Abstract
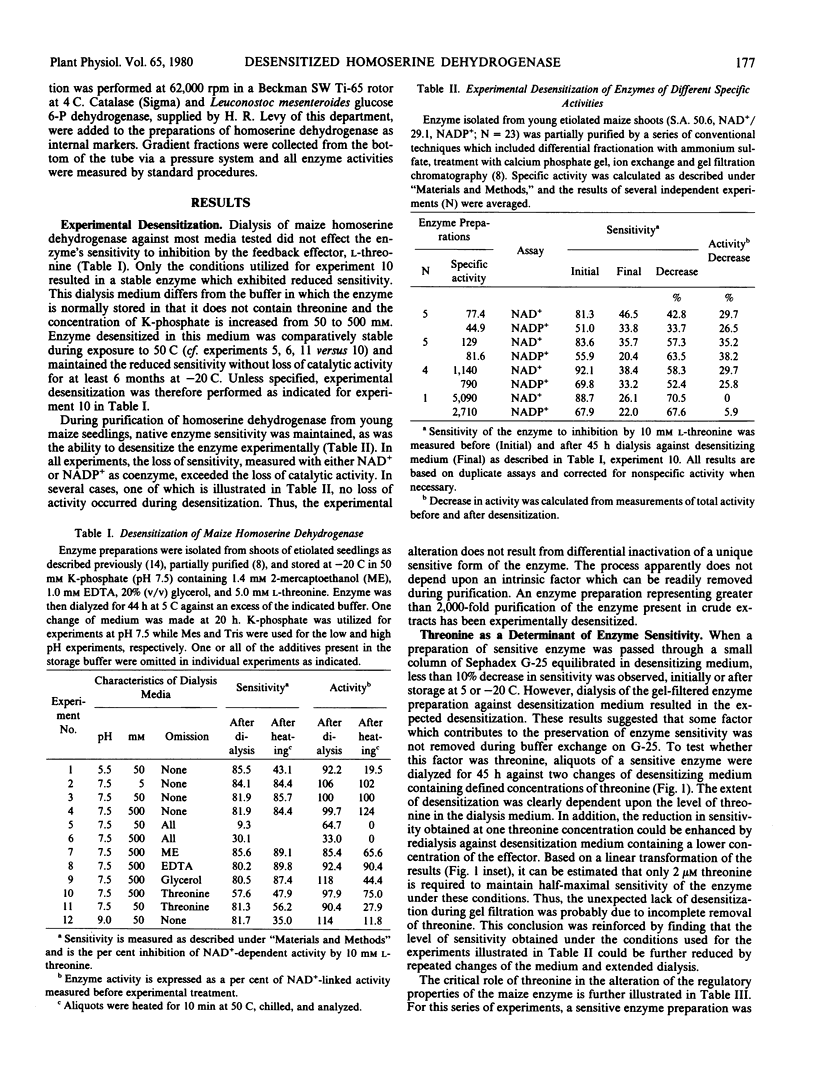
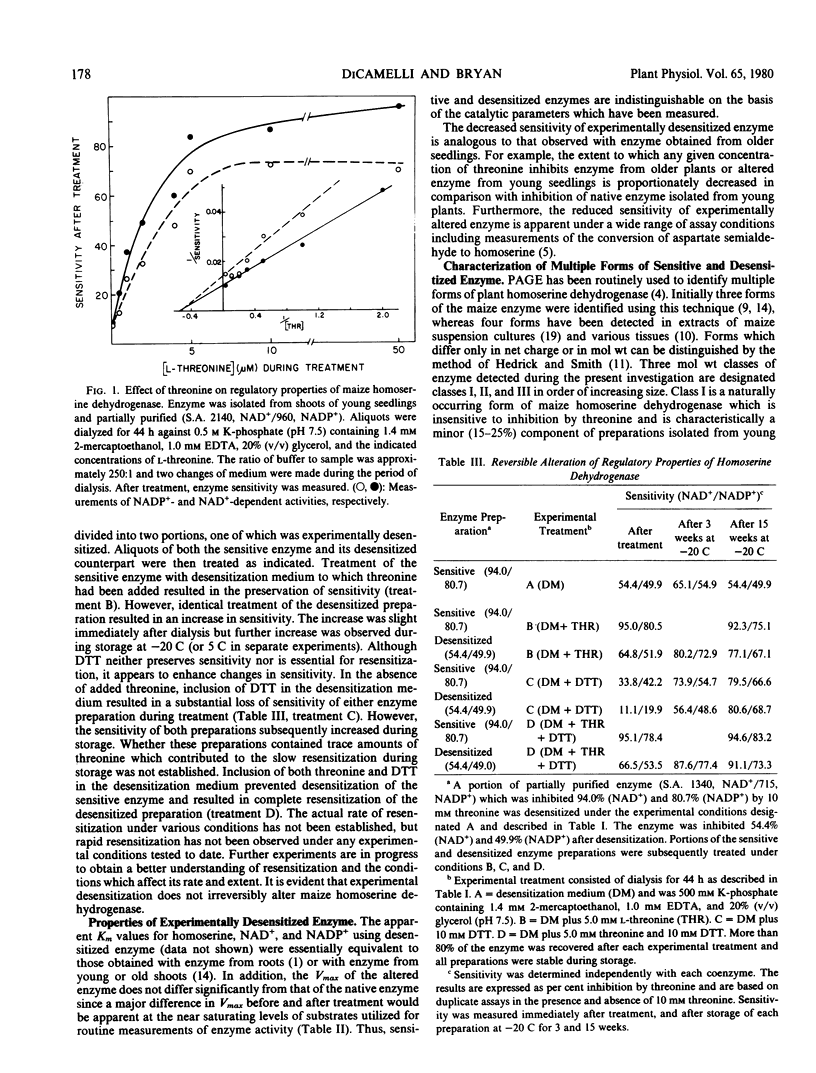
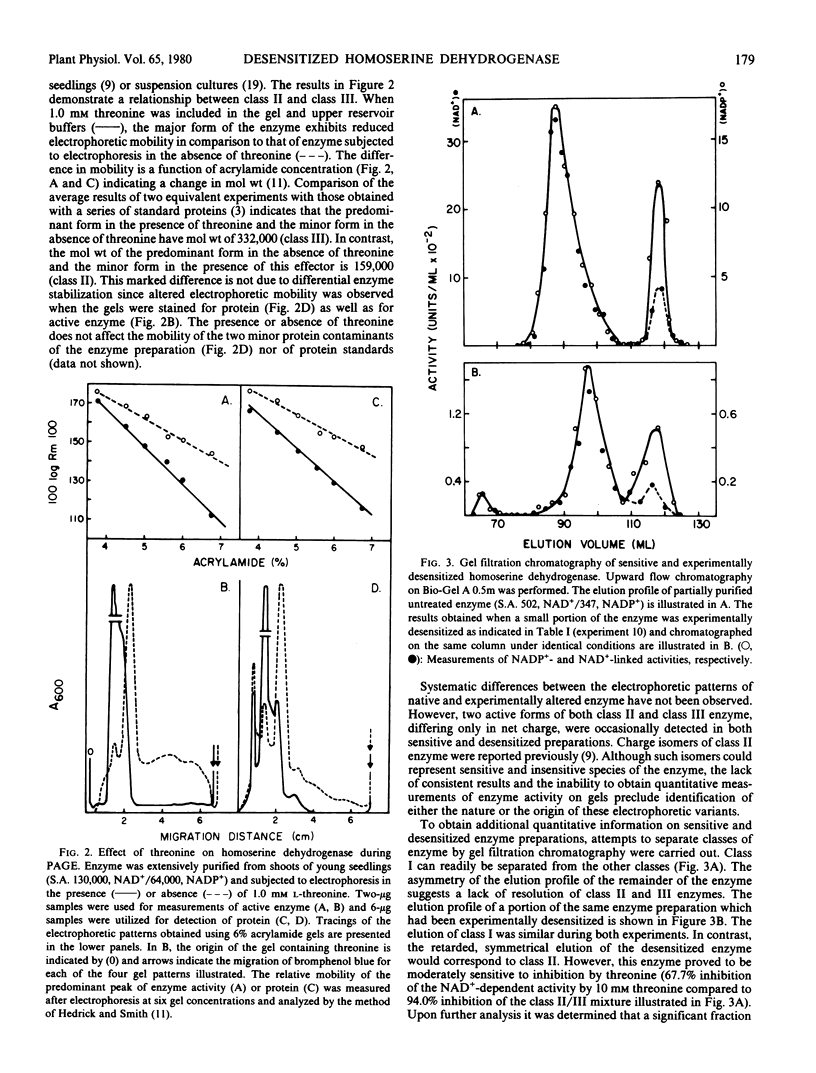
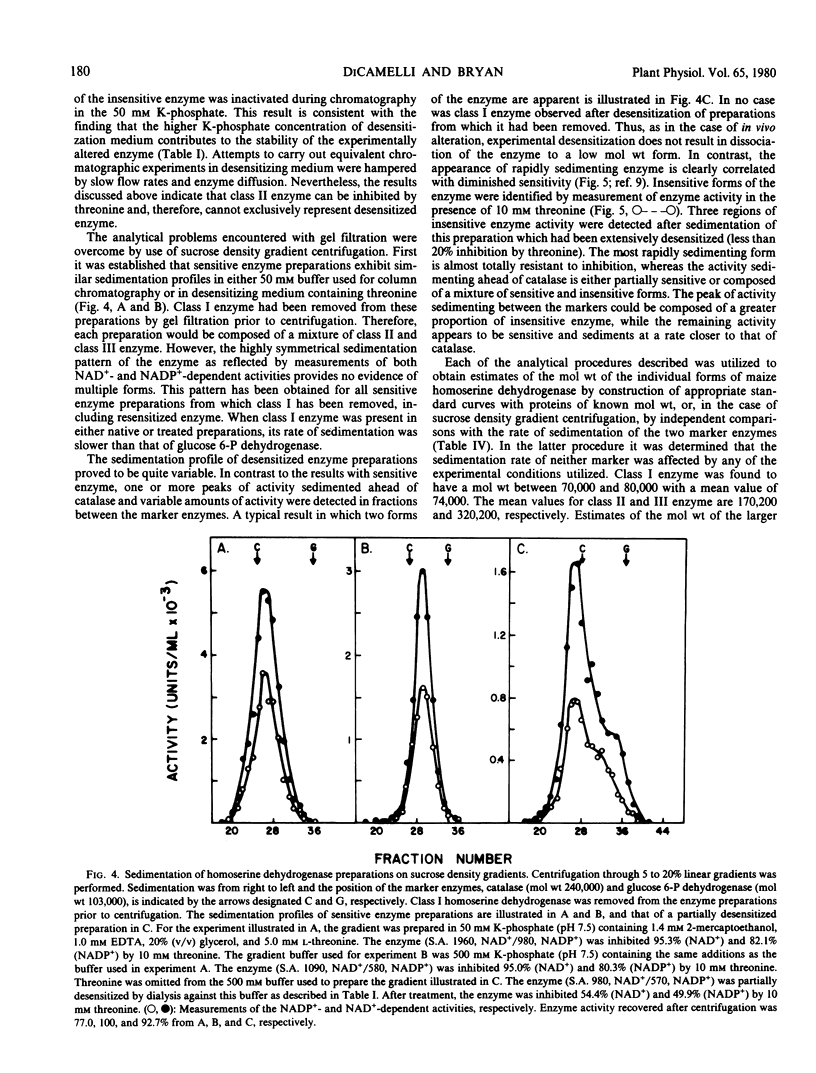
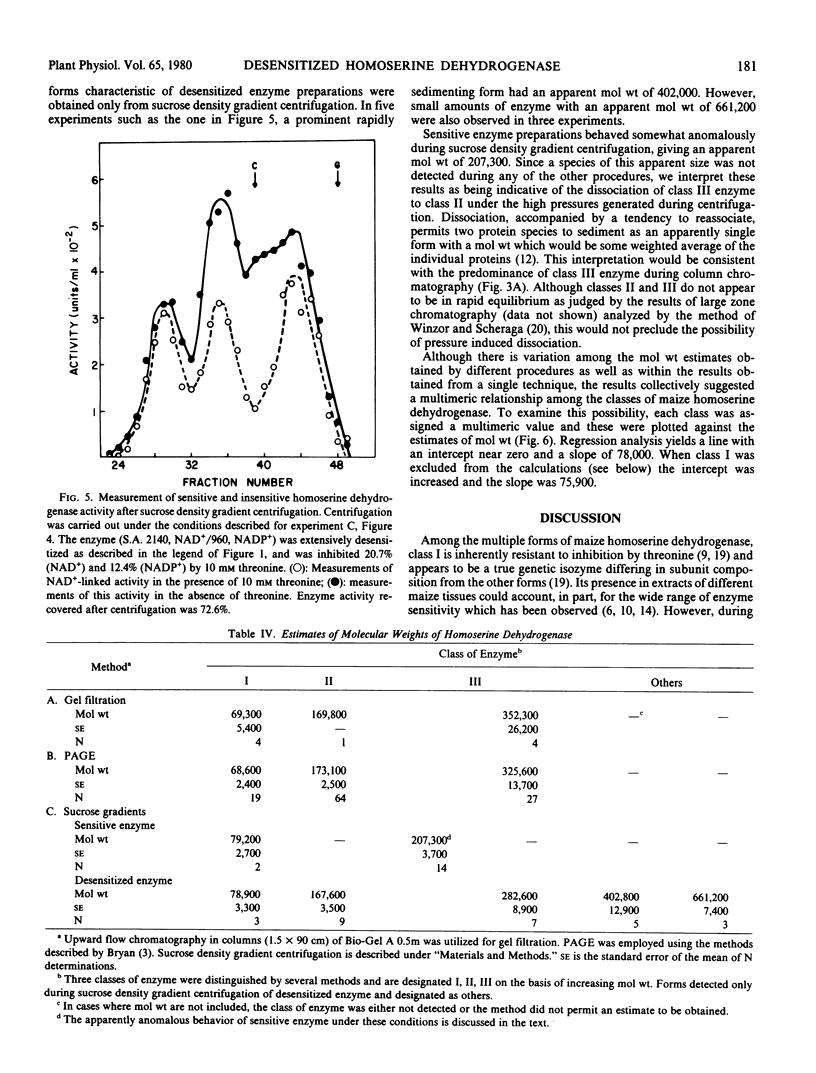
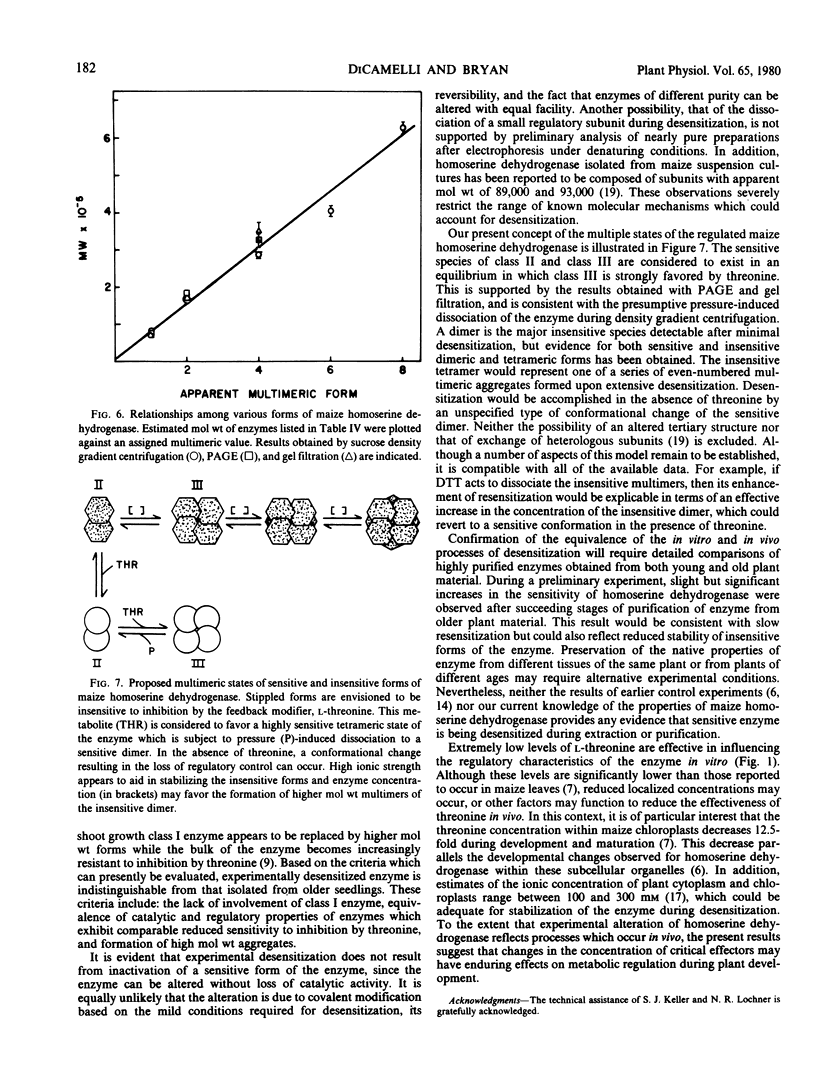
The properties of homoserine dehydrogenase (EC 1.1.1.3) isolated from shoots of young etiolated seedlings of Zea mays L. var. earliking can be reversibly altered by dialysis against an appropriate buffer. Treatment with 500 millimolar potassium phosphate buffer (pH 7.5) in the absence of l-threonine results in diminished regulatory control such that the enzyme becomes less sensitive to feedback inhibition. The physical and regulatory properties of experimentally altered and unaltered enzymes are compared with those of enzyme isolated from shoots of older seedlings. Multiple forms of both sensitive and insensitive enzymes are identified, and a model which is consistent with the observed isozymes and the difference in regulatory properties of enzymes obtained from seedlings of different ages is proposed. The initially sensitive enzyme is postulated to undergo a conformational change followed by formation of insensitive multimeric aggregated forms. The experimental conditions which facilitate alteration of the enzyme are discussed in relation to conditions which could occur in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan J. K., Lissik E. A., Matthews B. F. Changes in Enzyme Regulation during Growth of Maize: III. Intracellular Localization of Homoserine Dehydrogenase in Chloroplasts. Plant Physiol. 1977 Apr;59(4):673–679. doi: 10.1104/pp.59.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. K. Molecular weights of protein multimers from polyacrylamide gel electrophoresis. Anal Biochem. 1977 Apr;78(2):513–519. doi: 10.1016/0003-2697(77)90111-7. [DOI] [PubMed] [Google Scholar]
- Bryan J. K. Studies on the catalytic and regulatory properties of homoserine dehydrogenase of Zea mays roots. Biochim Biophys Acta. 1969 Feb 11;171(2):205–216. doi: 10.1016/0005-2744(69)90154-5. [DOI] [PubMed] [Google Scholar]
- Chapman D. J., Leech R. M. Changes in Pool Sizes of Free Amino Acids and Amides in Leaves and Plastids of Zea mays during Leaf Development. Plant Physiol. 1979 Mar;63(3):567–572. doi: 10.1104/pp.63.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicamelli C. A., Bryan J. K. Changes in Enzyme Regulation during Growth of Maize: II. Relationships among Multiple Molecular Forms of Homoserine Dehydrogenase. Plant Physiol. 1975 Jun;55(6):999–1005. doi: 10.1104/pp.55.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Krauss M. Dissociation of ribosomes induced by centrifugation: evidence for doubting conformational changes in ribosomes. Biochim Biophys Acta. 1971 Aug 12;246(1):81–99. [PubMed] [Google Scholar]
- Matthews B. F., Gurman A. W., Bryan J. K. Changes in Enzyme Regulation during Growth of Maize: I. Progressive Desensitization of Homoserine Dehydrogenase during Seedling Growth. Plant Physiol. 1975 Jun;55(6):991–998. doi: 10.1104/pp.55.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Walter T. J., Connelly J. A., Gengenbach B. G., Wold F. Isolation and characterization of two homoserine dehydrogenases from maize suspension cultures. J Biol Chem. 1979 Feb 25;254(4):1349–1355. [PubMed] [Google Scholar]


