Abstract
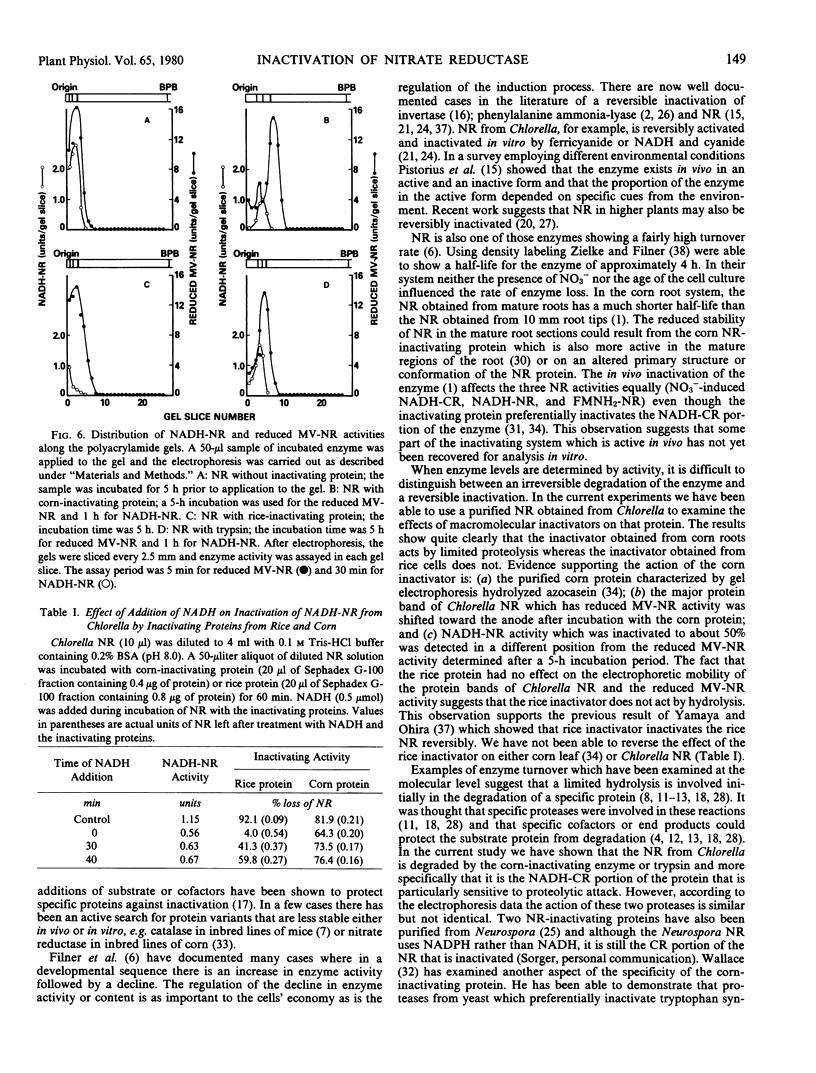
When nitrate reductase (NR) purified from Chlorella was incubated with NR-inactivating proteins purified from corn roots and rice cell suspension cultures or with trypsin there was a loss in NADH-NR and NADH cytochrome c reductase (NADH-CR) activities with time whereas the reduced methylviologen NR (MV-NR) remained active. When NADH-NR and NADH-CR activities were inactivated completely by the incubation with corn protein, the major protein band obtained by polyacrylamide gel electrophoresis shifted from an RF value of 0.12 to an RF of 0.25 and reduced MV-NR activity moved to the new position on the gel. When NADH-NR and NADH-CR activities were partially inactivated by the corn protein, NADH-NR activity was detected in an intermediate position (RF value of 0.18). Incubation with trypsin also caused a change in the NR protein migration pattern (RF value of 0.20). This protein band also had reduced MV-NR activity. Thus, the corn inactivator degrades NR in a fashion similar to but not identical with trypsin. The incubation of NR with rice inactivating protein resulted in a loss of NADH-NR but had no effect on the migration of NR protein or on the reduced MV-NR activity or mobility suggesting that the rice protein binds to Chlorella NR.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Oaks A. Comparative studies on the induction and inactivation of nitrate reductase in corn roots and leaves. Plant Physiol. 1976 Apr;57(4):572–576. doi: 10.1104/pp.57.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billett E. E., Wallace W., Smith H. A specific and reversible macromolecular inhibitor of phenylalanine ammonia-lyase and cinnamic acid-4-hydroxylase in gherkins. Biochim Biophys Acta. 1978 May 11;524(1):219–230. doi: 10.1016/0005-2744(78)90120-1. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Cohen G. N., Stadtman E. R. Some properties of Escherichia coli glutamine synthetase after limited proteolysis by subtilisin. J Biol Chem. 1979 Apr 25;254(8):3124–3128. [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Ganschow R. E., Schimke R. T. Independent genetic control of the catalytic activity and the rate of degradation of catalase in mice. J Biol Chem. 1969 Sep 10;244(17):4649–4658. [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly S. O., Tolbert N. E. NADH-Nitrate Reductase Inhibitor from Soybean Leaves. Plant Physiol. 1978 Aug;62(2):197–203. doi: 10.1104/pp.62.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsunuma T., Schött E., Elsässer S., Holzer H. Purification and properties of tryptophan-synthase-inactivating enzymes from yeast. Eur J Biochem. 1972 Jun 9;27(3):520–526. doi: 10.1111/j.1432-1033.1972.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Kominami E., Kobayashi K., Kominami S., Katunuma N. Properties of a specific protease for pyridoxal enzymes and its biological role. J Biol Chem. 1972 Nov 10;247(21):6848–6855. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lei M., Aebi U., Heidner E. G., Eisenberg D. Limited proteolysis of glutamine synthetase is inhibited by glutamate and by feedback inhibitors. J Biol Chem. 1979 Apr 25;254(8):3129–3134. [PubMed] [Google Scholar]
- Pressey R., Shaw R. Effect of temperature on invertase, invertase inhibitor, and sugars in potato tubers. Plant Physiol. 1966 Dec;41(10):1657–1661. doi: 10.1104/pp.41.10.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Sherrard J. H., Dalling M. J. In vitro stability of nitrate reductase from wheat leaves: I. Stability of highly purified enzyme and its component activities. Plant Physiol. 1979 Feb;63(2):346–353. doi: 10.1104/pp.63.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Lorimer G. H., Hall R. L., Borchers R., Bailey J. L. Reduced nicotinamide adenine dinucleotide-nitrate reductase of Chlorella vulgaris. Purification, prosthetic groups, and molecular properties. J Biol Chem. 1975 Jun 10;250(11):4120–4127. [PubMed] [Google Scholar]
- Solomonson L. P. Purification of NADH-Nitrate Reductase by Affinity Chromatography. Plant Physiol. 1975 Dec;56(6):853–855. doi: 10.1104/pp.56.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Spehar A. M. Stimulation of cyanide formation by ADP and its possible role in the regulation of nitrate reductase. J Biol Chem. 1979 Apr 10;254(7):2176–2179. [PubMed] [Google Scholar]
- Sorger G. J., Premakumar R., Gooden D. Demonstration in vitro of two intracellular inactivators of nitrate reductase from Neurospora. Biochim Biophys Acta. 1978 Apr 19;540(1):33–47. doi: 10.1016/0304-4165(78)90432-4. [DOI] [PubMed] [Google Scholar]
- Wallace W. A Re-evaluation of the Nitrate Reductase Content of the Maize Root. Plant Physiol. 1975 Apr;55(4):774–777. doi: 10.1104/pp.55.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W. Comparison of a nitrate reductase-inactivating enzyme from the maize root with a protease form yeast which inactivates tryptophan synthase. Biochim Biophys Acta. 1978 Jun 9;524(2):418–427. doi: 10.1016/0005-2744(78)90179-1. [DOI] [PubMed] [Google Scholar]
- Wallace W. Effects of a nitrate reductase inactivating enzyme and NAD(P)H on the nitrate reductase from higher plants and Neurospora. Biochim Biophys Acta. 1975 Feb 19;377(2):239–250. doi: 10.1016/0005-2744(75)90306-x. [DOI] [PubMed] [Google Scholar]
- Wallace W. Purification and properties of a nitrate reductase-inactivating enzyme. Biochim Biophys Acta. 1974 Mar 21;341(1):265–276. doi: 10.1016/0005-2744(74)90087-4. [DOI] [PubMed] [Google Scholar]
- Warner R. L., Hageman R. H., Dudley J. W., Lambert R. J. Inheritance of nitrate reductase activity in Zea mays L. Proc Natl Acad Sci U S A. 1969 Mar;62(3):785–792. doi: 10.1073/pnas.62.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya T., Oaks A., Boesel I. L. Characteristics of Nitrate Reductase-inactivating Proteins Obtained from Corn Roots and Rice Cell Cultures. Plant Physiol. 1980 Jan;65(1):141–145. doi: 10.1104/pp.65.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Roche A. I. Increase in linolenic Acid is not a prerequisite for development of freezing tolerance in wheat. Plant Physiol. 1979 Jan;63(1):5–8. doi: 10.1104/pp.63.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]