Abstract
Indoleacetaldehyde reductase catalyzes the conversion of indoleacetaldehyde to indole ethanol in extracts of Cucumis sativus L., with reduced pyridine nucleotide required as co-substrate. NADH and NADPH result in markedly different enzyme behavior, as reflected in reaction kinetics and in responses to inhibitors and activators. It is not yet clear whether there are two separate enzymes, one specific for NADH and the other for NADPH, or whether there is a single enzyme differentially influenced by the two co-substrates.
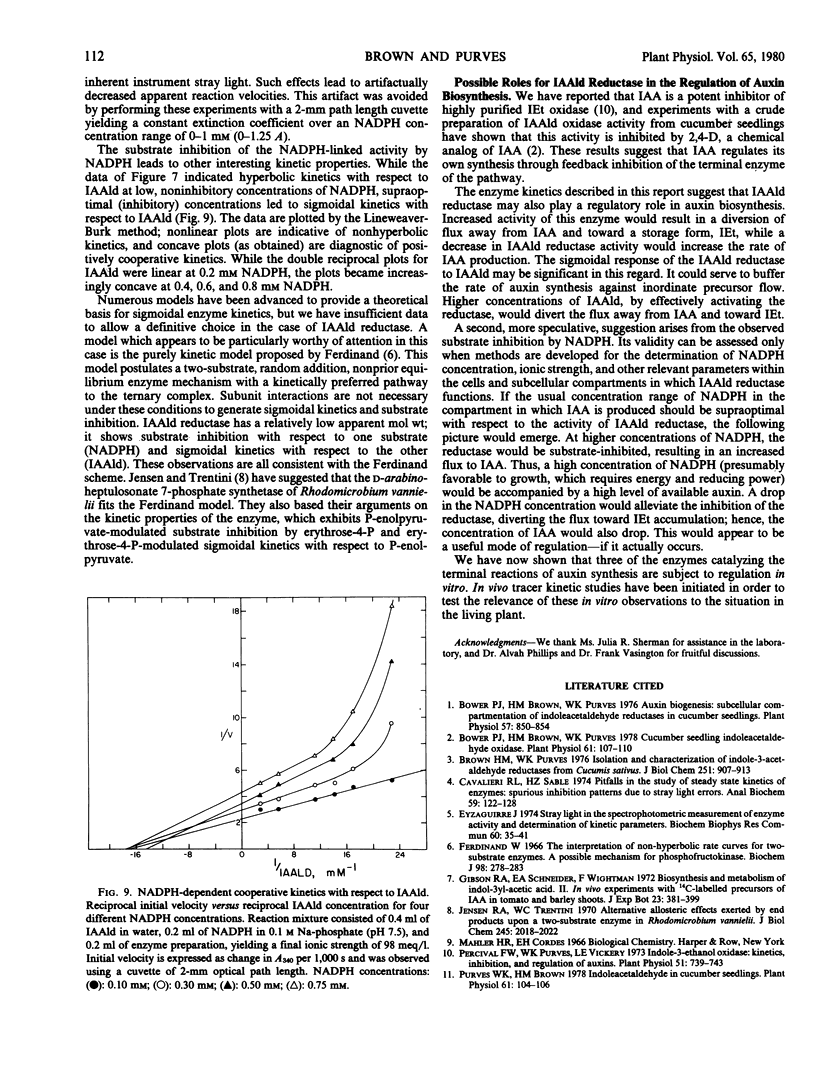
In the presence of NADH, the indoleacetaldehyde reductase activity was inhibited by NaCl and displayed hyperbolic kinetics under all conditions tested. However, in the presence of NADPH the enzyme was activated by NaCl at concentrations up to 0.1 molar. Under certain conditions with NADPH as co-substrate, the enzyme showed kinetics sigmoidal with respect to indoleacetaldehyde concentration and was strongly inhibited by high concentrations of NADPH. It is possible that this substrate inhibition of the NADPH-linked indoleacetaldehyde reductase activity by NADPH, as well as the sigmoidicity with respect to indoleacetaldehyde concentration, may function in the regulation of auxin biosynthesis.
Full text
PDF
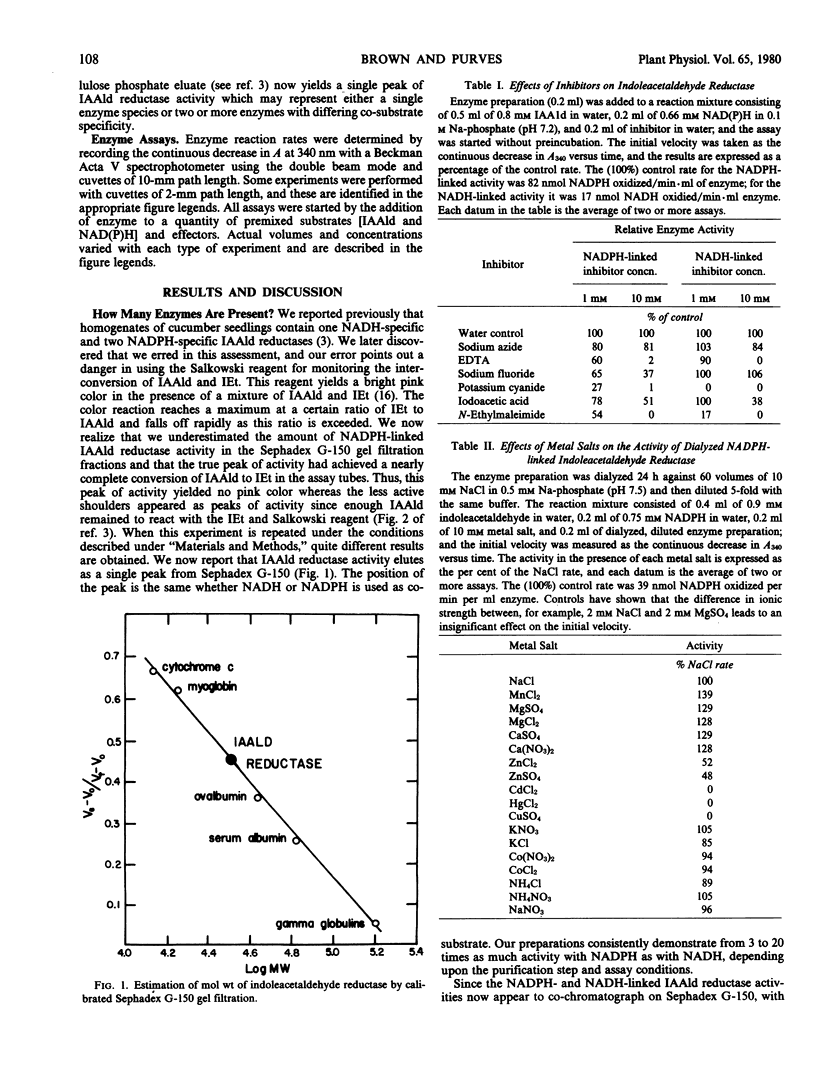
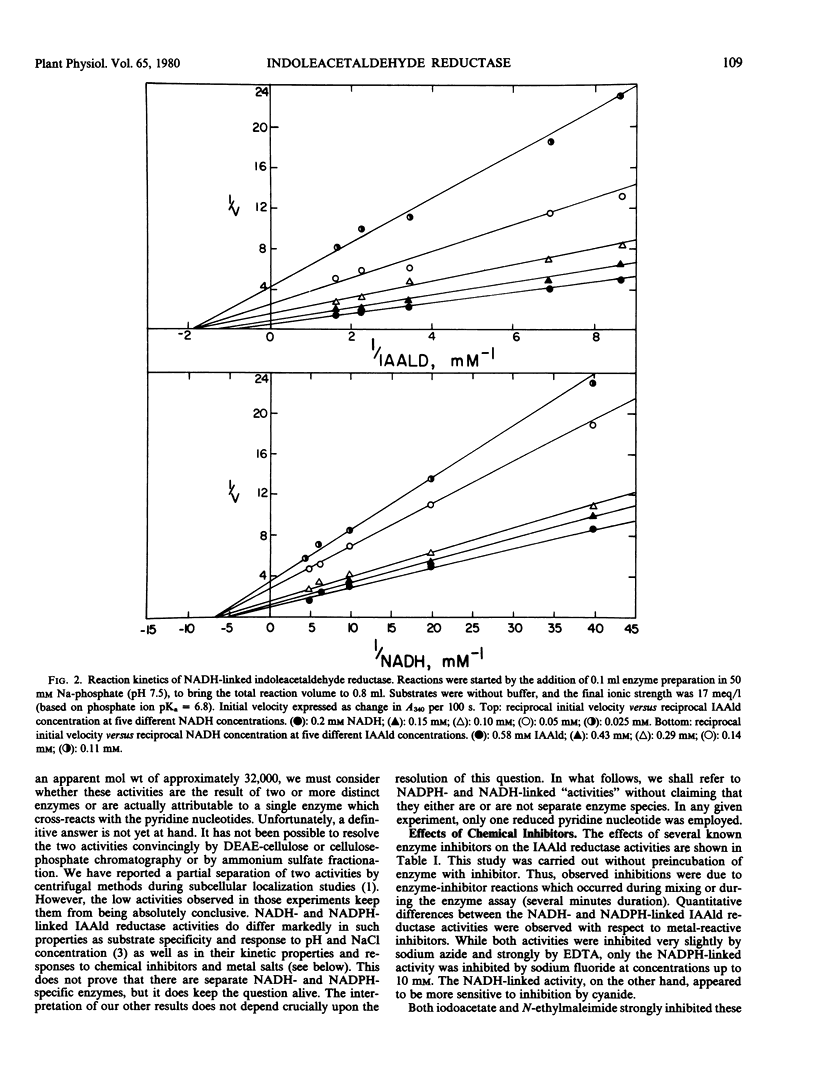
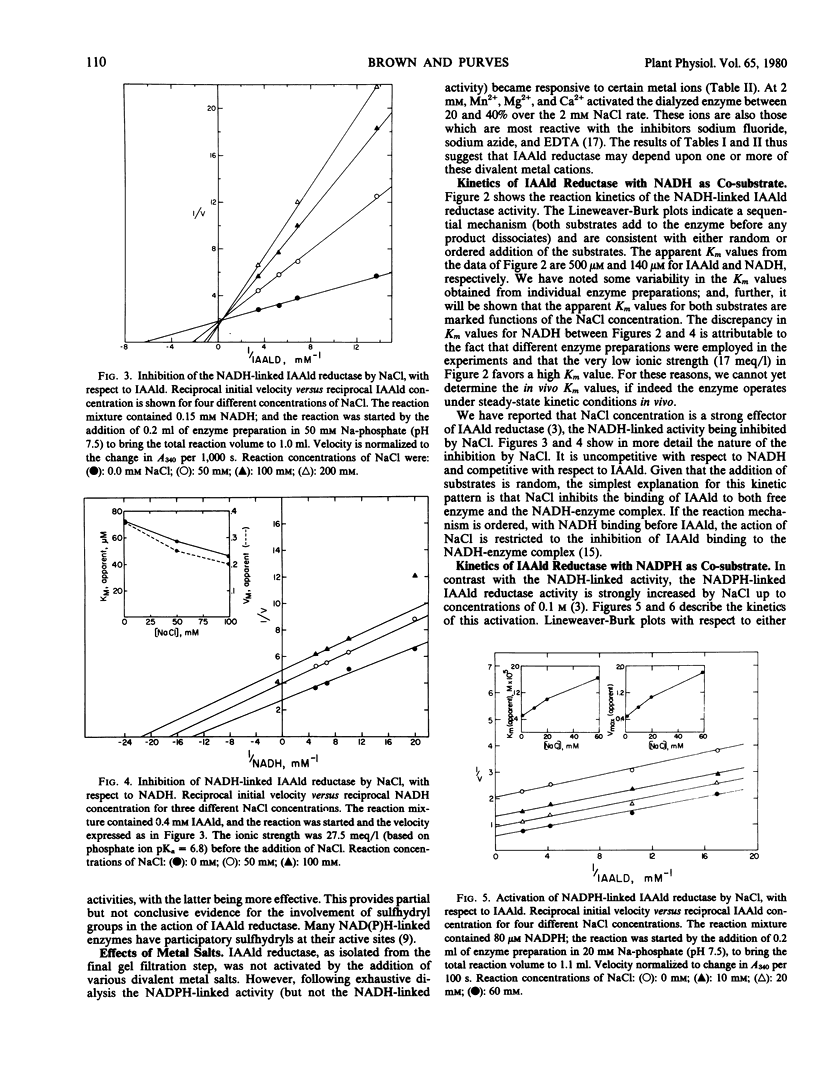
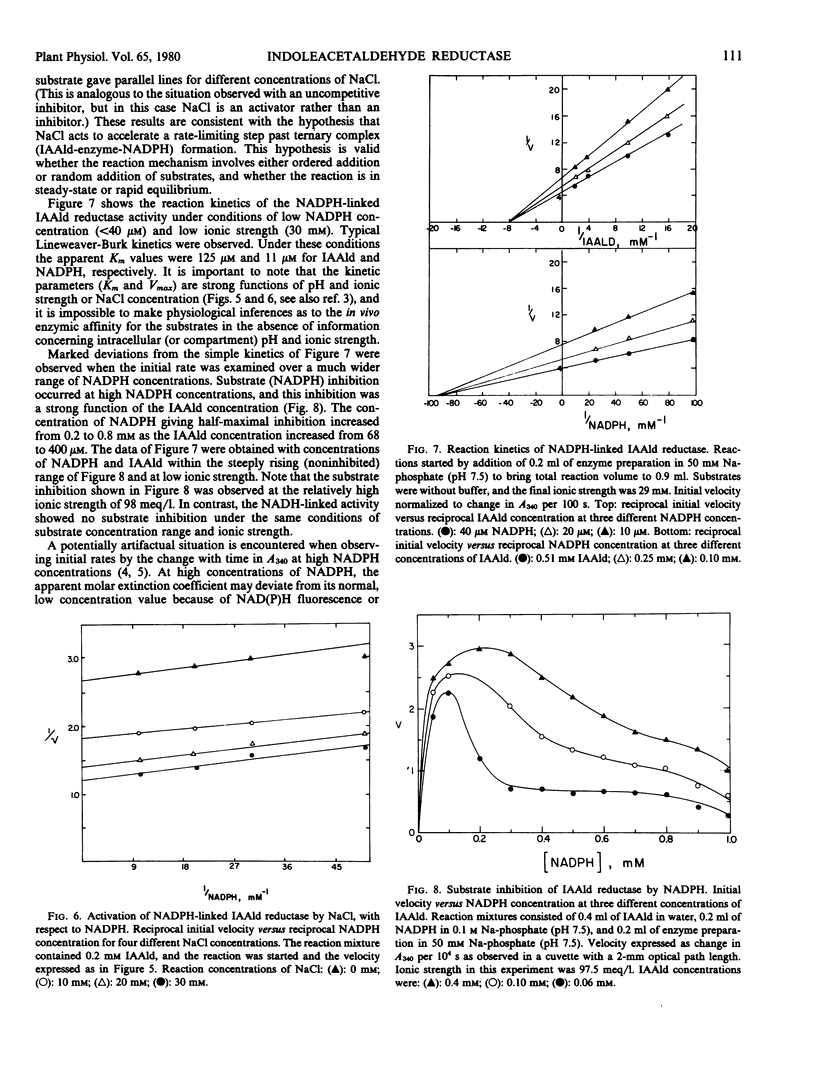

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bower P. J., Brown H. M., Purves W. K. Auxin biogenesis: subcellular compartmentation of indoleacetaldehyde reductases in cucumber seedlings. Plant Physiol. 1976 Jun;57(6):850–854. doi: 10.1104/pp.57.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower P. J., Brown H. M., Purves W. K. Cucumber seedling indoleacetaldehyde oxidase. Plant Physiol. 1978 Jan;61(1):107–110. doi: 10.1104/pp.61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Purves W. K. Isolation and characterization of indole-3-acetaldehyde reductases from Cucumis sativus. J Biol Chem. 1976 Feb 25;251(4):907–913. [PubMed] [Google Scholar]
- Cavalieri R. L., Sable H. Z. Pitfalls in the study of steady state kinetics of enzymes: spurious inhibition patterns due to stray light errors. Anal Biochem. 1974 May;59(1):122–128. doi: 10.1016/0003-2697(74)90016-5. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre J. Stray light interference in the spectrophotometric measurement of enzyme activity and determination of kinetic parameters. Biochem Biophys Res Commun. 1974 Sep 9;60(1):35–41. doi: 10.1016/0006-291x(74)90168-5. [DOI] [PubMed] [Google Scholar]
- Ferdinand W. The interpretation of non-hyperbolic rate curves for two-substrate enzymes. A possible mechanism for phosphofructokinase. Biochem J. 1966 Jan;98(1):278–283. doi: 10.1042/bj0980278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Trentini W. C. Alternative allosteric effects exerted by end products upon a two-substrate enzyme in Rhodomicrobium vannielii. J Biol Chem. 1970 Apr 25;245(8):2018–2022. [PubMed] [Google Scholar]
- Percival F. W., Purves W. K., Vickery L. E. Indole-3-ethanol Oxidase: Kinetics, Inhibition, and Regulation by Auxins. Plant Physiol. 1973 Apr;51(4):739–743. doi: 10.1104/pp.51.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves W. K., Brown H. M. Indoleacetaldehyde in cucumber seedlings. Plant Physiol. 1978 Jan;61(1):104–106. doi: 10.1104/pp.61.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Purves W. K. Isolation and identification of indole-3-ethanol (tryptophol) from cucumber seedlings. Plant Physiol. 1967 Apr;42(4):520–524. doi: 10.1104/pp.42.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery L. E., Purves W. K. Isolation of Indole-3-ethanol Oxidase from Cucumber Seedlings. Plant Physiol. 1972 May;49(5):716–721. doi: 10.1104/pp.49.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]


