRC Psycholinguistic Database.28 Each word was between three and seven letters in length and varied from the highest printed word frequency to 1 standard deviation below mean word frequency in the database. The words were divided into five blocks of 24 trials each. An additional set of 52 words was used for eight practice trials. The memory set of each trial consisted of six words presented serially for 500 ms each. Following an end-of-set signal, a probe word was presented and subjects were to respond as quickly and accurately as possible with a key press to indicate whether the probe word came from the immediately preceding set of six words (Figure 1C). On half the trials the probe item came from the memory set, with positive probes appearing equally often at each serial position. Words were randomly regrouped into memory sets and probes for each trial block.
Psychomotor Vigilance Test
Each PVT bout required 10 min of sustained attention, during which subjects were required to respond as quickly as possible, by pressing a button, to a simple visual stimulus that occurred at random intertrial intervals of 2 to 10 s. Sustained attention was quantified by the number of lapses of attention, defined as reaction times (RTs) > 500 ms.18
Skin Conductance Response
Administration of the reversal learning decision task included assessment of skin conductance response (SCR) amplitude, which is an established psychophysiologic index of affective reactions to stimulus processing.29–31 SCR was recorded by means of an SC5 skin conductance monitor (Contact Precision Instruments, Boston, MA). Conducting electrodes about 1 cm in diameter were attached to the nondominant hand on the interior of the medial phalanx of the index finger and the middle finger. Conductivity gel was applied between the skin and each of the two electrodes to ensure consistent ohmic contact. The electrodes were secured to the fingers by double adhesive tape rings. Skin conductance level was sampled at 20 Hz. SCR amplitude was defined as the difference between the peak skin conductance level and the baseline level for a given trial. Due to technical errors, SCR recordings were not obtained for three control subjects and four with sleep deprivation. Thus, the SCR data analyses reported here were performed on a smaller sample than the other data sets.
It is important to note that our use of SCR amplitude as an affective measure differs from uses of skin conductance level (SCL) in previous sleep deprivation research. Earlier studies have shown that tonic measures such as SCL indicate overall changes in arousal or attentiveness, but do not seem to reflect sleepiness in general.32 More recent studies have found that individual differences in SCL lability are a good indicator of subjective sleepiness.33 However, prior work did not isolate SCR amplitude to decision choices and choice outcome feedback. Our focus in this study was on SCR amplitudes to feedback because of the possibility that the sleep deprivation could produce changes in how feedback is valued even if the information provided by the feedback is understood cognitively. Our sampling procedure for SCR collection included markers of trial events so that we could isolate the reactions to feedback from the prefeedback portions of the trial.31
RESULTS
Figure 2 shows the d' discriminability values for each session of the reversal learning decision task, along with the hits and false alarm rates by trial block. The d' data were analyzed with mixed-design (repeated-measures) analysis of variance (ANOVA) of Group by Session. Task version ordering was included as a covariate. Prereversal, there was a significant main effect of Group (F1,23 = 12.38, P = 0.002, ηp2 = 0.35) and a Group by Session interaction (F2,46 = 5.33, P = 0.008, ηp2 = 0.33). Postreversal, the same effects were significant: Group (F1,23 = 13.23, P = 0.005, ηp2 = 0.37) and Group by Session interaction (F2,46 = 5.91, P = 0.005, ηp2 = 0.20).
Figure 2.
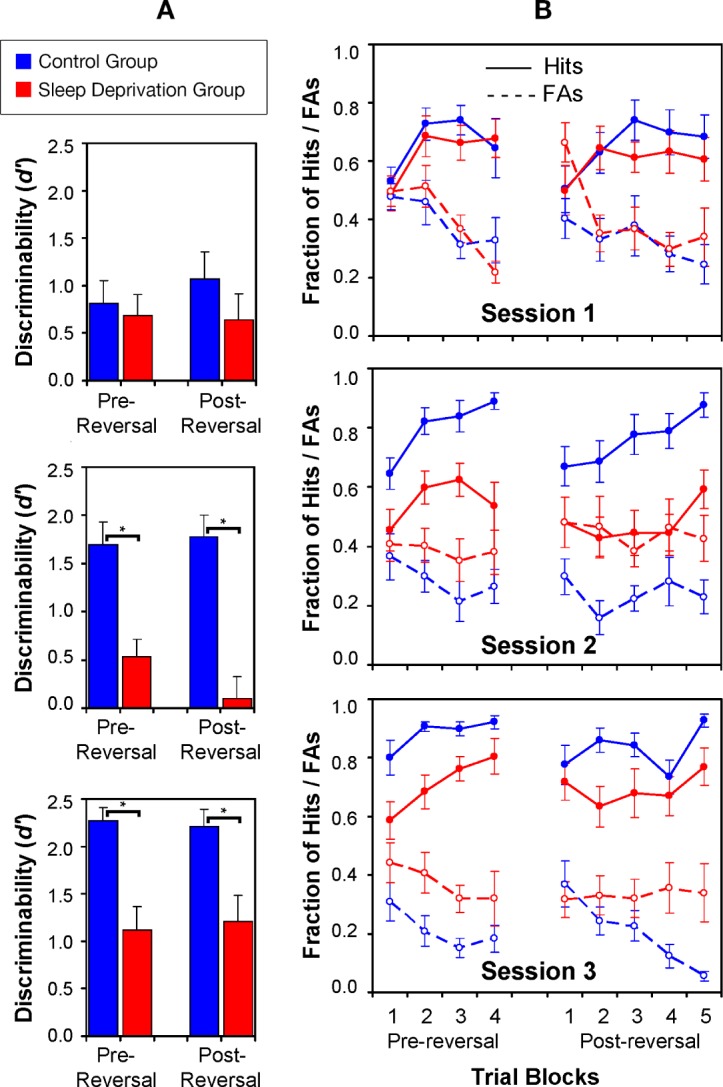
Performance on the reversal learning decision task. (A) Mean discriminability (d') values (and standard errors) for performance on the reversal learning decision task, before and after reversal of the stimulus-response mapping, in the control group (blue) and the sleep deprivation group (red). Brackets with asterisks indicate statistically significant pairwise differences. In Session 1 (baseline), performance was approximately equivalent between the two groups, both prereversal and postreversal (top panel). In Session 2 (sleep deprived or control), performance was degraded in the sleep deprivation group compared to the control group prereversal and especially postreversal (middle panel). In Session 3 (after recovery), performance prereversal and postreversal was improved in the sleep deprivation group compared to sleep deprivation, but still below performance in the controls (bottom panel). (B) Mean hits (solid lines) and false alarms (FAs; dashed lines) on the reversal learning decision task (and standard errors) across prereversal and postreversal trial blocks, in the control group (blue) and the sleep deprivation group (red), from which the d' values were derived. In Session 1 (baseline), both groups showed increasing hits and decreasing FAs over trial blocks before reversal as they learned the stimulus-response mapping. After reversal, both groups showed a temporary decrease in hits and increase in FAs, but they quickly learned to inhibit the previous responses and acquired the new stimulus-response mapping (top panel). In Session 2 (sleep deprived or control), before reversal, the control group showed the benefit of previous practice with a rapid increase in hits and decrease in FAs. The sleep deprivation group acquired the stimulus-response mappings, but did so more slowly than the control group. After reversal, the control group quickly returned to its previous high level of performance. In contrast, the subjects who have been sleep deprived were unable to distinguish between the go and no go stimuli for nearly the entire set of postreversal trials (middle panel). In Session 3 (after recovery), the control group quickly acquired the stimulus response mapping, and recovered easily from the reversal. The sleep deprivation group improved compared to their performance in Session 2, but their performance lagged behind that of the control group in Session 3.
In session 1, while all subjects were well rested, the sleep deprivation and control groups performed equivalently (P > 0.30 both prereversal and postreversal). Both groups were able to discriminate the go and no go stimulus sets before and after reversal (Figure 2A; top). In the prereversal phase, subjects gradually learned to differentiate go and no go sets until the reversal temporarily disrupted performance. Then, in the postreversal phase, feedback allowed for a return to the prereversal baseline (Figure 2B; top). The hit and false alarm patterns in the well-rested subjects mirror previous studies using contingency reversal in a go/no go decision context.34
In session 2, striking differences in performance emerged depending on whether the subjects were well rested or exposed to sleep deprivation (Figure 2, middle panel). Prereversal, the sleep deprivation group acquired the stimulus sets less effectively than the controls (F1,23 = 16.71, P < 0.001), but their mean d' value was significantly greater than zero (t12 = 2.81, P = 0.016). Postreversal, the sleep deprivation group not only performed more poorly than the controls (F1,23 = 25.81, P < 0.001), they showed a profound inability to differentiate go and no go stimuli over the 40 trials of the reversal phase, and their mean d' value was not significantly different from zero (t12 = 0.43, P = 0.67).
In session 3, which came after recovery sleep for the sleep deprivation group, subjects' performance was partially restored (Figure 2, bottom), but significant differences from the control group remained both prereversal (F1,23 = 16.71, P < 0.001) and postreversal (F1,23 = 9.76, P = 0.005).
Parallel analyses on the hit and false alarm rates to determine if the d' effects were dominated by selective effects on either hits or false alarms showed a statistical pattern that confirmed the impression conveyed by Figure 2B—the sleep deprivation effects on d' were due to both decreases in hits and increases in false alarms. Criterion scores c from the signal detection framework were analyzed in the same manner as the d' values. ANOVAs on c values produced no significant main effects or interactions (all P > 0.20). Thus, sleep deprivation effects on reversal learning performance were attributable to differences in the discriminability of go and no go sets rather than changes in response bias.
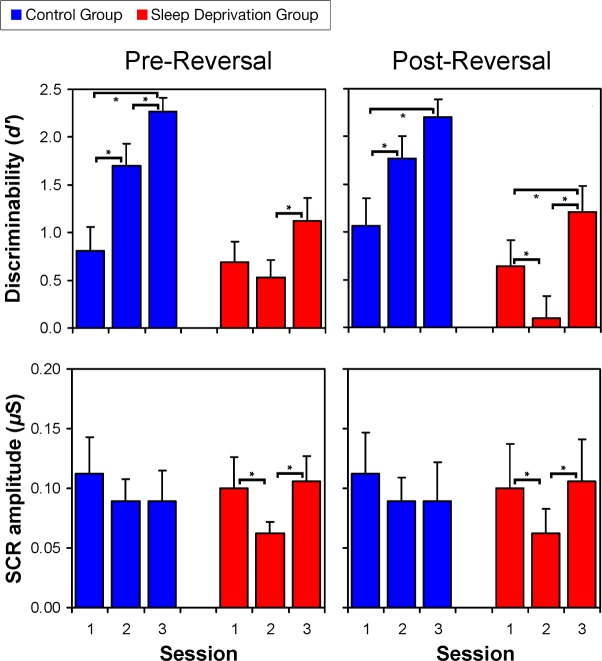
The sleep deprivation effects on performance in the reversal learning decision task were accompanied by effects on the SCRs elicited during feedback (Figure 3). Prereversal, SCRs showed no significant effects of Group, Session, or their interaction. In contrast, postreversal SCR data revealed a significant Group by Session interaction (F2,34 = 3.59, P = 0.038, ηp2 = 0.18). The control group had no significant changes in SCR over the sessions. The sleep deprivation group, however, showed substantially reduced SCR to feedback in Session 2 (sleep deprivation), compared to Sessions 1 (baseline) and 3 (recovery) and compared to Session 2 in the control group. This shows that during sleep deprivation, affective reactions to processing of feedback information were blunted.
Figure 3.
Performance and skin conductance response (SCR) reactions to feedback on the reversal learning decision task. The top panels show mean discriminability (d') values (and standard errors) for performance across the three sessions, in the control group (blue) and the sleep deprivation group (red), before reversal (top left) and after reversal (top right) of the stimulus-response mapping. These data are the same as those shown in the left panels of Figure 2. The bottom panels show mean SCR amplitude (and standard errors) across the three sessions, in the control group (blue) and the sleep deprivation group (red), before reversal (bottom left) and after reversal (bottom right). Brackets with asterisks indicate statistically significant pairwise differences. SCR responses were stable over sessions in the control group (bottom panels, blue), both prereversal and postreversal, and task performance showed a learning curve (progressive improvement) over sessions in this group (top panels, blue). In contrast, the sleep deprivation group showed substantially reduced SCR to feedback in Session 2 (bottom panels, red), both prereversal and postreversal, and task performance was degraded (especially postreversal) in Session 2 (when the subjects were sleep deprived) and showed less improvement due to learning in Session 3 (after recovery) (top panels, red). This indicates that during sleep deprivation, affective reactions to processing of feedback information were blunted, which was associated with profound and long-lasting deficits in reversal learning decision task performance.
As an exploratory analysis, we also examined the prefeed-back SCR trial data. These data reflect arousal during the period in which the stimulus is presented and the person makes a choice, but feedback has not yet been provided. In some decision contexts such data may reveal affective reactions that provide an index of anticipated outcomes that could guide choices.35 The prefeedback SCR data in this study displayed the same general pattern as the SCR to feedback data, with a dip in responses by the subjects with sleep deprivation in sessions 2. However, the prefeedback SCR data were more variable, and none of the effects approached significance.
Figure 4 shows the results for accuracy on the WM scanning task by serial position. The data were analyzed with mixed-design ANOVA of Group by Session by Position, with task version ordering as a covariate. For positive trials (in which the probe item appeared in the memory set), there were signifi-cant main effects of Group (F1,23 = 18.50, P < 0.001, ηp2 = 0.45) and Position (F5,115 = 11.01, P < 0.001, ηp2 = 0.32). These main effects were qualified by a significant Group by Session interaction (F2,46 = 9.84, P < 0.001, ηp2 = 0.30). The groups did not differ significantly in Sessions 1 and 3 (P > 0.10), but in Session 2, the sleep deprivation group exhibited significantly lower accuracy (t24 = 4.55, P < 0.001). Lower accuracy was consistently accompanied by longer RTs, with parallel patterns of statistical significance.
Figure 4.
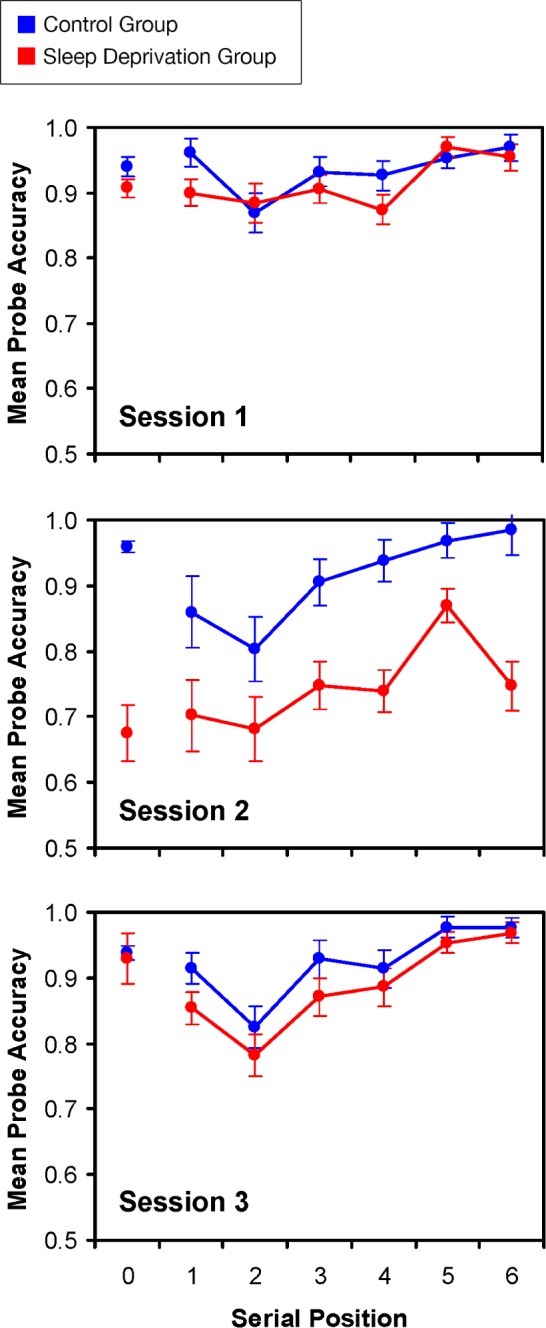
Performance on the working memory (WM) scanning task. Means (and standard errors) of accuracy are shown as a function of probe recency, for each of the three sessions in the control group (blue) and the sleep deprivation group (red). The means plotted at probe position 0 indicate responses on trials in which the probe item was not in the memory set. The sleep deprivation group exhibited significantly lower accuracy in Session 2 (when the subjects were sleep deprived).
Replicating earlier research,20 the general pattern of performance on the WM scanning task during baseline was that performance was best for the most recent memory set items, with a gradual decline in performance at earlier positions. During sleep deprivation, performance was approximately 20% below the level of the control group at each serial position, including the most recently presented stimuli. There were no interactions with Position in the omnibus ANOVA results, nor was there a Group by Position interaction for just the Session 2 data (P > 0.30). If the sleep deprived subjects were experiencing difficulty maintaining information that reached WM, we would have expected to see a growing disparity between the groups during Session 2 in going from the most to the least recent serial positions. Instead, the data show that for those stimuli that were successfully encoded, the sleep deprived subjects maintained the stimuli in WM as well as the control group.
Figure 5 shows the results for lapses of attention on the PVT. The data were analyzed with mixed-design ANOVA of Group by Test Bout. There were significant main effects of Group (F1,24 = 27.26, P < 0.001, ηp2 = 0.53), Test Bout (F41,984 = 15.76, P < 0.001, ηp2 = 0.40) and Group by Test Bout interaction (F41,984 = 13.03, P < 0.001, η p2 = 0.38). Consistent with other studies,23,24 the performance of the control group was stable over test bouts, but the sleep deprivation group showed substantial deficits in the ability to maintain sustained attention during test bouts that followed sleep loss. Near the time when Session 2 of the reversal learning decision task and the WM scanning task were administered, the overall rate of attentional lapses following sleep deprivation was approximately 20% of trials on average. Following recovery sleep, performance in the sleep deprivation group returned to baseline.
Figure 5.
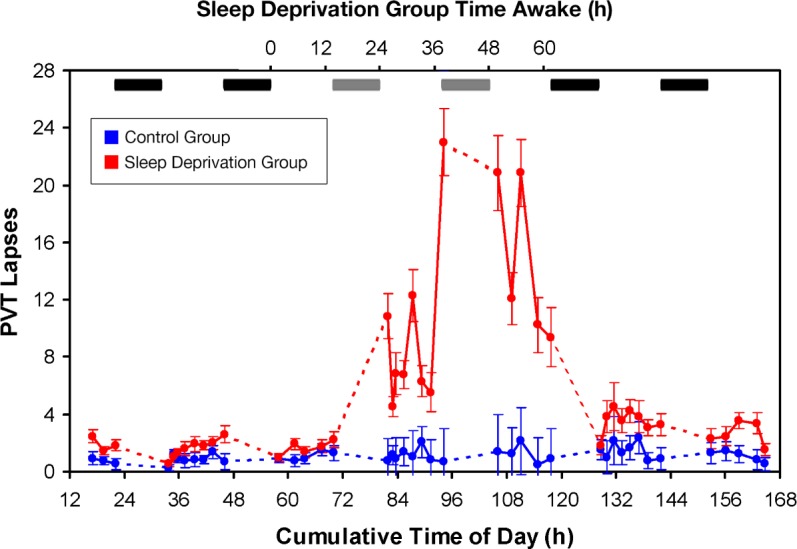
Performance on the psychomotor vigilance test (PVT). Means (and standard errors) of lapses of attention (reaction times > 500 ms) are shown as a function of time in the study, in the control group (blue) and the sleep deprivation group (red). On the top, black bars indicate scheduled sleep periods; gray bars indicate sleep periods in the control group only. In the sleep deprivation group, PVT lapses peaked at a rate of approximately 20% of trials.
DISCUSSION
The reversal learning decision task used in the current study was designed to capture elements of naturalistic decisions that require people to use outcome feedback to monitor and improve performance across trials. Consistent with the critical effects of sleep deprivation in many natural decision making contexts, in our experiment sleep deprivation had a powerful effect on decision making in the reversal learning decision task, especially when subjects had to adapt to abrupt changes in contingencies. In this task corrective feedback contributed to both short-term and long-term performance improvement, and both of these types of improvement were adversely affected by sleep deprivation.
Beginning with short-term performance, for well-rested subjects hit rate increased and false alarm rate decreased, producing a gradual improvement in d' within each session before the reversal of contingencies. The imposition of the reversal produced an immediate and substantial disruption of performance, which was gradually eliminated with further decision making trials. Sleep deprivation impaired short-term performance by slowing the expected increase in hit rate and decrease in false alarm rate, resulting in lower d', in the prereversal phase. The impairment caused by sleep deprivation was amplified after the reversal of contingencies, in that d' was reduced to near zero and sleep deprived subjects showed almost no improvement in performance for the remainder of the postreversal phase of the session (Figure 2).
Long-term improvement in performance on the reversal learning decision task was also evident. For the well-rested subjects, overall performance improved across each of the three sessions, revealing a beneficial effect of prior experience on each subsequent session. Sleep deprivation interfered with this long-term improvement. During Session 3, after recovery sleep, performance observed for sleep deprived subjects lagged well behind performance observed for well-rested subjects. In fact, for sleep deprived subjects performance was hardly better during Session 3 than during Session 1, whereas for rested subjects d' during Session 3 was about double its value during Session 1. Thus, for sleep deprived subjects the additional practice on the reversal learning decision task during Session 2 had no appreciable benefit for performance during Session 3 (Figure 2).
The large effects of sleep deprivation in the current study contrast with previous findings of sleep deprivation effects on performance on go/no go based tasks that do not involve dynamic updating of response contingencies based on outcome feedback.36–38 In the traditional go/no go tasks used in previous sleep deprivation studies, a prepotent tendency to respond to one stimulus is created by having a majority of trials require the go response. False alarms to no go trials in this context are taken as a measure of ability to inhibit a prepotent response. Compared to the go/no go based reversal learning decision task used in the current study, the burden on learning initial contingencies and on using feedback to acquire new contingencies is much lower in the traditional go/no go tasks, and sleep deprivation has more modest and inconsistent effects on performance.38,39
The WM scanning task provided an assessment of subjects' ability to encode and maintain information in the focus of attention. Both well-rested and sleep deprived subjects showed the expected serial position effect on the WM scanning task, i.e., higher accuracy and faster responses for more recent items in the memory set and a progressive decline in accuracy and response speed for more temporally remote items. The only group difference in this task was that accuracy for sleep deprived subjects was approximately 20% lower at all serial positions than for well-rested subjects (Figure 4). This result indicates that sleep deprivation did not cause problems in the maintenance of information in WM; otherwise we should have observed an increasing decline in accuracy for items in more remote positions in the memory set.
These findings are consistent with an earlier study showing that sleep deprived and well-rested controls have equivalent WM scanning rates, as indexed by the slope of RT over memory set size in a classic Sternberg WM scanning task.23 The implication of the current and previous WM data is that sleep deprived individuals may get less information into WM, but information that is encoded can be maintained and searched as effectively as in controls. It is therefore unlikely that sleep deprivation induced performance deficits in the reversal learning decision task due to failures of maintenance of stimulus information across trials.
In the absence of evidence of WM maintenance and scanning problems after sleep deprivation, accuracy differences between groups in the WM scanning task could still reflect failures of stimulus encoding due to lapses in attention during sleep deprivation. Results for our assay of sustained attention, the PVT, were comparable to those obtained in other sleep deprivation experiments. Near the timing of the WM scanning task and reversal learning decision task administrations (51–55 h of sleep deprivation), subjects showed lapses on about 20% of PVT trials (Figure 5). Note, too, that accuracy in the WM scanning task for sleep deprived subjects was about 20% lower than that for well-rested subjects (Figure 4). Thus, the loss of stimulus input due to instability of focal attention40 that is manifest on the PVT may account for the lower accuracy rate on the WM scanning task for sleep deprivation subjects as well.
If attentional lapsing is apparent in both the PVT and WM scanning tasks, such lapsing could also occur in the reversal learning decision task. As such, sleep deprivation could lead to a failure of encoding of stimulus or feedback information necessary to make correct decisions on approximately 20% of trials in the reversal learning decision task. This rate of lapsing might produce a decrement in acquiring the go and no go sets and delay recovery from the reversal by approximately 20% compared to the rested condition, but the actual sleep deprivation effects were considerably stronger than we would expect from lapsing alone. To provide further evidence for this conclusion, we conducted a control experiment (see supplemental material) that omitted feedback from a proportion of trials in order to simulate the effects of lapsing. Consistent with the finding that sleep deprivation effects on choices in an economic preference task are independent of sleep deprivation effects on sustained attention,14 the results from the control experiment confirmed that attentional lapsing alone does not explain the pattern and magnitude of performance deficits in the reversal learning decision task.
The pattern of deficits in reversal learning produced by sleep deprivation—poorer performance in the prereversal phase and severe disruption of performance in the postreversal phase—is also observed in cases of damage to the orbitofrontal cortex (OFC) and associated pathways including the amygdala and subcortical basal ganglia.16,41,42 These pathways appear to be crucial to integrating the cognitive and affective aspects of feedback in order to generate expectancies for choice outcomes, and it is the violation of such expectancies that allows for efficient recognition of a change in reward contingencies.43 From this perspective, our data suggest that sleep deprivation effects on reversal learning decision making involve compromised processing of the cognitive and/or affective dimensions of feedback needed to guide choice behavior. Specifically, our finding that sleep deprived subjects have blunted affective reaction to outcome feedback suggests two distinct, novel interpretations of the source of the sleep deprivation effects on reversal learning decision making. One possibility is that the blunted affective reactions to feedback under sleep deprivation, as observed with SCR (Figure 3), play a causal role in the inability to use feedback to adapt to changing contingencies. Another possibility is that blunted affective reactions are a consequence, rather than a cause, of the sleep deprivation effect on the ability to use feedback to learn the go/no go mappings.
Blunted affective reactions could play a direct, causal role in the inability of sleep deprived subjects to profit from feedback. This would be the case if lower SCR reactions to feedback represent dampening of the motivational properties of gains and losses. That is, lower attractiveness of gains and lower aversiveness of losses could make feedback less consequential as the affective reactions to the feedback are reduced. The idea that differences in affective reactions to outcomes can account for differences in decision making is reminiscent of decision making errors on measures such as the Iowa Gambling Task (IGT) by patients with damage to the OFC and other systems involved in dynamically updating reward expectancies.29,42,43 In the IGT, people make choices from decks of cards that produce gains and losses. The object is to learn to choose more often from “good” decks, which produce more gains, and less often from “bad” decks, which produce more losses. Unlike people who perform well on the task, patients with OFC damage fail to make advantageous choices even after 100 trials, and these patients fail to establish appropriate positive and negative valence for the deck options. In patients with damage to the amygdala, affective reactions to IGT outcomes are completely blunted. Accordingly, anticipatory SCR to choices is absent, and choice performance on the IGT shows little improvement over time. Like patients with damage to the OFC or amygdala, people who are sleep deprived demonstrate poor performance on the IGT.13 The blunted feedback reactions observed in the reversal learning decision task used here may thus be causal to sleep deprivation-induced deficits observed on this task, as well as on the IGT and related measures in other studies of healthy subjects.2,14,44
Further evidence for this interpretation may be found in studies that examined the processing of outcome feedback with event-related potentials (ERP). In one study using a flanker task, the amplitude of error-related negativity (ERN) was reduced after sleep deprivation, indicating reduced attention to error feedback information.45 In a related study using the flanker task, error positivity (Pe) and other ERPs indicative of error processing and correction were shown to be reduced after sleep deprivation, even in cases where ERN was unaffected, i.e., cases where the error feedback itself was attentionally registered.46 In a study using monetary incentives to try to offset the effect of sleep deprivation on error processing and error correction, the incentives somewhat reduced the sleep deprivation-induced decrease in ERN, thereby improving attention to error feedback.47 However, these incentives did not eliminate the effect of sleep deprivation on Pe or most aspects of performance. The implication of these studies is that even when outcome feedback is attended to and encoded, it may still have reduced efficacy under sleep deprivation. Thus, the fact that an error has occurred may be registering, but its effect on performance may nonetheless be diminished because of reduced affective salience when sleep deprived.
An alternative interpretation of the current results is that our sleep deprived subjects may have had blunted affective reactions to feedback as a consequence of problems with learning that rendered the feedback unhelpful. For example, if by the time feedback was delivered the subjects could no longer recall the stimulus shown or whether they chose to respond, then the feedback would serve no useful function for that trial, thus lowering the affective reaction to the feedback. This is consistent with the reduced d' values during sleep deprivation in the prereversal phase, signifying low signal-to-noise ratio (Figure 2), and would suggest that the inability to differentiate go and no go stimuli after reversal may be precipitated by incomplete learning of the go and no go stimuli before reversal. However, given that the ability to hold information in WM was largely intact in our sleep deprived subjects (after lapsing was taken into account), it seems unlikely that problems with maintenance of trial information in WM are responsible for the effects we observed.
Instead what may be affected by sleep deprivation is the ability to bind the stimulus, choice, and outcome in memory. If feedback and stimuli are not properly bound together in WM, it would be difficult to generate accurate expectancies about choice outcome. Failure to generate the needed outcome expectancies would, in turn, make feedback less meaningful and therefore dampen SCR reactivity to the feedback. There is increasing evidence that the hippocampus plays a key role in binding associations in both working and long-term memory,48 and thus it contributes to performance across a wide variety of tasks besides episodic memory.49 Our sleep deprivation effects in reversal learning decision making may likewise reflect impairment of hippocampus-dependent binding processes.50
Although the current study has a relatively small sample size and, to some extent, leaves open the question of what specific mechanisms underlie the effects we obtained, the data suggest that reversal learning tasks can serve as an important laboratory analog for studying how sleep deprivation produces errors in fast-paced operational environments in which information is emerging over time and feedback must be used to adapt to changing circumstances. Our findings have important implications for managing sleep deprivation-based impairment (“fatigue risk management”51,52) in emergency response, disaster management, military operations, and other dynamic real-world settings with uncertain outcomes and imperfect information. Inability to utilize feedback to evaluate decision outcomes may result in perseverative behavior,2 and it may be difficult to help oneself or someone else break free from this behavior because of that same inability to utilize feedback. Recent evidence suggests that stimulants such as caffeine may not be very effective as fatigue countermeasures in this context.53 It would be informative to investigate whether caffeine restores only the instability in sustained attention during sleep deprivation,40 but not the feedback blunting revealed in the current study.
Our data add to a growing body of evidence suggesting that understanding the effects of sleep deprivation on choice behavior requires the study of both “cold” cognitive information processes and “hot“ affective reactions to choice outcomes.54–56 As we learn more about how sleep deprivation affects feedback processing, it may be possible to further isolate and study in the laboratory the mechanisms by which sleep deprivation produces catastrophic errors in ecologically valid contexts outside the laboratory and find ways to manage and overcome this problem.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by National Institutes of Health grants R01HL105768 (MLJ) and R21CA167691 (JMH) and Office of Naval Research grant N00014-13-1-0302 (HVD). This work was performed at Washington State University. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the staff of the Sleep and Performance Research Center at Washington State University for their help conducting the study, and Peter Rosen for his technical assistance with collection of the skin conductance response data.
SUPPLEMENTAL MATERIAL
Lapsing of attention is a well-characterized effect of sleep deprivation (SD). Interruption in the bottom-up flow of information resulting from attentional lapsing could disrupt performance in the reversal learning decision task by reducing the number of opportunities for subjects to associatively bind responses and stimuli based on corrective outcome feedback. Thus, it is plausible to suggest that our reversal learning performance and skin conductance response (SCR) findings could be due to lapses of attention that accompany SD. If this suggestion is correct, the reversal learning decision task findings could simply be another example of the downstream effect of attentional lapses, similar to those found in other tasks that require sustained attention, such as the psychomotor vigilance test (PVT).
A direct way to examine this is to simulate attentional lapsing in people who are not sleep deprived. That is, we can experimentally omit information that would be lost if a lapse of attention were to occur. In the case of the reversal learning decision task, the most critical information is the outcome feedback on each trial that is necessary to correctly associate responses with stimuli, as omission of outcome feedback would render stimulus-response contingencies on a trial unin-formative. Therefore, we conducted an experiment to compare performance on the reversal learning decision task under conditions of full outcome feedback and partial outcome feedback (both prereversal and postreversal) in the absence of SD.
Seventy-five well-rested young adults (ages 18–25 y, 45 females) were randomly assigned to one of three experimental groups: 100% feedback (the original reversal learning task), 80% feedback (outcome feedback omitted on 20% randomly selected trials), or 60% feedback (outcome feedback omitted on 40% randomly selected trials). The 80% feedback condition was designed to provide rates of feedback omission (20%) that would simulate the lapse rates observed in the PVT and working memory scanning task. The 60% feedback condition was designed to simulate a much more severe lapsing rate (40%), roughly twice the lapsing rate found in our sustained attention tasks. Assignment to the groups was constrained to produce equal numbers of subjects (n = 25 per group). Data from four subjects (one in the 100% feedback group, one in the 80% feedback group, and two in the 60% feedback group) were excluded from analysis because these subjects stopped engaging in the task, as shown by hitting the space bar on every trial part way through the task.
The results of the experiment are summarized in Figure S1. Prereversal d' values varied by feedback condition (F2,68 = 4.58, P = 0.014). Using the Tukey honest significance difference test for pairwise comparisons, the 100% and 80% feedback conditions did not differ significantly (P = 0.435), and the 60% feedback condition had poorer performance than 100% feedback (P = 0.005) and 80% feedback (P = 0.035) conditions. Likewise, postreversal d' values varied significantly across groups (F2,68 = 4.40, P = 0.016), but in this case the only signifi-cant pairwise difference was between the 60% and the 100% conditions (P = 0.035). Again, performance did not differ significantly between the 100% and 80% conditions (P = 0.358). Separate analyses of the hit and false alarm rates over blocks led to the same conclusions as the d' analyses. Performance improved over trials both prereversal and postreversal, with poorer performance in the 60% feedback group.
Effect of attentional lapsing on reversal learning decision task performance. The reversal learning decision task was administered with either 100% feedback, 80% feedback, or 60% feedback. The left panel shows mean discriminability (d') values (and standard errors) for performance before and after reversal of the stimulus-response mapping. The right panel shows mean hits (solid lines) and false alarms (FAs; dashed lines) and standard errors across prereversal and postreversal trial blocks, from which the d' values were derived. Compared to the 100% feedback condition, the 80% feedback condition—simulating the approximately 20% lapsing expected of subjects with sleep deprivation (SD)—did not significantly impair discrimination of the go and no go sets. Doubling the expected lapsing rate by providing feedback on only 60% of trials produced poorer performance, but postreversal discrimination was still not reduced to near zero as was the case for the SD group in the main experiment (refer to Figure 2 in the main text).
Based on these data, a 20% rate of lapsing on the reversal learning decision task would have relatively little effect on overall performance. Doubling the rate of uninformative trials by eliminating 40% of the feedback would produce a notable decrement in performance, but even in that high rate of feedback omission the pattern of decrements was not as strong as that of the SD group in the main experiment (refer to Figure 2 in the main text). Thus, it appears that SD produces effects on reversal learning decision making beyond those of attentional lapsing alone.
This conclusion is strengthened by analyses of SCR to outcome feedback shown in Figure S2. Unlike the pattern obtained with sleep-deprived subjects, the feedback omission produced no significant differences in SCRs to feedback either prereversal (F2,57 = 0.98, P = 0.375) or postreversal (F2,57 = 1.26, P = 0.292). Note that these analyses were based on fewer subjects than those of the performance data—technical problems with recording of SCR resulted in missing data for 11 subjects (one from the 100% feedback condition, seven from the 80% feedback condition, and three from the 60% feedback condition). Nevertheless, perhaps more telling than the lack of significant differences across the feedback conditions is the fact that the postreversal difference in SCRs between the 100% condition and the 60% feedback condition (double the expected lapsing rate under SD) was less than 0.02 μS. In the main experiment, the postreversal difference in SCRs between the control condition and the SD condition was more than 2.5 times as large (refer to Figure 3, middle panel, in the main text). As such, it is unlikely that lapsing alone produced the blunted SCR reactions observed in the main experiment.
Skin conductance response (SCR) reactions to feedback on the reversal learning decision task administered with either 100% feedback, 80% feedback, or 60% feedback. Mean SCR amplitude (and standard errors) before and after reversal is shown. SCRs were quite variable across the feedback conditions, with no significant effect of feedback condition. Even when the trials without feedback were twice the expected lapsing rate under SD, we did not observe significant blunting of SCRs to feedback.
REFERENCES
- 1.Jackson ML, Gunzelmann G, Whitney P, et al. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev. 2013;17:215–25. doi: 10.1016/j.smrv.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Killgore WDS. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 3.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–89. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samkoff JS, Jacques CH. A review of studies concerning effects of sleep deprivation and fatigue on residents' performance. Acad Med. 1991;66:687–93. doi: 10.1097/00001888-199111000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Rosekind MR. Underestimating the societal costs of impaired alertness: safety, health, and productivity risks. Sleep Med. 2005;6:521–5. doi: 10.1016/s1389-9457(05)80005-x. [DOI] [PubMed] [Google Scholar]
- 6.Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep. 1994;17:84–93. doi: 10.1093/sleep/17.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Vila B. Impact of long work hours on police officers and the communities they serve. Am J Ind Med. 2006;49:972–80. doi: 10.1002/ajim.20333. [DOI] [PubMed] [Google Scholar]
- 8.Van Dongen HPA, Hursh SR. Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 5th edition. St. Louis, MO: Elsevier Saunders; 2010. Fatigue, performance, errors, and accidents; pp. 753–9. [Google Scholar]
- 9.Banderet LE, Stokes JW, Francesconi R, Kowal DM, Paul N. Artillery teams in simulated sustained combat: performance and other measures. In: Johnson DJ, Tepas WP, Colquhoun WP, Colligan MJ, editors. Biological rhythms, sleep and shiftwork. New York: Spectrum; 1981. pp. 459–77. [Google Scholar]
- 10.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–49. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 11.Wesensten NJ, Balkin TJ. The challenge of sleep management in military operations. US Army Med Dep J. 2013 Oct-Dec;:109–18. [PubMed] [Google Scholar]
- 12.Buelow MT, Suhr JA. The construct validity of the Iowa Gambling Task. Neuropsychol Rev. 2009;12:102–14. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- 13.Killgore WDS, Grugle NL, Reichardt RM, Killgore DB, Balkin TJ. Executive functions and the ability to sustain vigilance during sleep loss. Aviat Space Environ Med. 2009;80:81–7. doi: 10.3357/asem.2396.2009. [DOI] [PubMed] [Google Scholar]
- 14.Venkatraman V, Huettel SA, Chuah YML, Payne JW, Chee MWL. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci. 2011;31:3712–8. doi: 10.1523/JNEUROSCI.4407-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- 16.Frank MJ, Claus ED. Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev. 2006;113:300–26. doi: 10.1037/0033-295X.113.2.300. [DOI] [PubMed] [Google Scholar]
- 17.Dorrian J, Rogers N, Dinges DF. Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep de privation: clinical issues, pharmacology and sleep loss effects. New York: Marcel Dekker; 2005. pp. 39–70. [Google Scholar]
- 18.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 19.Balkin TJ, Bliese PD, Belenky G, et al. Comparative utility of instruments for monitoring sleepiness-related performance decrements in the operational environment. J Sleep Res. 2004;13:219–27. doi: 10.1111/j.1365-2869.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 20.Nee DE, Jonides J. Neural correlates of access to short-term memory. Proc Natl Acad Sci. 2008;105:14228–33. doi: 10.1073/pnas.0802081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manoach DS, Halpern EF, Kramer TS, et al. Rest-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiatry. 2001;158:955–8. doi: 10.1176/appi.ajp.158.6.955. [DOI] [PubMed] [Google Scholar]
- 22.Jonides J, Schumacher EH, Smith EE, et al. Verbal working memory load affects regional brain activation as measured by PET. J Cogn Neurosci. 1997;9:463–75. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- 23.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HPA. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 25.Green DM, Swets JA. Signal detection theory and psychophysics. 2nd edition. Huntington, NY: Krieger; 1974. [Google Scholar]
- 26.Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Meth Instr Comp. 1999;31:137–49. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- 27.Brosch T, Sander D, Pourtois G, Scherer KR. Beyond fear: rapid spatial orienting toward positive emotional stimuli. Psychol Sci. 2008;19:362–70. doi: 10.1111/j.1467-9280.2008.02094.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson MD. The MRC Psycholinguistic Database: machine readable dictionary, version 2. Behav Res Meth Instr Comp. 1988;20:6–11. [Google Scholar]
- 29.Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cort. 1996;6:215–25. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- 30.Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3rd edition. New York, NY: Cambridge University Press; 2007. pp. 159–81. [Google Scholar]
- 31.Whitney P, Hinson JM, Wirick A, Holben H. Somatic responses in behavioral inhibition. Cogn Affect Behav Neurosci. 2007;7:37–43. doi: 10.3758/cabn.7.1.37. [DOI] [PubMed] [Google Scholar]
- 32.Miró E, Cano-Lozano MC, Buela-Casal G. Electrodermal activity during total sleep deprivation and its relationship with other activation and performance measures. J Sleep Res. 2002;11:105–12. doi: 10.1046/j.1365-2869.2002.00286.x. [DOI] [PubMed] [Google Scholar]
- 33.Michael L, Passmann S, Becker R. Electrodermal lability as an indicator for subjective sleepiness during total sleep deprivation. J Sleep Res. 2012;21:470–8. doi: 10.1111/j.1365-2869.2011.00984.x. [DOI] [PubMed] [Google Scholar]
- 34.Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146:465–72. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- 35.Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Chuah YML, Venkatraman V, Dinges DF, Chee MWL. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J Neurosci. 2006;26:7156–62. doi: 10.1523/JNEUROSCI.0906-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ayalon L, Ancoli-Israel S, Drummond SPA. Altered brain activation during response inhibition in obstructive sleep apnea. J Sleep Res. 2009;18:204–8. doi: 10.1111/j.1365-2869.2008.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagaspe P, Taillard J, Amiéva H, et al. Influence of age, circadian and homeostatic processes on inhibitory motor control: a Go/Nogo task study. PLoS ONE. 2012;7:e39410. doi: 10.1371/journal.pone.0039410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acheson A, Richards JB, de Wit H. Effects of sleep deprivation on impulsive behaviors in men and women. Physiol Behav. 2007;91:579–87. doi: 10.1016/j.physbeh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 41.Fellows LK. The role of orbitofrontal cortex in decision making. Ann NY Acad Sci. 2007;1121:421–30. doi: 10.1196/annals.1401.023. [DOI] [PubMed] [Google Scholar]
- 42.Pauli WM, Hazy TE, O'Reilly RC. Expectancy, ambiguity, and behavioral flexibility: separable and complementary roles of the orbital frontal cortex and amygdala in processing reward expectancies. J Cogn Neurosci. 2011;24:351–66. doi: 10.1162/jocn_a_00155. [DOI] [PubMed] [Google Scholar]
- 43.Clark L, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 44.Pace-Schott EF, Nave G, Morgan A, Spencer RMC. Sleep-dependent modulation of affectively guided decision-making. J Sleep Res. 2012;21:30–9. doi: 10.1111/j.1365-2869.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh S, Cheng IC, Tsai LL. Immediate error correction process following sleep deprivation. J Sleep Res. 2007;16:137–47. doi: 10.1111/j.1365-2869.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 46.Murphy TI, Richard M, Masaki H, Segalowitz SJ. The effect of sleepiness on performance monitoring: I know what I am doing, but do I care? J Sleep Res. 2006;15:15–21. doi: 10.1111/j.1365-2869.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh S, Li TH, Tsai LL. Impact of monetary incentives on cognitive performance and error monitoring following sleep deprivation. Sleep. 2010;33:499–507. doi: 10.1093/sleep/33.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen RK, Rondina R, II, Riggs L, Meltzer JA, Ryan JD. Hippocampal and neocortical oscillatory contributions to visuospatial binding and comparison. J Exp Psych Gen. 2013;142:1–11. doi: 10.1037/a0034043. [DOI] [PubMed] [Google Scholar]
- 49.Yonelinas AP. The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behav Brain Res. 2013;254:34–44. doi: 10.1016/j.bbr.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akadhi K, Zagaar M, Alhaider I, Salim S, Aleisa A. Neurobiological consequences of sleep deprivation. Curr Neuropharmacol. 2013;11:231–49. doi: 10.2174/1570159X11311030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawson D, McCulloch K. Managing fatigue: it's about sleep. Sleep Med Rev. 2005;9:365–80. doi: 10.1016/j.smrv.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Satterfield BC, Van Dongen HPA. Occupational fatigue, underlying sleep and circadian mechanisms, and approaches to fatigue risk management. Fatigue Biomed Health Behav. 2013;1:118–36. [Google Scholar]
- 53.Killgore WDS, Grugle NL, Balkin TJ. Gambling when sleep deprived: don't bet on stimulants. Chronobiol Int. 2012;29:43–54. doi: 10.3109/07420528.2011.635230. [DOI] [PubMed] [Google Scholar]
- 54.Venkatraman V, Chuah YML, Huettel SA, Chee MWL. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–9. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 55.Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—a prefrontal amygdala disconnect. Curr Biol. 2007;17:R877–8. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Motomura Y, Kitamura S, Oba K, et al. Sleep debt elicits negative emotional reaction through diminished amygdale-anterior cingulated functional connectivity. PLoS ONE. 2013;8:e56578. doi: 10.1371/journal.pone.0056578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of attentional lapsing on reversal learning decision task performance. The reversal learning decision task was administered with either 100% feedback, 80% feedback, or 60% feedback. The left panel shows mean discriminability (d') values (and standard errors) for performance before and after reversal of the stimulus-response mapping. The right panel shows mean hits (solid lines) and false alarms (FAs; dashed lines) and standard errors across prereversal and postreversal trial blocks, from which the d' values were derived. Compared to the 100% feedback condition, the 80% feedback condition—simulating the approximately 20% lapsing expected of subjects with sleep deprivation (SD)—did not significantly impair discrimination of the go and no go sets. Doubling the expected lapsing rate by providing feedback on only 60% of trials produced poorer performance, but postreversal discrimination was still not reduced to near zero as was the case for the SD group in the main experiment (refer to Figure 2 in the main text).
Skin conductance response (SCR) reactions to feedback on the reversal learning decision task administered with either 100% feedback, 80% feedback, or 60% feedback. Mean SCR amplitude (and standard errors) before and after reversal is shown. SCRs were quite variable across the feedback conditions, with no significant effect of feedback condition. Even when the trials without feedback were twice the expected lapsing rate under SD, we did not observe significant blunting of SCRs to feedback.