Abstract
Alcoholic liver disease is a leading cause of morbidity and mortality worldwide. Alcoholic fatty liver disease can progress to steatohepatitis, alcoholic hepatitis, fibrosis, and cirrhosis. Patients with alcohol abuse show quantitative and qualitative changes in the composition of the intestinal microbiome. Furthermore, patients with alcoholic liver disease have increased intestinal permeability and elevated systemic levels of gut-derived microbial products. Maintaining eubiosis, stabilizing the mucosal gut barrier or preventing cellular responses to microbial products protect from experimental alcoholic liver disease. Therefore, intestinal dysbiosis and pathological bacterial translocation appear fundamental for the pathogenesis of alcoholic liver disease. This review highlights causes for intestinal dysbiosis and pathological bacterial translocation, their relationship and consequences for alcoholic liver disease. We also discuss how the liver affects the intestinal microbiota.
Keywords: alcoholic liver disease, microbiome, bacterial translocation, intestinal bacterial dysbiosis, metabolome
Introduction
Alcohol-related liver cirrhosis was responsible for 0.9% of all global deaths and 47.9% of all liver cirrhosis-attributable deaths in 2010 (Hartmann et al., 2012, Rehm et al., 2013). Alcoholic liver disease (ALD) encompasses fatty liver, or hepatic steatosis, and the more serious entities alcoholic steatohepatitis, alcoholic hepatitis, fibrosis, cirrhosis, and liver cancer (Gao and Bataller, 2011). Already 50 years ago, Lieber et al. showed that alcohol-induced hepatic steatosis resolves within several weeks of abstinence (Lieber et al., 1965). In case of continued consumption of alcohol, fatty liver can progress to fibrosis and cirrhosis which can lead to portal hypertension or liver failure (Gao and Bataller, 2011, Liu, 2014). 10% of heavy drinkers will develop alcoholic liver cirrhosis (Levene and Goldin, 2012, Liu, 2014). Alcoholics and subjects with alcoholic liver cirrhosis display higher levels of bacterial products in their blood than healthy humans (Parlesak et al., 2000, Bajaj et al., 2014c). In addition, bacterial infections caused by pathological bacterial translocation increase the mortality in cirrhotic patients tremendously. 30% of these patients expire within one month, another 30% die by one year (Arvaniti et al., 2010).
This review provides an overview of causes for intestinal dysbiosis and describes changes in the intestinal microbiome during alcoholic liver disease. We will further discuss the relationship between dysbiosis and the onset of pathological bacterial translocation, as well as their contribution to ALD in animals and humans.
1. Intestinal dysbiosis
The intestine harbors a diverse community of bacteria. Microbial members of this community are beneficial for host metabolism and digestion, thereby creating a symbiotic relationship with the host. Intestinal dysbiosis is defined as an imbalance of the different microbial entities in the intestine with a disruption of symbiosis (McLoughlin and Mills, 2011). Intestinal dysbiosis can present as quantitative (intestinal bacterial overgrowth) and qualitative changes in the intestinal microbiota. It has been associated with ALD both in experimental animal models and patients (Kirpich et al., 2008, Mutlu et al., 2009, Chen et al., 2011, Yan et al., 2011, Mutlu et al., 2012, Hartmann et al., 2013, Leclercq et al., 2014, Chen et al., 2015).
1.1. Intestinal bacterial overgrowth in alcoholic liver disease
Chronic alcohol ingestion leads to small and large intestinal bacterial overgrowth and dysbiosis in animals and humans (Bode et al., 1984, Casafont Morencos et al., 1996, Yan et al., 2011, Hartmann et al., 2013). Intestinal bacterial overgrowth is defined as increased numbers of bacteria in the intestine, in animals most commonly evidenced by quantitative polymerase chain reaction (qPCR) using universal 16S ribosomal RNA bacterial primer sets in cecal samples; alternatively conventional culturing techniques of small and large intestinal contents or fecal samples can be used as well (Adachi et al., 1995, Yan et al., 2011, Hartmann et al., 2013). In humans, it is classically defined as at least 105 cultured colony forming units of bacteria per ml from jejunal aspirates (Kerlin and Wong, 1988, Bauer et al., 2000, Simren and Stotzer, 2006). The increase of both aerobic and anaerobic bacteria was most pronounced in the proximal small intestine following intragastric feeding of alcohol in mice (Yan et al., 2011). Large intestinal bacterial overgrowth develops as early as one week after intragastric alcohol feeding (Hartmann et al., 2013), and is also present in end-stage liver disease in rodents (Guarner et al., 1997, Sanchez et al., 2005). Similarly, subjects with moderate alcohol consumption as well as patients with alcoholic liver cirrhosis display small intestinal bacterial overgrowth (Casafont Morencos et al., 1996, Gabbard et al., 2014). Small intestinal bacterial overgrowth correlates well with the severity of the alcoholic cirrhosis (Casafont Morencos et al., 1996). Probiotics are live, non-pathogenic microorganisms promoting the growth of other beneficial microorganisms (Hartmann et al., 2012, Cicenia et al., 2014). Intriguingly, probiotics VSL#3 decrease small intestinal bacterial overgrowth in cirrhotic patients (Lunia et al., 2014). This finding is important since small intestinal bacterial overgrowth has been shown to be a risk factor for the occurrence of hepatic encephalopathy besides the Child-Pugh-Score itself (Lunia et al., 2014). Interestingly, selective intestinal decontamination with antibiotics as prevention and intervention can abrogate large intestinal bacterial overgrowth and alleviate subsequent liver damage in rodents (Adachi et al., 1995, Chen et al., 2014). However, a randomized, double-blind, placebo-controlled clinical trial in patients with alcoholic liver disease (27 with cirrhosis, 23 without cirrhosis) using the non-absorbable, broad-spectrum antibiotic Paromomycin did not show an improvement in liver damage relative to placebo treated patients (Bode et al., 1997). Since endotoxin was not reduced after 4 weeks of treatment, the antimicrobial treatment might not have effectively reduced intestinal bacterial overgrowth, or the treatment length was not long enough. Another possible explanation could be that antibiotics induce dysbiosis (Cho et al., 2012) and possible pathogenic bacteria have been selected by the treatment with Paromomycin. There are indications that broad spectrum antibiotic treatment alone might decrease the expression of intestinal tight junction proteins (Cresci et al., 2013). Taken together, chronic alcohol abuse results in small and large intestinal bacterial overgrowth that (at least in rodents) represents an attractive target for therapy.
1.2. Qualitative changes in the microbiome
The microbiome consists of several phyla which comprise classes that encompass orders. Orders consist of a number of families that finally comprise certain genera and species of bacteria (see Table 1, 2, and Supplementary Table 1). Gram staining can help to distinguish different phyla from each other: Firmicutes and Actinobacteria are generally Gram-positive, while Proteobacteria, Fusobacteria and Bacteroidetes are usually Gram-negative. However, members of the Firmicutes-class Negativicutes for example are, as the name implies, Gram-negative (Vesth et al., 2013). Therefore, more advanced technologies (e.g. deep pyrosequencing of bacterial 16S rRNA) are used to track microbial changes occurring in the intestine in alcoholic liver disease, as described below.
Table 1.
Changes in intestinal microbiota associated with experimental alcoholic liver disease in animals
| Implicated microbiota
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Condition | Comparison | Phylum | Class | Order | Family | Genus/Species | Methodology | Reference |
| Intragastric Tsukamoto-French mouse model for 3 weeks | Isocaloric vs. ethanol fed (n=3 vs. 3) | Bacteroidetes ↑ Firmicutes ↓ |
Bacteroidia ↑ Bacilli |
Bacteroidales Lactobacillales ↓ | Bacteroidaceae Enterococcaceae Lactobacillaceae ↓ |
Bacteroides spp. ↑ Enterococcus spp. ↑ Lactobacillus spp. Pediococcus spp. Leuconostoc spp. Lactococcus spp. ↓ |
16S rRNA gene pyrosequencing, quantitative real-time PCR Cecum samples | Yan and colleagues (2011) |
| Proteobacteria ↑ Verrucomicrobia ↑ |
Erysipelotrichia Verrucomicrobiae |
Erysipelotrichales Verrucomicrobiales |
Leuconostocaceae Streptococcaceae Erysipelotrichaceae ↑ Verrucomicrobiaceae |
Akkermansia muciniphila ↑ |
||||
|
| ||||||||
| Intragastric Tsukamoto-French mouse model for 1 week | Isocaloric vs. ethanol fed (n=7 vs 9, and 3 vs. 5) | Firmicutes Verrucomicrobia | Bacilli Verrucomicrobiae | Lactobacillales Verrucomicrobiales | Lactobacillaceae Verrucomicrobiaceae |
Lactobacillus spp. Akkermansia muciniphila ↑ |
Quantitative real-time PCR Cecum samples | Hartmann and colleagues (2013) |
|
| ||||||||
| Lieber-DeCarli Liquid diet mice for 6 weeks | Isocaloric vs. ethanol fed (n=8 vs. 8) | Actinobacteria ↑ Bacteroidetes ↓ Firmicutes ↓ Proteobacteria ↑ |
Actinobacteria Bacteroidia Bacilli Clostridia Erysipeltrichia Betaproteobacteria |
Actinomycetales Bacteroidales Bacillales Lactobacillales Clostridiales Erysipeltrichales Burkholderiales |
Corynebacteriaceae Bacteroidaceae Porphyromonadaceae Prevotellaceae Listeriaceae Aerococcaceae Lactobacillaceae Ruminococcaceae Erysipeltrichaceae Alcaligenaceae |
Corynebacterium spp. ↑ Bacteroides spp. Parabacteroides spp. Tannerella spp. Hallella sp. Listeria spp. ↑ Aerococcus spp. ↑ Lactobacillus spp. ↑ Acetivibrio spp. ↑ Incertae Sedis Allobaculum spp. ↑ Alcaligenes spp. ↑ |
16S rRNA gene pyrosequencing Stool sample | Bull-Otterson and colleagues (2013) |
| Ethanol fed vs. ethanol fed+ Lb. rhamnosus (n=8 vs. 4) | Actinobacteria ↓ Firmicutes ↑ Proteobacteria ↓ |
Actinobacteria Bacilli Clostridia Betaproteobacteria | Actinomycetales Lactobacillales Clostridiales Burkholderiales | Corynebacteriaceae Lactobacillaceae Ruminococcaceae Alcaligenaceae |
Corynebacterium spp. ↓ Lactobacillus spp. ↑ Incertae Sedis ↑ Alcaligenes spp. ↓ |
|||
|
| ||||||||
| Ethanol liquid diet for 7d + oral binge on day 7 in mice | Isocaloric vs. ethanol fed (n=5-7) | Proteobacteria Firmicutes | Gammabacteria Bacilli | Enterobacteriales Lactobacillales ↑ | Enterobacteriaceae ↑ Enterococcaceae |
Enterococcus spp. ↑ | Quantitative culturing of stool samples | Campos Canesso and colleagues (2014) |
|
| ||||||||
| Intragastric Tsukamoto-French mouse model including supple- ments of un- saturated or saturated fatty acids for 3 weeks | Isocaloric vs. ethanol fed with unsaturated FAs | Bacteroidetes ↑ Firmicutes ↓ |
Bacilli | Lactobacillales | Lactobacillaceae |
Lb. rhamnosus
Lactobacillus spp. ↓ |
16S rRNA gene pyrosequencing, quantitative real-time PCR Cecum samples | Chen and colleagues (2015) |
| Ethanol fed with unsaturated Fas vs. ethanol fed with saturated FAs (n=2-7) | Bacteroidetes ↓ Firmicutes ↑ |
Bacilli | Lactobacillales | Lactobacillaceae |
Lb. rhamnosus ↑ Lactobacillus spp. ↑ |
|||
A comparison of condition A vs condition B: ↑, increase in condition B relative to condition A; ↓, decrease in condition B relative to condition A.
Taxonomy was updated using the National Center for Biotechnology Information (NCBI) Taxonomy Browser.
Table 2.
Changes in intestinal microbiota associated with alcoholic liver disease in humans – Selection, for full list see Supplementary Table 1
| Implicated microbiota
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Disease | Comparison | Phylum | Class | Order | Family | Genus/Species | Methodology | Reference |
| Healthy (n=24) Alcoholic patients (n=66) |
Healthy vs. alcoholic patients | Actinobacteria Firmicutes | Actinobacteria Bacilli | Bifidobacteriales Lactobacillales | Bifidobacteriaceae Enterococcaceae Lactobacillaceae |
Bifidobacterium spp. ↓ Enterococcus spp. Lactobacillus spp. ↓ |
Quantitative culturing of stool samples | Kirpich and colleagues (2008) |
| Alcoholic patients without probiotics vs. alcoholic patients with probiotics | Actinobacteria Firmicutes | Actinobacteria Bacilli | Bifidobacteriales Lactobacillales | Bifidobacteriaceae Enterococcaceae Lactobacillaceae |
Bifidobacterium spp. ↑ Enterococcus spp. ↑ Lactobacillus spp. ↑ |
|||
|
| ||||||||
| Healthy (n=18) Alcoholics with- out cirrhosis (n=29) Alcoholics with cirrhosis (=19) |
Healthy vs. alcoholic cirrhotic patients | Bacteroidetes | Bacteroidia | Bacteroidales | Bacteroidaceae ↓ | 16S rRNA gene pyrosequencing Sigmoid mucosal biopsy | Mutlu and colleagues (2012) | |
|
Healthy vs. alcoholics without cirrhosis |
Bacteroidetes |
Bacteroidia |
Bacteroidales |
Bacteroidaceae ↓ |
||||
|
Nondysbiotic alcoholics vs. dysbiotic alcoholics |
Bacteroidetes Firmicutes Proteobacteria Verrucomicrobia |
Bacteroidia ↓ Sphingobacteria ↑ Bacilli ↑ Clostridia ↓ Gammaproteobacteria ↑ Verrucomicrobiae ↓ |
||||||
|
| ||||||||
| Healthy (n=25) Cirrhotic patients (only alcoholic=43, not alcoholic=170) |
Cirrhotic patients other than solely alcoholic vs. alcoholic cirrhotic patients | Firmicutes Proteobacteria |
Clostridia Gammabacteria |
Clostridiales Enterobacteriales Oceanospirillales |
Family XIV Incertae Sedis ↓ Lachnospiraceae ↓ Ruminococcaceae ↓ Enterobacteriaceae ↑ Halomonadaceae ↑ |
16S rRNA gene pyrosequencing Stool sample only | Bajaj and colleagues (2014c) | |
|
| ||||||||
| Nonalcoholic cirrhotic patients (n=5) Alcoholic cirrhotic patients with active alcohol abuse (n=5) | Nonalcoholic cirrhotic patients vs. alcoholic cirrhotic patients | Bacteroidetes Firmicutes | Bacteroidia Negativicutes | Bacteroidales Selenomonadales | Bacteroidaceae ↓ Veillonellaceae ↑ |
16S rRNA gene pyrosequencing Stool sample | Kakiyama and colleagues (2014) | |
|
| ||||||||
| Healthy (n=15) Alcohol dependent patients with high or low intestinal perme- ability (n=26, or 34, out of whom 6 or 7 were used for micro- biota studies, respectively) | Alcohol dependent patients with low intestinal perme- ability vs. alcohol dependent patients with high intestinal permeability | Actinobacteria Firmicutes | Actinobacteria Clostridia Negativicutes |
Bifidobacteriales Clostridiales Selenomonadales |
Bifidobacteriaceae Clostridiaceae Family XIII Incertae Sedis ↓ Family XIV Incertae Sedis ↑ Lachnospiraceae ↑ Oscillospiraceae Ruminococcaceae ↓ Veillonellaceae |
Bifidobacterium spp. ↓ Clostridium spp. ↓ Blautia spp. ↑ Dorea spp. ↑ Oscillibacter spp. F. prausnitzii ↓ Ruminococcus spp. Subdoligranulum spp. Megasphaera spp. ↑ |
16S rRNA gene pyrosequencing, quantitative real-time PCR Stool sample | Leclercq and colleagues (2014) |
| Alcohol dependent patients with high intestinal perme- ability pre- vs. post- alcohol abstinence | Actinobacteria Firmicutes | Actinobacteria Bacilli Clostridia Erysipeltrichia | Bifidobacteriales Lactobacillales Clostridiales Erysipeltrichales | Bifidobacteriaceae Lactobacillaceae Ruminococcaceae ↑ Erysipeltrichaceae ↓ |
Bifidobacterium spp. ↑ Lactobacillus spp. ↑ Holdemania spp. ↓ |
|||
A comparison of condition A vs condition B: ↑, increase in condition B relative to condition A; ↓, decrease in condition B relative to condition A.
Taxonomy was updated using the National Center for Biotechnology Information (NCBI) Taxonomy Browser.
1.2.1. Qualitative changes in the microbiome in animals following chronic alcohol feeding
Although only a small number of enteric bacteria can be cultured using conventional culturing techniques (Gill et al., 2006, Yan et al., 2011, Hartmann et al., 2013), recent advances such as Length Heterogeneity PCR (LH-PCR) (Mutlu et al., 2009) and deep pyrosequencing of bacterial 16S rRNA (Yan et al., 2011, Chen et al., 2015) helped to explore the gut microbiome further. Alcohol administration for 10 weeks results in colonic dysbiosis in rats, which can be prevented by probiotic and prebiotic feeding (Mutlu et al., 2009). In mice ethanol feeding reduces the phylum Firmicutes (Yan et al., 2011, Bull-Otterson et al., 2013) and the genus Lactobacillus spp. within the phylum Firmicutes (Yan et al., 2011, Hartmann et al., 2013) (Table 1). Enterococcus spp., also belonging to Firmicutes, increases after alcohol administration (Yan et al., 2011, Campos Canesso et al., 2014). There is evidence that alcohol-treated mice show higher intestinal levels of Verrucomicrobia and one of their genera Akkermansia muciniphila, Actinobacteria with their genus Corynebacterium spp., and Proteobacteria and their genus Alcaligenes spp. (Yan et al., 2011, Bull-Otterson et al., 2013, Hartmann et al., 2013). Several studies in rodents (Yan et al., 2011, Bull-Otterson et al., 2013) and humans (Loguercio et al., 2005, Lata et al., 2007, Kirpich et al., 2008, Dhiman et al., 2014) demonstrate that supplementation with probiotic bacteria alleviates ALD and liver cirrhosis in general. Interestingly, administration of probiotic Lactobacillus rhamnosus GG during the last two weeks of a six week alcohol feeding experiment to mice reversed the aforementioned microbial findings so that Actinobacteria and Corynebacterium spp., and Proteobacteria and Alcaligenes spp., as well as Firmicutes and their genera Lactobacillus spp. and Ruminococcaceae Incertae Sedis increased significantly relative to mice fed alcohol alone (Bull-Otterson et al., 2013). Supplementation with saturated fatty acids prevents alcoholic liver injury by restoring levels of Bacteroidetes, Firmicutes and Lactobacillus rhamnosus and spp. in the intestine of mice (Chen et al., 2015). Prebiotics, as complex carbohydrates that cannot be digested by the host but can specifically serve “good” bacteria as an energy source, reduce small intestinal bacterial overgrowth and ameliorate experimental alcoholic liver disease in an intragastric feeding model of ethanol (Yan et al., 2011).
1.2.2. Intestinal dysbiosis in alcoholics
Quantitative and qualitative changes in the intestinal microbiota occur in subjects with moderate alcohol consumption, alcoholics and alcoholic cirrhotics (Bajaj et al., 2014c, Gabbard et al., 2014, Leclercq et al., 2014). The term cirrhosis dysbiosis ratio, or CDR (Bajaj et al., 2014c), was proposed to reflect the changes of “good” vs. “bad” bacteria occurring in the intestine of cirrhotic patients. This ratio consists of the amount of the beneficial autochthonous bacteria Lachnospiraceae, Ruminococcaceae and the Clostridiales Family XIV Incertae Sedis divided by the amount of the potentially pathogenic taxa Enterobacteriaceae and Bacteroidaceae. It is postulated the lower the CDR, the more advanced is the cirrhosis (Bajaj et al., 2014c). The Lachnospiraceae (Chen et al., 2011, Bajaj et al., 2012b, Bajaj et al., 2014c), the Ruminococcaceae (Bajaj et al., 2012b, Kakiyama et al., 2013, Bajaj et al., 2014c), the Clostridiales Family XIV Incertae Sedis (Bajaj et al., 2012b, Bajaj et al., 2014c) are typically found at lower intestinal levels in subjects with at least partly alcohol-related liver cirrhosis, whereas Enterobacteriaceae (Chen et al., 2011, Bajaj et al., 2012b, Kakiyama et al., 2013, Bajaj et al., 2014c) including their prominent genus Escherichia coli (Liu et al., 2004) are found at higher levels (Table 2 and Supplementary Table 1). The Bacteroidaceae family showed a trend toward expansion in cirrhotic patients in some reports (Kakiyama et al., 2013, Bajaj et al., 2014c). Other studies showed a decreased abundance of Bacteroidaceae in patients with liver cirrhosis, in particular in alcoholic cirrhotics (Chen et al., 2011, Mutlu et al., 2012, Kakiyama et al., 2014). The CDR is lowest in alcoholic cirrhotic patients compared with cirrhotic subjects of another etiology; similarly, endotoxemia is higher and correlates with the expanding Gram-negative Enterobacteriaceae in these alcoholic patients (Bajaj et al., 2014c). Interestingly, administration of Lactobacillus rhamnosus GG to cirrhotic patients for four weeks resulted in an increase in Lachnospiraceae and the Clostridiales Family XIV Incertae Sedis, and a decrease in Enterobacteriaceae with an associated reduction of endotoxemia and serum TNF-alpha levels (Bajaj et al., 2014b). Clostridium spp. (Zhao et al., 2004, Bajaj et al., 2012a) as well as Enterococcaceae (Bajaj et al., 2014c) and their genus Enterococcus spp. (Zhao et al., 2004, Chen et al., 2011, Bajaj et al., 2012a) are found at greater quantities in the stools and colonic biopsy samples from cirrhotic patients. Fecal analysis in these patients also demonstrated a higher abundance of Fusobacteriaceae (Chen et al., 2011, Bajaj et al., 2012b), Staphylococcaceae (Bajaj et al., 2014c) and their genus Staphylococcus spp. (Liu et al., 2004). As mentioned above, alcoholics exhibit reduced numbers of the beneficial Lactobacillus spp. (Kirpich et al., 2008), and, similarly to cirrhotics, show lower fecal amounts of Bifidobacterium spp. (Zhao et al., 2004, Kirpich et al., 2008, Leclercq et al., 2014). Administration of probiotic Lactobacillus spp. and Bifidobacterium spp. to alcoholics increased levels of intestinal Lactobacillus spp. and Bifidobacterium spp., and improved liver enzymes (Kirpich et al., 2008). Likewise, mixtures of pre- and probiotics, or synbiotics (Cocktail 2000; Medipharm, Kagerod, Sweden; including Lactobacillus spp.), reduced amounts of Staphylococcus spp., Fusobacterium spp., E. coli, increased the abundance of Lactobacillus spp., and improved liver function in cirrhotic subjects (partly due to alcohol) (Liu et al., 2004). Intriguingly, although some microbial changes were non-reversible such as a reduced amount of Faecalibacterium prausnitzii, alcohol abstinence alone resulted in a partial restoration of eubiosis. Suppressed levels of Bifidobacterium spp., Lactobacillus spp., and Ruminococcaceae recovered in alcohol dependent patients (Leclercq et al., 2014). These findings were associated with lower scores of depression, anxiety, and craving after 3 weeks of abstinence as well as a significantly improved intestinal permeability (Leclercq et al., 2014). The abundance of Veillonellaceae (Chen et al., 2011, Kakiyama et al., 2013, Kakiyama et al., 2014) and their genus Megasphera spp. (Leclercq et al., 2014) is greater in stools of alcoholics and cirrhotic patients compared with healthy subjects. On the other hand, treatment with Rifaximin causes a reduction of the Gram-negative Veillonellaceae and reduces endotoxemia in partially alcohol-induced cirrhotic subjects (Bajaj et al., 2013). Streptococcaceae seem to expand in patients with liver cirrhosis related to HBV and alcohol (Chen et al., 2011) which gets exacerbated after treatment with proton-pump inhibitors (PPI) (Bajaj et al., 2014a). The reduction of fecal levels of Lachnospiraceae and Ruminococcaceae worsens after PPI treatment, too (Bajaj et al., 2014a). Taken together, dysbiosis occurs after chronic alcohol administration and is commonly associated with a decrease in “good” bacteria. “Good” commensals such as Lactobacillus spp. diminish, and possible pathogenic, in this sense “bad” bacteria such as Enterobacteriaceae increase. Preventing dysbiosis or restoring eubiosis (e.g. by supplementing probiotics, prebiotics or synbiotics) appears a valid strategy for treatment of alcoholic liver disease. None of the intestinal bacteria that are induced after chronic alcohol administration was causatively linked to the onset or progression of alcoholic liver disease.
2. Factors contributing to intestinal dysbiosis after chronic alcohol consumption
How can we explain intestinal changes in the microbiota following chronic alcohol consumption? A number of factors might contribute to alcohol-associated dysbiotic changes (Figure 1).
Figure 1. Contributing factors to intestinal dysbiosis after chronic alcohol consumption.
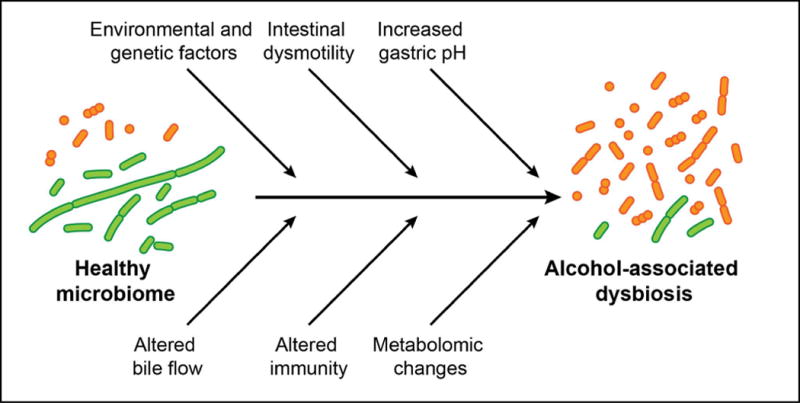
Chronic alcohol administration results in a quantitative increase of intestinal bacteria and a qualitative change in the bacterial composition of the microbiota. Several factors might contribute to alcohol-associated dysbiotic changes in the intestine.
Environmental factors
Environmental factors such as dietary habits, medications or xenobiotics are among the strongest determinants affecting the composition of the intestinal microbiome. For example, a western diet changes the gut microbiome dramatically (Ley et al., 2006). Alcohol and obesity synergistically worsen liver disease in experimental animal models and humans (Loomba et al., 2009, Xu et al., 2011). Whether microbiome changes contribute to this synergistic effect on steatohepatitis is not known. In addition, to what extent ethanol is used or produced by intestinal bacteria directly following chronic alcohol administration is also not known.
Genetics
Fatty liver disease develops in the majority of patients with chronic alcohol abuse, while fibrosis and cirrhosis occur in 40–60% of alcoholics (O’Shea et al., 2010). Genetic determinants are thought to contribute to the risk of developing progressive alcoholic liver disease. Women are more susceptible to alcohol-induced liver disease than men (Sato et al., 2001). Polymorphisms in cytochrome P4502E1 (CYP2E1) and alcohol-dehydrogenase-3 (ADH-3) genes are risk factors for developing liver disease among alcoholics (Monzoni et al., 2001). Furthermore, variations in patatin-like phospholipase domain-containing protein 3 (PNPLA3) affect the risk of developing alcoholic liver cirrhosis as well (Tian et al., 2010). It is not clear whether genetic variants contribute to alcoholic liver disease by affecting the composition of the intestinal microbiota. Host genetics in general can influence the composition of the human gut microbiome, which subsequently can impact host metabolism (Goodrich et al., 2014). Further studies are needed to elucidate the impact of genetics on microbiota and ALD.
Intestinal dysmotility
Ethanol reduces the intestinal motility that might lead to a proliferation of luminal bacteria. Social drinkers demonstrate an increased orocecal transit time relative to teetotalers; alcoholics exhibit an even longer orocecal transit time than social drinkers (Addolorato et al., 1997). Similarly, cirrhotic patients exhibit small intestinal bacterial overgrowth with a prolonged transit time (Gupta et al., 2010). Cisapride as a prokinetic agent improves small intestinal motility, and, interestingly, inhibits bacterial proliferation in subjects with liver cirrhosis (Madrid et al., 2001). To which degree impaired intestinal motility contributes to alcoholic liver disease, is not known.
Increased gastric pH
Ethanol either has no impact on gastric acid release or even increases it in non-alcoholic subjects (Chari et al., 1993). Alcoholics, however, exhibit hypochlorhydria, or the state of reduced gastric acid production (Dinoso et al., 1972, Chari et al., 1993). This might be due to their significantly altered gastric histology with higher rates of superficial and atrophic gastritis (Dinoso et al., 1972, Chari et al., 1993). Hypochlorhydria is associated with small intestinal bacterial overgrowth in cirrhotic patients (Shindo et al., 1993). It is not known whether ethanol-induced hypochlorhydria alters the progression of alcoholic liver disease.
Altered bile flow
Chronic alcohol abuse leads to higher total bile acid levels in the stool (Kakiyama et al., 2014). However, once the alcoholic patient develops cirrhosis, the fecal amount of total bile acids decreases significantly (Kakiyama et al., 2014). This might be due to the diminished bile secretion into the intestine observed in cirrhotics (Raedsch et al., 1983). The major receptor for bile acids in intestinal cells, the nuclear receptor Farnesoid X Receptor (FXR), influences several antimicrobials, amongst them angiogenin 1 and RNAse family member 4. A reduction of these bactericidal proteins was linked to small intestinal bacterial overgrowth in mice (Inagaki et al., 2006). Remarkably, oral administration of bile acids to cirrhotic rats abolished the small intestinal bacterial overgrowth (Lorenzo-Zuniga et al., 2003). Therefore, a decreased bile flow in subjects with liver cirrhosis (Raedsch et al., 1983) could contribute to quantitative microbiome changes.
Altered immune response
Chronic alcohol consumption has profound effects on the host immune system. Host bactericidal molecules are central effectors of the intestinal innate immune system contributing to the composition of the intestinal microbiome. Antimicrobial molecules are secreted by Paneth cells and intestinal epithelial cells. Two of these antimicrobials, Regenerating islet derived (Reg)-3b and Reg3g, were suppressed in murine and human small intestine after alcohol feeding and chronic ethanol abuse, respectively (Yan et al., 2011, Hartmann et al., 2013). The intestinal mucus layer serves as a means of protection in the gut, and its thickness increases in alcoholics (Hartmann et al., 2013). Whether alcohol-induced effects on the host immune system have either direct or indirect effects on the composition of the intestinal microbiome is an area that deserves to be studied in more detail.
Although all of these factors are affected by chronic alcohol consumption, more mechanistic studies are required to identify, whether these determinants and associated changes in the gut microbiome indeed impact alcoholic liver disease. Similarly, it is also not clear to what extent changes in liver function contribute to dysbiosis. Carefully designed future studies are required to determine whether pre-cirrhotic alcoholic liver disease affects the composition of the intestinal microbiome.
3. Consequences of intestinal dysbiosis in alcoholic liver disease
3.1. Pathological bacterial translocation
Pathological bacterial translocation is defined as the passage of viable bacteria or microbial products from the gastrointestinal tract to mesenteric lymph nodes or other extraintestinal organs (Berg and Garlington, 1979). The contribution of bacteria to liver disease is emphasized by an experiment where small intestinal bacterial overgrowth was experimentally induced which alone resulted in bacterial translocation and subsequent liver injury (Lichtman et al., 1990). Inversely, selective intestinal decontamination with antibiotics can reduce pathological bacterial translocation and endotoxemia, and ameliorate hepatic damage in rodents (Adachi et al., 1995, Chen et al., 2014).
Bacterial translocation is initiated when the intestinal epithelium is damaged and the intestine becomes more permeable (Parlesak et al., 2000, Purohit et al., 2008). What mechanisms are involved in the pathogenesis of that increased intestinal permeability? The ethanol metabolite acetaldehyde but not ethanol itself increases the permeability of Caco-2 cell monolayers (Rao, 1998). Furthermore, alcohol feeding to rats leads to acute injury of the colonic epithelial barrier via acetaldehyde, the metabolite of ethanol generated by gut bacteria, and an associated activation of mast cells (Ferrier et al., 2006). In addition, intestinal Cyp2E1 appears to play a role in alcohol-induced intestinal oxidative stress and intestinal permeability (Abdelmegeed et al., 2013). Taken together, possibly alcohol and its metabolite acetaldehyde directly cause tight junction disruption.
Intestinal inflammation is another mediator of intestinal barrier dysfunction. Pro-inflammatory mediators such as IL-1-beta or TNF-alpha are increased in the small intestine of mice after ethanol feeding (Fleming et al., 2001). Lamina propria monocytes and macrophages appear to be the source for increased cytokine production. These cells increase TNF-alpha production in the small intestine of mice and in the duodenum of humans after chronic alcohol consumption (Chen et al., 2014). Intestinal inflammation precedes the onset of alcohol-induced increased intestinal permeability in mice (Chen et al., 2014). Most importantly, alcohol-associated dysbiosis triggers this local intestinal inflammatory response. This was demonstrated by using non-absorbable antibiotics, which reduce large intestinal bacterial overgrowth, intestinal inflammation and intestinal permeability. This evidently links intestinal dysbiosis with gut barrier dysfunction, although the triggering microbial metabolite or product is currently not known. TNF-receptor 1 mutant mice are protected from intestinal barrier dysfunction and alcoholic liver disease. Reactivation of TNF-receptor 1 on intestinal epithelial cells resulted in increased intestinal permeability and liver disease that is similar to wild type mice after alcohol feeding, suggesting that enteric TNF-receptor 1 promotes intestinal barrier dysfunction and mediates ALD (Chen et al., 2014). Mice lacking myosin-light chain kinase (MLCK), a intracellular downstream target of TNF-receptor 1 in intestinal epithelial cells, show partial protection from intestinal barrier dysfunction and ALD (Chen et al., 2014), suggesting that other intracellular signaling molecules are involved to mediate tight junction disruption downstream of the TNF-receptor 1. Inducible nitric oxide synthases (iNOS) could be a candidate, because intestinal iNOS expression is dependent on TNF-receptor 1 on enterocytes after chronic alcohol feeding (Chen et al., 2014). iNOS expression correlates with barrier function disruption in differentiated Caco-2 cells (Banan et al., 1999). Furthermore, an iNOS inhibitor attenuated alcohol-induced gut permeability, endotoxemia, and liver injury (Tang et al., 2009). Whether iNOS (possibly as genetic variant) affects the composition of the gut microbiota, is currently not known, but deserves future investigation.
Interestingly, not all patients with alcohol dependence show increased intestinal permeability. The group of alcoholics with increased gut permeability also had an altered composition and activity of the gut microbiota such as lower amounts of Bifidobacterium spp., Clostridiales Family XIV Incertae Sedis, and Ruminococcaceae when compared with healthy controls. Levels of these bacteria were not changed in alcoholics with a low intestinal permeability (Leclercq et al., 2014). This raises the question whether other factors than dysbiosis induce gut permeability. There are indications that host genetics influence the composition of the intestinal microbiome and the host metabolism (Goodrich et al., 2014). This might be involved in inducing gut permeability associated with ALD as well.
As a consequence of increased intestinal permeability, pathological bacterial translocation can occur and plasma levels of gut-derived microbial products increase. Lipopolysaccharide (LPS), or endotoxin, is a critical component of the outer membrane of Gram-negative bacteria (Fadl et al., 2005). Many studies have shown that alcohol administration correlates with plasma endotoxin levels in animal models (Nanji et al., 1993, Adachi et al., 1995, Tamai et al., 2000). Elevations in plasma LPS can be observed during early stages of ALD (Parlesak et al., 2000) as well as during advanced stages of cirrhosis (Bajaj et al., 2014c). The degree of liver injury correlates with endotoxemia in patients with liver cirrhosis (Lin et al., 1995), and is higher in alcoholic cirrhosis compared with other etiologies (Bajaj et al., 2014c). Additionally, peptidoglycan, the major cell wall component in Gram-positive bacteria, is elevated in rat plasma after acute alcohol administration (Tabata et al., 2002).
We recently used a genetic mouse model with an enhanced intestinal innate immune response and with resistance to alcohol-induced large intestinal bacterial overgrowth (Hartmann et al., 2013). Despite more permeable intestines due to the mechanical absence of mucin-2 (Muc2), Muc2-deficient mice had decreased plasma LPS levels and were consequently protected from alcoholic liver disease (Hartmann et al., 2013). This suggests that failure of a physical barrier can be compensated by controlling the luminal bacterial burden.
The innate immune system has conserved pattern recognition receptors, e.g. Toll-like receptors (TLRs), that recognize specific pathogen-associated molecular patterns (PAMPs) such as LPS, peptidoglycan, or bacterial DNA (Akira et al., 2006). The cellular receptor for LPS is TLR4 which plays an eminent role in the immune response to pathological bacterial translocation. TLR4 mutant C3H/Hej mice and TLR4-knockout mice – although exhibiting a similar gut permeability after alcohol feeding compared with wild-type mice – show less hepatic steatosis, inflammation and cell death following ethanol feeding relative to wild type mice (Uesugi et al., 2001, Hritz et al., 2008). Deficiency of its cellular co-receptor cluster of differentiation 14 (CD14) results in alleviated alcohol-induced liver injury (Yin et al., 2001). LPS binding to TLR4 initiates an intracellular downstream signaling cascade in immune cells and other liver cells. Kupffer cells, amongst other cells, are important in the pathogenesis of ALD. Inactivation of these cells via gadolinium chloride injections potently decreases ethanol-induced liver injury (Adachi et al., 1994). Furthermore, hepatic stellate cells (HSCs) play a central role in the pathogenesis of liver fibrosis (Karaa et al., 2008). Oxidative stress mediated by ethanol and its metabolite acetaldehyde sensitizes HSCs to activation by endotoxin, which results in liver fibrosis after chronic ethanol feeding combined with LPS (Karaa et al., 2008). TLR4 signaling in non-bone marrow-derived liver cells including HSCs is required for liver steatosis, inflammation, and a fibrogenic response after chronic alcohol treatment HSCs (Inokuchi et al., 2011). LPS induces apoptosis in hepatocytes, in particular in synergy with other hepatotoxic agents (Kudo et al., 2009). For subsequent cellular events in the liver following pathological bacterial translocation, we would like to refer to other in depth reviews (Seki and Schnabl, 2012, Szabo, 2015).
Interestingly, we recently showed that certain aspects of the commensal microbiota might protect against chronic liver disease. In the absence of the microbiota, liver injury and fibrosis induced by oral administration of thioacetamide or intraperitoneal injections of carbon tetrachloride is more pronounced in germ-free mice compared with conventional mice (Mazagova et al., 2014). Strikingly, hepatocytes were more susceptible to toxin-induced cell death in the absence of the microbiota or when lacking innate immune signaling (Mazagova et al., 2014). Future studies need to determine whether alcohol-induced hepatocyte death is similarly exacerbated in the absence of the microbiota. Furthermore, it will be crucial to identify microbial products or metabolites with cytoprotective properties.
3.2. Changes in intestinal metabolites
Metabolomic studies can determine intestinal metabolites that will reflect functional changes of the intestinal microbiota. Chronic ethanol administration to rats over 8 weeks results in a reduction of almost all amino acids including all three branched-chain amino acids (leucine, isoleucine, valine), perturbations of the steroid, lipid and carnitine metabolism (Xie et al., 2013b), as well as the bile acid metabolism (Xie et al., 2013a) in the intestine. Certain metabolites belonging to alcohols, alkanes, and benzenes (such as 1-nonanol, hexane, and styrene, respectively) could only be detected in the feces of alcohol dependent subjects but not in healthy controls. In contrast, other volatile organic compounds such as 2-methyl-1-butanol, methyl-cyclopentane, and methanethiol were detectable in the feces of healthy humans but non-detectable in alcohol dependent patients (Leclercq et al., 2014).
Short-chain fatty acids (SCFAs) are bacterial fermentation products, and dysbiosis might cause differences in intestinal fermentation. And indeed, intestinal levels of SCFAs are lower after ethanol administration except for levels of acetic acid, which increases as metabolite of ethanol (Xie et al., 2013b). Supplementation of the SCFA butyrate improved the intestinal barrier function following acute, short-term or chronic alcohol exposure in mice, but liver injury was reduced only if ethanol was administered acute or short-term (Cresci et al., 2014).
Furthermore, ethanol directly decreases the biosynthesis of saturated LCFAs by gut microbiota as shown by metagenomics analysis. As a consequence, intestinal amounts of saturated long-chain fatty acids (LCFAs) diminish after alcohol feeding (Chen et al., 2015). Since Lactobacillus spp. are able to utilize saturated LCFAs in vivo and in culture for growth, lower levels of saturated LCFAs (Chen et al., 2015) could explain suppressed amounts of Lactobacillus spp. following chronic alcohol feeding (Kirpich et al., 2008, Yan et al., 2011, Hartmann et al., 2013, Leclercq et al., 2014). Administration of LCFAs to alcohol-fed mice increased levels of Lactobacillus spp., reduced intestinal inflammation, improved intestinal barrier function (Chen et al., 2015), and reduced alcoholic liver disease (Nanji et al., 1995, Nanji et al., 1997, Ronis et al., 2004, You et al., 2005, Kirpich et al., 2012, Zhong et al., 2013, Chen et al., 2015). Additionally, supernatant of Lactobacillus spp. alone has been shown to improve epithelial barrier function in vitro (Cicenia et al., 2014, Chen et al., 2015). Thus, microbial products or metabolites together with reduced amounts of Lactobacillus trigger intestinal inflammation, barrier dysfunction and liver disease following chronic alcohol feeding. This is a good example of how a connection between the microbial metabolome and host has been established. Although the taxonomic composition of the alcohol-associated gut microbiome has been characterized and has advanced our knowledge, we are just starting to understand the functional consequences of dysbiosis. Future studies are required to establish urgently needed links between microbial metabolites and the host that either confer protection against disease or mediate disease.
3.3. Bile acid metabolism
Bile acids are important communicators between the liver and the intestine. Conjugated bile acids are secreted from the hepatic biliary system into the duodenum, are modified in the intestine by bacteria and return to the liver via the enterohepatic circulation. As described earlier, patients with liver cirrhosis exhibit a reduced bile flow (Raedsch et al., 1983). Bile acids induce antimicrobial molecules by activating FXR in intestinal epithelial cells (Inagaki et al., 2006), therefore a reduced bile flow might contribute to intestinal bacterial overgrowth. Further evidence of the interplay between the intestinal microbiome and the bile acid metabolism is given by alcohol feeding experiments in rodents: Ethanol administration to rats results in decreased taurine-conjugated bile acids in liver and intestine, while levels of unconjugated and glycine-conjugated bile acids increase (Xie et al., 2013a). This could be partly explained by intestinal bacterial overgrowth because patients with gastrointestinal bacterial overgrowth exhibit an increased deconjugation of bile acids (Theisen et al., 2000). Patients with chronic alcohol abuse show higher total bile acids, higher lithocholic acid and deoxycholic acid, higher secondary bile acids and a higher secondary-to-primary bile acid ratio in the stool (Kakiyama et al., 2014). However, once the patient develops cirrhosis (in particular if advanced), the fecal amount of total bile acids decreases significantly and serum levels of conjugated bile acids increase (Kakiyama et al., 2013, Kakiyama et al., 2014). Both phenomena might be due to the diminished bile acid secretion into the intestine observed in cirrhotics (Raedsch et al., 1983). Further studies are needed to clarify the interplay of gut microbiota and bile acids in ALD to better understand the pathogenesis and to develop novel pharmaceutical agents to ameliorate the treatment of patients with chronic alcohol abuse. This will better define how the liver communicates to the intestine, since this crosstalk is bidirectional.
Consequences of intestinal dysbiosis that are relevant to the pathogenesis of alcoholic liver disease are illustrated and summarized in Figure 2.
Figure 2. Consequences of intestinal dysbiosis relevant to the pathogenesis of alcoholic liver disease.
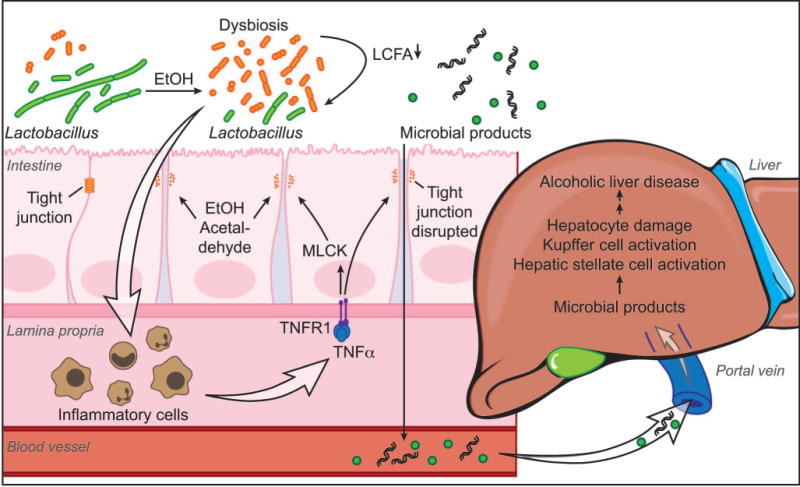
Chronic alcohol consumption leads to intestinal bacterial overgrowth and dysbiosis. Metabolomic changes such as lower bacterial synthesis of long-chain fatty acids (LCFA) result in smaller amounts of ‘good’ bacteria, e.g. Lactobacillus spp. Yet unknown microbial metabolites or products cause intestinal inflammation. While anti-inflammatory properties of intestinal lactobacilli suppress intestinal inflammation during health, reduced amounts of lactobacilli associated with chronic alcohol administration are not any longer able to maintain intestinal homeostasis. Inflammatory cells of the intestinal lamina propria are activated and secrete tumor necrosis factor (TNF)-alpha. TNF-alpha then binds to its receptor TNFR1 on enterocytes, which results in a disruption of tight junctions, partly mediated via myosin-light chain kinase (MLCK). Ethanol and its metabolite acetaldehyde might contribute to a dysfunction of the gut barrier. Microbial products can therefore translocate from the intestinal lumen to the portal venous blood. Translocated microbial products activate hepatic stellate cells and Kupffer cells, and damage hepatocytes. This synergizes with a direct hepatotoxic effect of alcohol and its metabolites to cause progression of alcoholic liver disease.
Conclusion
Chronic alcohol consumption results in small and large intestinal bacterial overgrowth and changes in the taxonomic composition of the intestinal microbiome. Factors that shape the alcohol-associated microbiome are largely unknown. Ethanol and/or acetaldehyde could contribute to a dysfunction of the mucosal barrier by disrupting epithelial tight junctions. Recently, intestinal inflammation has been causatively linked to increased intestinal permeability. Intestinal inflammation precedes heightened gut permeability, and intestinal decontamination prevents intestinal inflammation and increased intestinal permeability. Which products or metabolites from the dysbiotic microbiota initiate intestinal inflammation requires further studies. Pathological bacterial translocation appears to be the only currently known pathogenic factor linking intestinal dysbiosis to progression of alcoholic liver disease. Given excellent examples of how metagenomic or metabolomic factors affect the progression of other liver diseases such as non-alcoholic fatty liver disease and steatohepatitis (NAFLD/NASH) (Schnabl and Brenner, 2014, Boursier and Diehl, 2015), it is unlikely that an increased gut permeability is the only critical intestinal component for progression of alcoholic liver disease. We have recently discovered metabolites, i.e. saturated long-chain fatty acids, whose reduced intestinal concentrations contribute to alcohol-associated dysbiosis and affect alcoholic liver disease (Chen et al., 2015). Identification of other pathways linking the microbiota to alcoholic liver disease is challenging, but could be a key for a better understanding of the gut-liver axis and for designing interventional trials.
Supplementary Material
Acknowledgments
We thank Diantha La Vine for help with the illustration.
The manuscript was supported in part by the Deutsche Forschungsgemeinschaft (DFG) grant 7336/1-1 (to PH), NIH grants K08 DK081830, R01 AA020703, U01 AA021856 (to BS), and by Award Number I01BX002213 from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (to BS).
Abbreviations
- ADH
alcohol dehydrogenase
- ALD
alcoholic liver disease
- CDR
cirrhosis dysbiosis ratio
- CYP
cytochrome P450 enzyme
- FXR
Farnesoid X Receptor
- IL
interleukin
- iNOS
inducible nitric oxide synthases
- LCFAs
long-chain fatty acids
- LPS
lipopolysaccharide
- MLCK
myosin-light chain kinase
- PAMPs
pathogen-associated molecular patterns
- Reg3
regenerating islet-derived 3
- SCFAs
short-chain fatty acids
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
Footnotes
Conflict of interest: The authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- ABDELMEGEED MA, BANERJEE A, JANG S, YOO SH, YUN JW, GONZALEZ FJ, KESHAVARZIAN A, SONG BJ. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free radical biology & medicine. 2013;65:1238–45. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADACHI Y, BRADFORD BU, GAO W, BOJES HK, THURMAN RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–60. [PubMed] [Google Scholar]
- ADACHI Y, MOORE LE, BRADFORD BU, GAO W, THURMAN RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–24. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- ADDOLORATO G, MONTALTO M, CAPRISTO E, CERTO M, FEDELI G, GENTILONI N, STEFANINI GF, GASBARRINI G. Influence of alcohol on gastrointestinal motility: lactulose breath hydrogen testing in orocecal transit time in chronic alcoholics, social drinkers and teetotaler subjects. Hepato-gastroenterology. 1997;44:1076–81. [PubMed] [Google Scholar]
- AKIRA S, UEMATSU S, TAKEUCHI O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- ARVANITI V, D’AMICO G, FEDE G, MANOUSOU P, TSOCHATZIS E, PLEGUEZUELO M, BURROUGHS AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–56. 1256 e1–5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- BAJAJ JS, COX IJ, BETRAPALLY NS, HEUMAN DM, SCHUBERT ML, RATNESWARAN M, HYLEMON PB, WHITE MB, DAITA K, NOBLE NA, SIKAROODI M, WILLIAMS R, CROSSEY MM, TAYLOR-ROBINSON SD, GILLEVET PM. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. American journal of physiology. Gastrointestinal and liver physiology. 2014a;307:G951–7. doi: 10.1152/ajpgi.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAJAJ JS, HEUMAN DM, HYLEMON PB, SANYAL AJ, PURI P, STERLING RK, LUKETIC V, STRAVITZ RT, SIDDIQUI MS, FUCHS M, THACKER LR, WADE JB, DAITA K, SISTRUN S, WHITE MB, NOBLE NA, THORPE C, KAKIYAMA G, PANDAK WM, SIKAROODI M, GILLEVET PM. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2014b;39:1113–25. doi: 10.1111/apt.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAJAJ JS, HEUMAN DM, HYLEMON PB, SANYAL AJ, WHITE MB, MONTEITH P, NOBLE NA, UNSER AB, DAITA K, FISHER AR, SIKAROODI M, GILLEVET PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. Journal of hepatology. 2014c;60:940–7. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAJAJ JS, HEUMAN DM, SANYAL AJ, HYLEMON PB, STERLING RK, STRAVITZ RT, FUCHS M, RIDLON JM, DAITA K, MONTEITH P, NOBLE NA, WHITE MB, FISHER A, SIKAROODI M, RANGWALA H, GILLEVET PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PloS one. 2013;8:e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAJAJ JS, HYLEMON PB, RIDLON JM, HEUMAN DM, DAITA K, WHITE MB, MONTEITH P, NOBLE NA, SIKAROODI M, GILLEVET PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. American journal of physiology. Gastrointestinal and liver physiology. 2012a;303:G675–85. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAJAJ JS, RIDLON JM, HYLEMON PB, THACKER LR, HEUMAN DM, SMITH S, SIKAROODI M, GILLEVET PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. American journal of physiology. Gastrointestinal and liver physiology. 2012b;302:G168–75. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANAN A, CHOUDHARY S, ZHANG Y, FIELDS JZ, KESHAVARZIAN A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. The Journal of pharmacology and experimental therapeutics. 1999;291:1075–85. [PubMed] [Google Scholar]
- BAUER TM, SCHWACHA H, STEINBRUCKNER B, BRINKMANN FE, DITZEN AK, KIST M, BLUM HE. Diagnosis of small intestinal bacterial overgrowth in patients with cirrhosis of the liver: poor performance of the glucose breath hydrogen test. Journal of hepatology. 2000;33:382–6. doi: 10.1016/s0168-8278(00)80273-1. [DOI] [PubMed] [Google Scholar]
- BERG RD, GARLINGTON AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infection and immunity. 1979;23:403–11. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODE C, SCHAFER C, FUKUI H, BODE JC. Effect of treatment with paromomycin on endotoxemia in patients with alcoholic liver disease–a double-blind, placebo-controlled trial. Alcoholism, clinical and experimental research. 1997;21:1367–73. [PubMed] [Google Scholar]
- BODE JC, BODE C, HEIDELBACH R, DURR HK, MARTINI GA. Jejunal microflora in patients with chronic alcohol abuse. Hepato-gastroenterology. 1984;31:30–4. [PubMed] [Google Scholar]
- BOURSIER J, DIEHL AM. Implication of gut microbiota in nonalcoholic Fatty liver disease. PLoS pathogens. 2015;11:e1004559. doi: 10.1371/journal.ppat.1004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULL-OTTERSON L, FENG W, KIRPICH I, WANG Y, QIN X, LIU Y, GOBEJISHVILI L, JOSHI-BARVE S, AYVAZ T, PETROSINO J, KONG M, BARKER D, MCCLAIN C, BARVE S. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PloS one. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS CANESSO M, LACERDA QUEIROZ N, MARCANTONIO C, LAUAR J, ALMEIDA D, GAMBA C, CASSALI G, PEDROSO S, MOREIRA C, SANTOS MARTINS F, NICOLI J, MARTINS TEIXEIRA M, BRUNIALTI GODARD A, THOMAZ VIEIRA A. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC microbiology. 2014;14:240. doi: 10.1186/s12866-014-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASAFONT MORENCOS F, DE LAS HERAS CASTANO G, MARTIN RAMOS L, LOPEZ ARIAS MJ, LEDESMA F, PONS ROMERO F. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Digestive diseases and sciences. 1996;41:552–6. doi: 10.1007/BF02282340. [DOI] [PubMed] [Google Scholar]
- CHARI S, TEYSSEN S, SINGER MV. Alcohol and gastric acid secretion in humans. Gut. 1993;34:843–7. doi: 10.1136/gut.34.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN P, STARKEL P, TURNER JR, HO SB, SCHNABL B. Dysbiosis-induced intestinal inflammation activates TNFRI and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–894. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN P, TORRALBA M, TAN J, EMBREE M, ZENGLER K, STARKEL P, VAN PIJKEREN JP, DEPEW J, LOOMBA R, HO SB, BAJAJ JS, MUTLU EA, KESHAVARZIAN A, TSUKAMOTO H, NELSON KE, FOUTS DE, SCHNABL B. Supplementation of Saturated Long-Chain Fatty Acids Maintains Intestinal Eubiosis and Reduces Ethanol-induced Liver Injury in Mice. Gastroenterology. 2015;148:203–214 e16. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y, YANG F, LU H, WANG B, LEI D, WANG Y, ZHU B, LI L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–72. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- CHO I, YAMANISHI S, COX L, METHE BA, ZAVADIL J, LI K, GAO Z, MAHANA D, RAJU K, TEITLER I, LI H, ALEKSEYENKO AV, BLASER MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CICENIA A, SCIROCCO A, CARABOTTI M, PALLOTTA L, MARIGNANI M, SEVERI C. Postbiotic activities of lactobacilli-derived factors. Journal of clinical gastroenterology. 2014;48(Suppl 1):S18–22. doi: 10.1097/MCG.0000000000000231. [DOI] [PubMed] [Google Scholar]
- CRESCI G, NAGY LE, GANAPATHY V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN. Journal of parenteral and enteral nutrition. 2013;37:763–74. doi: 10.1177/0148607113486809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESCI GA, BUSH K, NAGY LE. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcoholism, clinical and experimental research. 2014;38:1489–501. doi: 10.1111/acer.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHIMAN RK, RANA B, AGRAWAL S, GARG A, CHOPRA M, THUMBURU KK, KHATTRI A, MALHOTRA S, DUSEJA A, CHAWLA YK. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147:1327–37 e3. doi: 10.1053/j.gastro.2014.08.031. [DOI] [PubMed] [Google Scholar]
- DINOSO VP, JR, CHEY WY, BRAVERMAN SP, ROSEN AP, OTTENBERG D, LORBER SH. Gastric secretion and gastric mucosal morphology in chronic alcoholics. Archives of internal medicine. 1972;130:715–9. [PubMed] [Google Scholar]
- FADL AA, SHA J, KLIMPEL GR, OLANO JP, NIESEL DW, CHOPRA AK. Murein lipoprotein is a critical outer membrane component involved in Salmonella enterica serovar typhimurium systemic infection. Infection and immunity. 2005;73:1081–96. doi: 10.1128/IAI.73.2.1081-1096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRIER L, BERARD F, DEBRAUWER L, CHABO C, LANGELLA P, BUENO L, FIORAMONTI J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. The American journal of pathology. 2006;168:1148–54. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING S, TORATANI S, SHEA-DONOHUE T, KASHIWABARA Y, VOGEL SN, METCALF ES. Pro- and anti-inflammatory gene expression in the murine small intestine and liver after chronic exposure to alcohol. Alcoholism, clinical and experimental research. 2001;25:579–89. [PubMed] [Google Scholar]
- GABBARD SL, LACY BE, LEVINE GM, CROWELL MD. The impact of alcohol consumption and cholecystectomy on small intestinal bacterial overgrowth. Digestive diseases and sciences. 2014;59:638–44. doi: 10.1007/s10620-013-2960-y. [DOI] [PubMed] [Google Scholar]
- GAO B, BATALLER R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILL SR, POP M, DEBOY RT, ECKBURG PB, TURNBAUGH PJ, SAMUEL BS, GORDON JI, RELMAN DA, FRASER-LIGGETT CM, NELSON KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODRICH JK, WATERS JL, POOLE AC, SUTTER JL, KOREN O, BLEKHMAN R, BEAUMONT M, VAN TREUREN W, KNIGHT R, BELL JT, SPECTOR TD, CLARK AG, LEY RE. Human genetics shape the gut microbiome. Cell. 2014;159:789–99. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUARNER C, RUNYON BA, YOUNG S, HECK M, SHEIKH MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. Journal of hepatology. 1997;26:1372–8. doi: 10.1016/s0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- GUPTA A, DHIMAN RK, KUMARI S, RANA S, AGARWAL R, DUSEJA A, CHAWLA Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. Journal of hepatology. 2010;53:849–55. doi: 10.1016/j.jhep.2010.05.017. [DOI] [PubMed] [Google Scholar]
- HARTMANN P, CHEN P, WANG HJ, WANG L, MCCOLE DF, BRANDL K, STARKEL P, BELZER C, HELLERBRAND C, TSUKAMOTO H, HO SB, SCHNABL B. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–19. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMANN P, CHEN WC, SCHNABL B. The intestinal microbiome and the leaky gut as therapeutic targets in alcoholic liver disease. Frontiers in physiology. 2012;3:402. doi: 10.3389/fphys.2012.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRITZ I, MANDREKAR P, VELAYUDHAM A, CATALANO D, DOLGANIUC A, KODYS K, KURT-JONES E, SZABO G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INAGAKI T, MOSCHETTA A, LEE YK, PENG L, ZHAO G, DOWNES M, YU RT, SHELTON JM, RICHARDSON JA, REPA JJ, MANGELSDORF DJ, KLIEWER SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3920–5. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOKUCHI S, TSUKAMOTO H, PARK E, LIU ZX, BRENNER DA, SEKI E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcoholism, clinical and experimental research. 2011;35:1509–18. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKIYAMA G, HYLEMON PB, ZHOU H, PANDAK WM, HEUMAN DM, KANG DJ, TAKEI H, NITTONO H, RIDLON JM, FUCHS M, GURLEY EC, WANG Y, LIU R, SANYAL AJ, GILLEVET PM, BAJAJ JS. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. American journal of physiology. Gastrointestinal and liver physiology. 2014;306:G929–37. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKIYAMA G, PANDAK WM, GILLEVET PM, HYLEMON PB, HEUMAN DM, DAITA K, TAKEI H, MUTO A, NITTONO H, RIDLON JM, WHITE MB, NOBLE NA, MONTEITH P, FUCHS M, THACKER LR, SIKAROODI M, BAJAJ JS. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. Journal of hepatology. 2013;58:949–55. doi: 10.1016/j.jhep.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARAA A, THOMPSON KJ, MCKILLOP IH, CLEMENS MG, SCHRUM LW. S-adenosyl-L-methionine attenuates oxidative stress and hepatic stellate cell activation in an ethanol-LPS-induced fibrotic rat model. Shock. 2008;30:197–205. doi: 10.1097/shk.0b013e318160f417. [DOI] [PubMed] [Google Scholar]
- KERLIN P, WONG L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–8. doi: 10.1016/0016-5085(88)90173-4. [DOI] [PubMed] [Google Scholar]
- KIRPICH IA, FENG W, WANG Y, LIU Y, BARKER DF, BARVE SS, MCCLAIN CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcoholism, clinical and experimental research. 2012;36:835–46. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRPICH IA, SOLOVIEVA NV, LEIKHTER SN, SHIDAKOVA NA, LEBEDEVA OV, SIDOROV PI, BAZHUKOVA TA, SOLOVIEV AG, BARVE SS, MCCLAIN CJ, CAVE M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–82. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUDO H, TAKAHARA T, YATA Y, KAWAI K, ZHANG W, SUGIYAMA T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. Journal of hepatology. 2009;51:168–75. doi: 10.1016/j.jhep.2009.02.032. [DOI] [PubMed] [Google Scholar]
- LATA J, NOVOTNY I, PRIBRAMSKA V, JURANKOVA J, FRIC P, KROUPA R, STIBUREK O. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. European journal of gastroenterology & hepatology. 2007;19:1111–3. doi: 10.1097/MEG.0b013e3282efa40e. [DOI] [PubMed] [Google Scholar]
- LECLERCQ S, MATAMOROS S, CANI PD, NEYRINCK AM, JAMAR F, STARKEL P, WINDEY K, TREMAROLI V, BACKHED F, VERBEKE K, DE TIMARY P, DELZENNE NM. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4485–93. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVENE AP, GOLDIN RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61:141–52. doi: 10.1111/j.1365-2559.2011.04145.x. [DOI] [PubMed] [Google Scholar]
- LEY RE, TURNBAUGH PJ, KLEIN S, GORDON JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- LICHTMAN SN, SARTOR RB, KEKU J, SCHWAB JH. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414–23. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- LIEBER CS, JONES DP, DECARLI LM. Effects of Prolonged Ethanol Intake: Production of Fatty Liver Despite Adequate Diets. The Journal of clinical investigation. 1965;44:1009–21. doi: 10.1172/JCI105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN RS, LEE FY, LEE SD, TSAI YT, LIN HC, LU RH, HSU WC, HUANG CC, WANG SS, LO KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. Journal of hepatology. 1995;22:165–72. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- LIU J. Ethanol and liver: recent insights into the mechanisms of ethanol-induced fatty liver. World journal of gastroenterology : WJG. 2014;20:14672–85. doi: 10.3748/wjg.v20.i40.14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Q, DUAN ZP, HA DK, BENGMARK S, KURTOVIC J, RIORDAN SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–9. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- LOGUERCIO C, FEDERICO A, TUCCILLO C, TERRACCIANO F, D’AURIA MV, DE SIMONE C, DEL VECCHIO BLANCO C. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. Journal of clinical gastroenterology. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- LOOMBA R, BETTENCOURT R, BARRETT-CONNOR E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Alimentary pharmacology & therapeutics. 2009;30:1137–49. doi: 10.1111/j.1365-2036.2009.04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORENZO-ZUNIGA V, BARTOLI R, PLANAS R, HOFMANN AF, VINADO B, HAGEY LR, HERNANDEZ JM, MANE J, ALVAREZ MA, AUSINA V, GASSULL MA. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551–7. doi: 10.1053/jhep.2003.50116. [DOI] [PubMed] [Google Scholar]
- LUNIA MK, SHARMA BC, SHARMA P, SACHDEVA S, SRIVASTAVA S. Probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12:1003–8 e1. doi: 10.1016/j.cgh.2013.11.006. [DOI] [PubMed] [Google Scholar]
- MADRID AM, HURTADO C, VENEGAS M, CUMSILLE F, DEFILIPPI C. Long-Term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. The American journal of gastroenterology. 2001;96:1251–5. doi: 10.1111/j.1572-0241.2001.03636.x. [DOI] [PubMed] [Google Scholar]
- MAZAGOVA M, WANG L, ANFORA AT, WISSMUELLER M, LESLEY SA, MIYAMOTO Y, ECKMANN L, DHUNGANA S, PATHMASIRI W, SUMNER S, WESTWATER C, BRENNER DA, SCHNABL B. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015 Mar;29(3):1043–55. doi: 10.1096/fj.14-259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLOUGHLIN RM, MILLS KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. The Journal of allergy and clinical immunology. 2011;127:1097–107. doi: 10.1016/j.jaci.2011.02.012. quiz 1108–9. [DOI] [PubMed] [Google Scholar]
- MONZONI A, MASUTTI F, SACCOCCIO G, BELLENTANI S, TIRIBELLI C, GIACCA M. Genetic determinants of ethanol-induced liver damage. Molecular medicine. 2001;7:255–62. [PMC free article] [PubMed] [Google Scholar]
- MUTLU E, KESHAVARZIAN A, ENGEN P, FORSYTH CB, SIKAROODI M, GILLEVET P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcoholism, clinical and experimental research. 2009;33:1836–46. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUTLU EA, GILLEVET PM, RANGWALA H, SIKAROODI M, NAQVI A, ENGEN PA, KWASNY M, LAU CK, KESHAVARZIAN A. Colonic microbiome is altered in alcoholism. American journal of physiology. Gastrointestinal and liver physiology. 2012;302:G966–78. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANJI AA, KHETTRY U, SADRZADEH SM, YAMANAKA T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. The American journal of pathology. 1993;142:367–73. [PMC free article] [PubMed] [Google Scholar]
- NANJI AA, SADRZADEH SM, YANG EK, FOGT F, MEYDANI M, DANNENBERG AJ. Dietary saturated fatty acids: a novel treatment for alcoholic liver disease. Gastroenterology. 1995;109:547–54. doi: 10.1016/0016-5085(95)90344-5. [DOI] [PubMed] [Google Scholar]
- NANJI AA, ZAKIM D, RAHEMTULLA A, DALY T, MIAO L, ZHAO S, KHWAJA S, TAHAN SR, DANNENBERG AJ. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatology. 1997;26:1538–45. doi: 10.1002/hep.510260622. [DOI] [PubMed] [Google Scholar]
- O’SHEA RS, DASARATHY S, MCCULLOUGH AJ. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- PARLESAK A, SCHAFER C, SCHUTZ T, BODE JC, BODE C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. Journal of hepatology. 2000;32:742–7. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- PUROHIT V, BODE JC, BODE C, BRENNER DA, CHOUDHRY MA, HAMILTON F, KANG YJ, KESHAVARZIAN A, RAO R, SARTOR RB, SWANSON C, TURNER JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–61. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAEDSCH R, STIEHL A, GUNDERT-REMY U, WALKER S, SIEG A, CZYGAN P, KOMMERELL B. Hepatic secretion of bilirubin and biliary lipids in patients with alcoholic cirrhosis of the liver. Digestion. 1983;26:80–8. doi: 10.1159/000198872. [DOI] [PubMed] [Google Scholar]
- RAO RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcoholism, clinical and experimental research. 1998;22:1724–30. [PubMed] [Google Scholar]
- REHM J, SAMOKHVALOV AV, SHIELD KD. Global burden of alcoholic liver diseases. Journal of hepatology. 2013;59:160–8. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- RONIS MJ, KOROURIAN S, ZIPPERMAN M, HAKKAK R, BADGER TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. The Journal of nutrition. 2004;134:904–12. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- SANCHEZ E, CASAFONT F, GUERRA A, DE BENITO I, PONS-ROMERO F. Role of intestinal bacterial overgrowth and intestinal motility in bacterial translocation in experimental cirrhosis. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva. 2005;97:805–14. doi: 10.4321/s1130-01082005001100005. [DOI] [PubMed] [Google Scholar]
- SATO N, LINDROS KO, BARAONA E, IKEJIMA K, MEZEY E, JARVELAINEN HA, RAMCHANDANI VA. Sex difference in alcohol-related organ injury. Alcoholism, clinical and experimental research. 2001;25:40S–45S. doi: 10.1097/00000374-200105051-00007. [DOI] [PubMed] [Google Scholar]
- SCHNABL B, BRENNER DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–24. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKI E, SCHNABL B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. The Journal of physiology. 2012;590:447–58. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINDO K, MACHIDA M, MIYAKAWA K, FUKUMURA M. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. The American journal of gastroenterology. 1993;88:2084–91. [PubMed] [Google Scholar]
- SIMREN M, STOTZER PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO G. Gut-Liver Axis in Alcoholic Liver Disease. Gastroenterology. 2015;148:30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABATA T, TANI T, ENDO Y, HANASAWA K. Bacterial translocation and peptidoglycan translocation by acute ethanol administration. Journal of gastroenterology. 2002;37:726–31. doi: 10.1007/s005350200118. [DOI] [PubMed] [Google Scholar]
- TAMAI H, KATO S, HORIE Y, OHKI E, YOKOYAMA H, ISHII H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcoholism, clinical and experimental research. 2000;24:390–4. [PubMed] [Google Scholar]
- TANG Y, FORSYTH CB, FARHADI A, RANGAN J, JAKATE S, SHAIKH M, BANAN A, FIELDS JZ, KESHAVARZIAN A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcoholism, clinical and experimental research. 2009;33:1220–30. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEISEN J, NEHRA D, CITRON D, JOHANSSON J, HAGEN JA, CROOKES PF, DEMEESTER SR, BREMNER CG, DEMEESTER TR, PETERS JH. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2000;4:50–4. doi: 10.1016/s1091-255x(00)80032-3. [DOI] [PubMed] [Google Scholar]
- TIAN C, STOKOWSKI RP, KERSHENOBICH D, BALLINGER DG, HINDS DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nature genetics. 2010;42:21–3. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- UESUGI T, FROH M, ARTEEL GE, BRADFORD BU, THURMAN RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- VESTH T, OZEN A, ANDERSEN SC, KAAS RS, LUKJANCENKO O, BOHLIN J, NOOKAEW I, WASSENAAR TM, USSERY DW. Veillonella, Firmicutes: Microbes disguised as Gram negatives. Standards in genomic sciences. 2013;9:431–48. doi: 10.4056/sigs.2981345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE G, ZHONG W, LI H, LI Q, QIU Y, ZHENG X, CHEN H, ZHAO X, ZHANG S, ZHOU Z, ZEISEL SH, JIA W. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013a;27:3583–93. doi: 10.1096/fj.13-231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE G, ZHONG W, ZHENG X, LI Q, QIU Y, LI H, CHEN H, ZHOU Z, JIA W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. Journal of proteome research. 2013b;12:3297–306. doi: 10.1021/pr400362z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU J, LAI KK, VERLINSKY A, LUGEA A, FRENCH SW, COOPER MP, JI C, TSUKAMOTO H. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. Journal of hepatology. 2011;55:673–82. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAN AW, FOUTS DE, BRANDL J, STARKEL P, TORRALBA M, SCHOTT E, TSUKAMOTO H, NELSON KE, BRENNER DA, SCHNABL B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN M, BRADFORD BU, WHEELER MD, UESUGI T, FROH M, GOYERT SM, THURMAN RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. Journal of immunology. 2001;166:4737–42. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- YOU M, CONSIDINE RV, LEONE TC, KELLY DP, CRABB DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–77. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO HY, WANG HJ, LU Z, XU SZ. Intestinal microflora in patients with liver cirrhosis. Chinese journal of digestive diseases. 2004;5:64–7. doi: 10.1111/j.1443-9573.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- ZHONG W, LI Q, XIE G, SUN X, TAN X, JIA W, ZHOU Z. Dietary fat sources differentially modulate intestinal barrier and hepatic inflammation in alcohol-induced liver injury in rats. American journal of physiology. Gastrointestinal and liver physiology. 2013;305:G919–32. doi: 10.1152/ajpgi.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


