Abstract
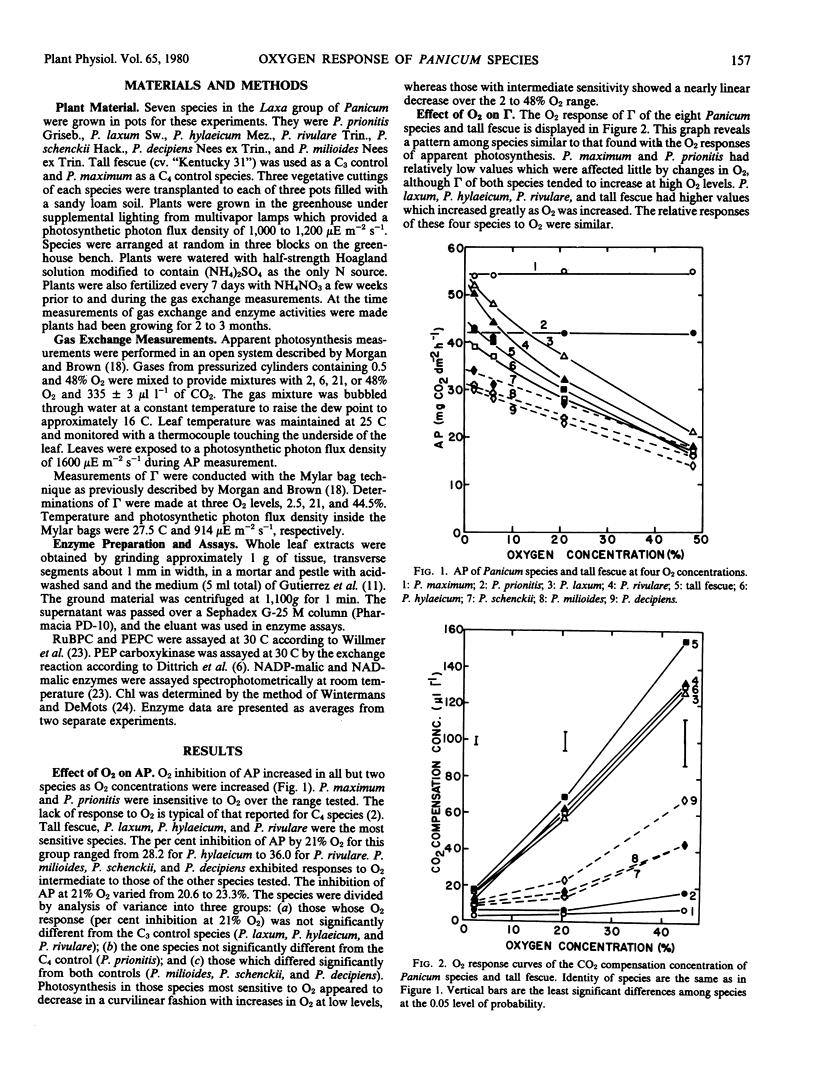
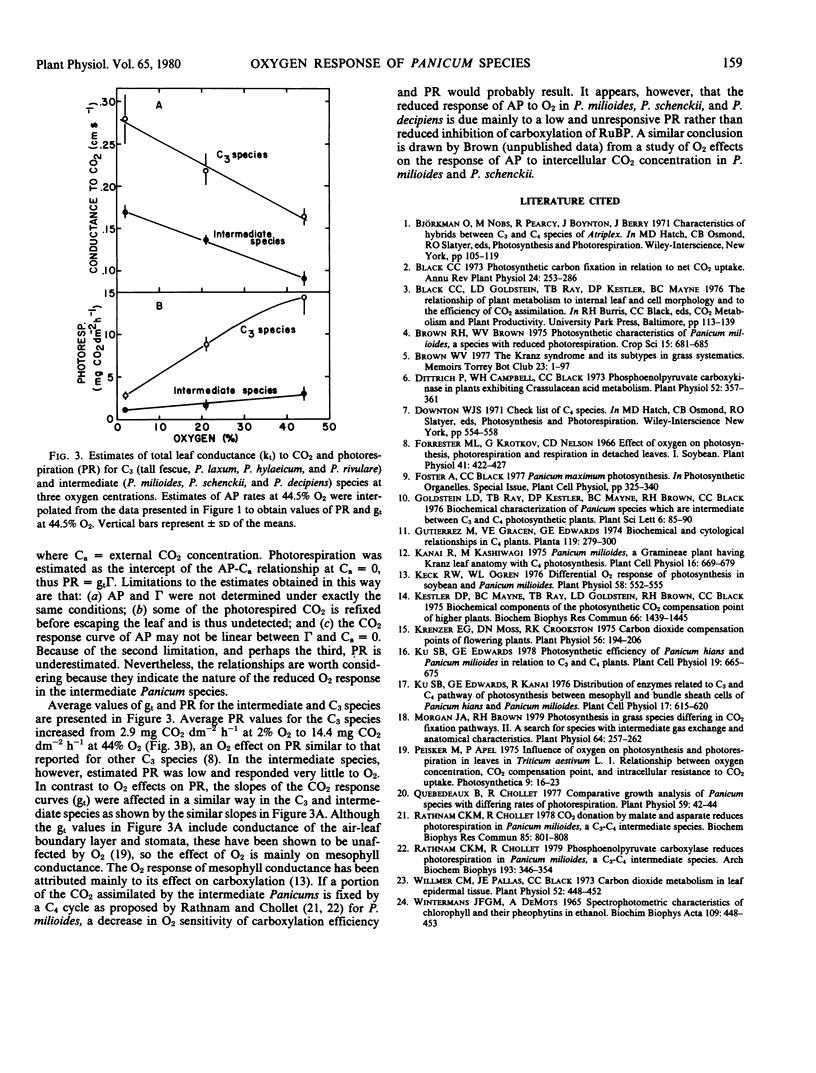
Measurements of CO2 exchange at varying O2 concentrations in seven grass species of the Laxa group of Panicum and activities of five photosynthetic enzymes were compared to values obtained for these characters in a cool season C3 grass, tall fescue (Festuca arundinacea Schreb.) and a C4 grass, P. maximum Jacq. Plants were divided into three groups on the basis of the inhibition of apparent photosynthesis by 21% O2. Rates of apparent photosynthesis in P. prionitis Griseb. and P. maximum were virtually unaffected by changes in O2 concentration. In another group consisting of P. hylaeicum Mez., P. rivulare Trin., P. laxum Sw., and tall fescue apparent photosynthesis was inhibited by 28.2 to 36.0% at 21% O2. An intermediate inhibition of 20.6 to 23.3% at 21% O2 was exhibited by P. milioides Nees ex Trin., P. schenckii Hack., and P. decipiens Nees ex Trin. The CO2 compensation concentration for P. prionitis and P. maximum was low (≤6 microliters per liter CO2 at 21% O2) and affected little by O2, whereas values for P. hylaeicum, P. rivulare, P. laxum, and tall fescue were much greater, and increased almost linearly from 2 to 48% O2. Values for P. milioides, P. schenckii, and P. decipiens were intermediate to the other two groups. The effect of O2 on total leaf conductance to CO2 was similar to the C3 grasses and the intermediate Panicums. However, estimates of photorespiration in the intermediate species were low and changed little with O2 in comparison to estimates for the C3 species which were higher and increased greatly with increased O2.
Activities of phosphoenolpyruvate carboxylase were greatest in P. maximum and P. prionitis and one-fourth or less in the remaining species. Activity of ribulose bisphosphate carboxylase was 548 micromoles per mg chlorophyll per hour in tall fescue; activity in the remaining species was approximately one-fourth or less of that in tall fescue, with the exception of P. rivulare, in which it was 440 micromoles per milligram chlorphyll per hour. High activities of two C4 decarboxylating enzymes, phosphoenolpyruvate carboxykinase and NADP-malic enzyme, were observed in P. maximum (1,988 micromoles per milligram chlorophyll per hour) and P. prionitis (125 micromoles per milligram chlorophyll per hour), respectively. Only minimal activities of decarboxylating enzymes were detected in the remaining species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dittrich P., Campbell W. H., Black C. C. Phosphoenolpyruvate carboxykinase in plants exhibiting crassulacean Acid metabolism. Plant Physiol. 1973 Oct;52(4):357–361. doi: 10.1104/pp.52.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck R. W. Differential Oxygen Response of Photosynthesis in Soybean and Panicum milioides. Plant Physiol. 1976 Oct;58(4):552–555. doi: 10.1104/pp.58.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler D. P., Mayne B. C., Ray T. B., Goldstein L. D., Brown R. H., Black C. C. Biochemical components of the photosynthetic CO2 compensation point of higher plants. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1439–1446. doi: 10.1016/0006-291x(75)90520-3. [DOI] [PubMed] [Google Scholar]
- Krenzer E. G., Moss D. N., Crookston R. K. Carbon dioxide compensation points of flowering plants. Plant Physiol. 1975 Aug;56(2):194–206. doi: 10.1104/pp.56.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A., Brown R. H. Photosynthesis in Grass Species Differing in Carbon Dioxide Fixation Pathways: II. A Search for Species with Intermediate Gas Exchange and Anatomical Characteristics. Plant Physiol. 1979 Aug;64(2):257–262. doi: 10.1104/pp.64.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B., Chollet R. Comparative growth analyses of panicum species with differing rates of photorespiration. Plant Physiol. 1977 Jan;59(1):42–44. doi: 10.1104/pp.59.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Chollet R. CO2 donation by malate and aspartate reduces photorespiration in Panicum milioides, a C3-C4 intermediate species. Biochem Biophys Res Commun. 1978 Nov 29;85(2):801–808. doi: 10.1016/0006-291x(78)91233-0. [DOI] [PubMed] [Google Scholar]
- Rathnam C. K., Chollet R. Phosphoenolpyruvate carboxylase reduces photorespiration in Panicum milioides, a C3-C4 intermediate species. Arch Biochem Biophys. 1979 Apr 1;193(2):346–354. doi: 10.1016/0003-9861(79)90039-0. [DOI] [PubMed] [Google Scholar]
- Willmer C. M., Pallas J. E., Black C. C. Carbon dioxide metabolism in leaf epidermal tissue. Plant Physiol. 1973 Nov;52(5):448–452. doi: 10.1104/pp.52.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]



