Abstract
Bone morphogenic protein 1 (BMP1), a metalloproteinase, is known to cleave a wide variety of extracellular matrix proteins, suggesting that a consensus substrate cleavage amino acid sequence might exist. However, while such a consensus sequence has been proposed based on P4 to P4′ (i.e., the four amino acids flanking either side of the BMP1 cleavage site; P4P3P2P1|P1′P2′P3′P4′) sequence homologies between two BMP1 substrates, dentin matrix protein 1 and dentin sialoprotein phosphophoryn (DSP-PP) (i.e., xMQx | DDP), no direct testing has so far been attempted. Using an Sf9 cell expression system, we have been able to produce large amounts of uncleaved DSP-PP,. Point mutations introduced into this recombinant DSP-PP were then tested for their affects on DSP-PP cleavage by either Sf9 endogenous tolloid-related protein 1 (TLR-1) or by its human homolog, BMP1. Here we have measured DSP-PP cleavage efficiencies after modifications based on P4-P4′ sequence comparisons with dentin matrix protein 1, as well as for prolysyl oxidase and chordin, two other BMP1 substrates. Our results demonstrate that any mutations within or outside of the DSP-PP P4 to P4′ cleavage site can block, impair or accelerate DSP-PP cleavage, and suggest that its BMP1 cleavage site is highly conserved in order to regulate its cleavage efficiency, possibly with additional assistance from its conserved exosites. Thus, BMP1 cleavage cannot be based on a consensus substrate cleavage site.
Keywords: Dentin Sialophosphoprotein, DMP1, Chordin, Prolysyl Oxidase, mutations
Introduction
The dentin sialoprotein phosphophoryn (DSP-PP) gene produces a precursor protein, which undergoes cleavage to produce mature dentin sialoprotein (DSP) and phosphophoryn (PP) proteins that are necessary for dentin formation. During the early stages of dentin formation, this DSP-PP precursor protein cleavage activity must be tightly regulated in order to assure normal tooth development. Our understanding of this critical cleavage process has been hampered by the limited quantities of DSP-PP precursor protein available from cultured cells (1). In order to overcome this limitation, we recently described a method using the Sf9 baculovirus expression system that enables researchers to produce high yields of DSP-PP precursor protein, which then allows MS/MS analysis of isolated tryptic fragments of the N-terminal PP sequence. Using this method, we reported that the DSP-PP cleavage site was G447 | D448, based on our analysis of isolated tryptic fragments of the PP240 cleavage product (1). We were also able to demonstrate that Sf9 cells express a tolloid-related-1 (TLR1) peptidase, which cleaves DSP-PP at the same cleavage site (i.e., SMQG447| D448DPN) as its human homolog, BMP1, which is believed to be the human physiological processing enzyme for DSP-PP. In addition to DSP-PP, BMP1 also cleaves a wide variety of other extracellular matrix proteins, such as procollagen types I–IV, prolysyl oxidase, and DMP1 (see BMP1/Tolloid cleavage table in the MEROPS database: http://merops.sanger.ac.uk/cgi-bin/substrates?id=M12.005), suggesting that a consensus sequence might exist for the P4 to P4′ (Schechter, I and Berg, A. On the size of the active site in protease. I. Papain. Biochem. Biophy. Res. Comm. 1967; 27:157–162) BMP1 cleavage site. Here we expand our earlier DSP-PP mutation results (2) by asking whether selective DSP-PP mutations that mimic those sequences present in other BMP1 substrate cleavage sites can affect DSP-PP cleavage efficiency.
Materials and methods
Site-directed mutagenesis
Using the Stratagene QuickChange Site-Directed Mutagenesis protocol (Stratagene, La Jolla, CA), mutations were generated and verified (1).
Baculovirus Expression System
As described previously, various mutations generated by site-directed mutagenesis were subcloned into bacculovirus and expressed in Sf9 cells. Insect baculovirus expression system can produce sufficient DSP-PP240 recombinant protein, mature DSP and PP proteins (1).
Partial Purification of recombinant DSP-PP240 proteins and the mutated recombinant proteins using polyanion extraction
Acidic proteins such as DSP-PP240 precursor protein, DSP and PP are soluble in 5% trichloroacetic acid (TCA). We used 5% TCA to precipitate the majority of culture medium proteins. The TCA-soluble portion was neutralized and precipitated with 1.0 M CaCl2. This precipitate was dissolved in 1/10 volume of 0.1 M EDTA and stored at −20°C. This partially purified recombinant proteins were separated on PAGE gels and stained with Stains-All as previously described. Details see Yang et al. (2).
Quantification of DSP-PP240 and PP240 proteins
NIH Image J was used to quantify intensity of Stains-All stained bands. These intensity values were then used to calculate the ratio of PP240:(PP240+DSP-PP240). A higher ratio represents a higher efficiency of DSP-PP precursor cleavage.
Results
Alignment of P4 – P4′ sequences for BMP1 substrates
Table 1 (Supplement) lists the P4 to P4′ cleavage sites for 14 BMP1 substrates, including DSP-PP. From this table, it is readily apparent that only the P1′ amino acid Asp is shared among these 14 substrates. The DMP1 cleavage site has the greatest homology to the DSP-PP cleavage site. Two other BMP1 substrates, prolysyl oxidase and the C-terminal cleavage site of chordin also share some sequence homology to DSP-PP within their P4 to P4′ cleavage sites. Based on sequences homologies between DSP-PP and each of these 3 matrix protein BMP1 substrates, we then tested whether the substitution of selected amino acids from DMP1, prolysyl oxidase, and human chordin cleavage sites into the same P4 to P4′ DSP-PP cleavage sites, could affect DSP-PP cleavage.
Testing the proposed DMP1 consensus sequence (xMQx/DDP) with DSP-PP
In addition to DSP-PP, dentin matrix protein 1 is also essential for the formation of dentin. Recent evidence suggests that DSP-PP is a downstream effector molecule that mediates the roles of DMP1 in dentinogenesis (3, 4). Based on these two BMP1 substrate cleavage sites, we and others (5), proposed that xMQx | DDP could be a BMP1 consensus cleavage site (Figure 1A). To test this possible consensus sequence, we generated mutations in the rat DSP-PP240 cDNA sequence that changed Gly447 found in the P1 position of DSP-PP in all species except opossum, to S447 (i.e., G447S) found in the P1 position of DMP1. Similarly we also changed S444 found in the P4 position of DSP-PP to G444 (S444G) found in the P4 position of DMP1. As shown in Fig 1B, both substitutions result in double PP bands.
Figure 1. Testing the proposed xMQx.
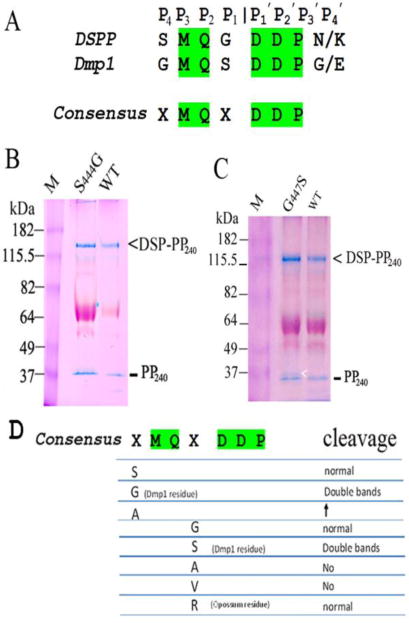
DDP consensus sequence between DSPP and Dmp1 cleavage sites in Fig. 1A. As shown in Fig. 1D, a number of mutations were generated in the P4 and P1 positions of DSP-PP. Samples were run on PAGE and stained with Stains-All. The DSP-PP240 and PP240 bands are stained blue. The S444G (Fig. 1B) and the G447S (Fig. 1C) mutations resulted in double PP240 bands (see arrow in Fig. 1C). Gel data from the other substitutions are derived from Yang, et al., 2013 (2).
Additional mutations were also created (2), as described in Fig. 1C. The P4 substitution S444A caused an increase in DSP-PP cleavage efficiency, while P1 substitutions G447A and G447V completely blocked DSP-PP cleavage. Interestingly, opossum DSP-PP contains an arginine residue at position 447. When we made a G447R substitution, DSP-PP cleavage was unchanged, thus demonstrating that this 180 million year old Arg mutation in opossum was neutral. These results indicate that the P4 and P1 conserved amino acids in DSP-PP cannot be exchanged with any of the P4 or P1 residues shown in Fig. 1C. Thus the proposed consensus cleavage site, xMQx | DDP, does not provide an accurate model for cleavage by BMP1.
Lysyl oxidase and DSP-PP
Lysyl oxidase (LOX) is important for the stability of connective tissues. The secreted proLOX is enzymatically quiescent and is activated through proteolytic cleavage between residues Gly(162) and Asp(163) (residue numbers according to the mouse LOX) by BMP1 (6). By comparing the P4 to P4′ cleavage sites in these two proteins, a likely consensus cleavage sequence would be xMxG | DDP. To test this consensus sequence we made a Q446K substitution in the P2 position of DSP-PP. As shown in Table 2 (Supplement), this substitution blocked DSP-PP cleavage. Similarly, a Q446E substitution reported by von Marschall et al., (2010) (5) also blocked DSP-PP cleavage. These results demonstrate that the DSP-PP P2 position cannot be altered suggesting that the proposed consensus sequence xMxG | DDP does not provide an accurate model for cleavage by BMP1.
Chordin and DSP-PP
During embryogenesis, communication between vertebrate dorsal and ventral sides is mediated by the action of growth factor antagonists, such as the BMP antagonist Chordin, that regulate the flow of BMPs during embryogenesis. Chordin contains 4 cystiene-rich (CR) BMP binding modules and is cleaved by BMP-1 at sites similar to procollagen C-propeptide cleavage sites (7). We chose to compare the C-terminal P4 to P4′ Chordin cleavage site with DSP-PP. Based on these two cleavage sequences, we propose that the consensus cleavage sequence would be xMQx | DxP. To test this consensus sequence we made four P2′ substitutions in DSP-PP. As shown in Fig. 2, the D449G substitution decreased DSP-PP cleavage to 1/3 normal; the D449E substitution increased DSP-PP cleavage by 2.5 fold; the D449A substitution also increased DSP-PP cleavage by 2 fold; and the D449N substitution decreased DSP-PP cleavage to 1/6 normal (2). These results suggest that the DSP-PP P2′ position cannot be altered without significant effect on DSP-PP cleavage efficiency, and suggest that the proposed xMQx | DxP consensus sequence does not provide an accurate model for cleavage by BMP1.
Figure 2. Testing the proposed xMQx.
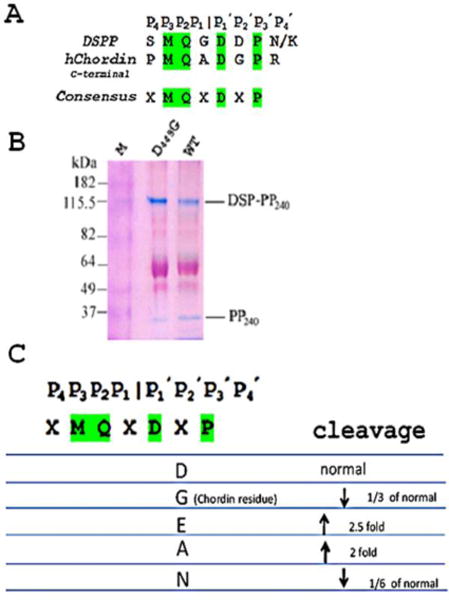
DxP consensus sequence. Fig. 2A showed the consensus sequence between DSPP and human Chordin C-terminal cleavage sites. As shown in Fig. 2C, a number of between mutations were generated in the P2′ position of DSP-PP. Samples were run on PAGE and stained with Stains-All. The D449G (Fig. 2B) mutation resulted in a PP240 band 1/3 normal. Gel data from the other substitutions are derived from Yang, et al., 2013 (2).
Discussion
We have fully characterized the Sf9 baculovirus expression system with respect to DSP-PP240 synthesis and secretion (1). Newly synthesized DSP-PP240 is secreted into the conditioned medium where it undergoes accurate cleavage (i.e., SMQG447| D448DPN), most likely by an endogenous Sf9 tolloid-like (TLR1) protease (a BMP1 homolog), into DSP430 and PP240. The Sf9 system therefore allows studies of BMP1 sequence specificity to be undertaken.
The apparent lack of BMP1 substrate specificity has confounded researchers for many years since BMP1 metalloproteinase has a highly conserved amino acid sequence in its catalytic domain, and there appears to be little homology between the many BMP1 substrate P4 to P4′ cleavage sites. Our amino acid substitution results suggest that at least within DSP-PP, the P4 to P4′ cleavage site sequence is highly conserved. Amino acid substitutions into this cleavage site that mimic the conserved P4 to P4′ cleavage sites of DMP1, prolysyl oxidase, or human Chordin result in altered DSP-PP240 cleavage efficiency. Similarly, amino acid substitutions in several conserved DSP-PP240 exosites also alter DSP-PP240 cleavage efficiency (2). Thus it is possible that each BMP1 substrate may have its own unique P4-P4′ cleavage sequence, which may coordinate with their conserved exosites, to facilitate the cleavage of that protein. Mutations within or outside of these cleavage sites might block, impair or accelerate BMP1 substrate cleavage. Finally, it can be noted that the amount of physiologically active DSP-PP products (amounts of the many BMP1 cleavage products) depend upon the efficiency of BMP1 substrate cleavage. Hence the quality of dentin mineralization, or extracellular matrix organization most likely requires the maintenance of conserved P4 to P4′ substrate sequences.
Supplementary Material
Acknowledgments
This work was supported by DE18901 to HHR. We thank Dr. David Ritchie for valuable discussions and help for this manuscript. We also thank Dr. Zhihong Dong for help with Endnote and manuscript submission.
Footnotes
Declaration of interest: The authors have no conflicts of interest.
References
- 1.Ritchie HH, Yee CT, Tang XN, Dong ZH, Fuller RS. DSP-PP Precursor Protein Cleavage by Tolloid-Related-1 Protein and by Bone Morphogenetic Protein-1. PLoS ONE. 2012;7:e41110. doi: 10.1371/journal.pone.0041110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang R, Lim G, Dong Z, Lee A, Yee C, Fuller R, Ritchie H. The Efficiency of Dentin Sialoprotein-Phosphophoryn Processing Is Affected by Mutations Both Flanking and Distant from the Cleavage Site. J Biol Chem. 2013;288:6024–33. doi: 10.1074/jbc.M112.382952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson M, Zhu Q, Wang S, Liu Q, Liu Y, Wang X, Yuan B, Ruest L, Feng J, D’Souza R, Qin C, Lu Y. The Rescue of Dentin Matrix Protein 1 (DMP1)-deficient Tooth Defects by the Transgenic Expression of Dentin Sialophosphoprotein (DSPP) Indicates That DSPP Is a Downstream Effector Molecule of DMP1 in Dentinogenesis. J Biol Chem. 2013;288:7204–14. doi: 10.1074/jbc.M112.445775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayanan K, Gajjeraman S, Ramachandran A, Hao J, George A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. J Biol Chem. 2006;281:19064–71. doi: 10.1074/jbc.M600714200. [DOI] [PubMed] [Google Scholar]
- 5.von Marschall Z, Fisher L. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix 2 products by three isoforms of bone morphogenetic protein-1 (BMP1) Matrix Biology. 2010;29:295–303. doi: 10.1016/j.matbio.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cronshaw A, Fothergill-Gilmore L, Hulmes D. The proteolytic processing site of the precursor of lysyl oxidase. The Biochemical journal. 1995;306:279–84. doi: 10.1042/bj3060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott I, Blitz I, Pappano W, Imamura Y, Clark T, Steiglitz B, Thomas C, Maas S, Takahara K, Cho K, Greenspan D. Mammalian BMP-1/Tolloid-Related Metalloproteinases, Including Novel Family Member Mammalian Tolloid-Like 2, Have Differential Enzymatic Activities and Distributions of Expression Relevant to Patterning and Skeletogenesis. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


