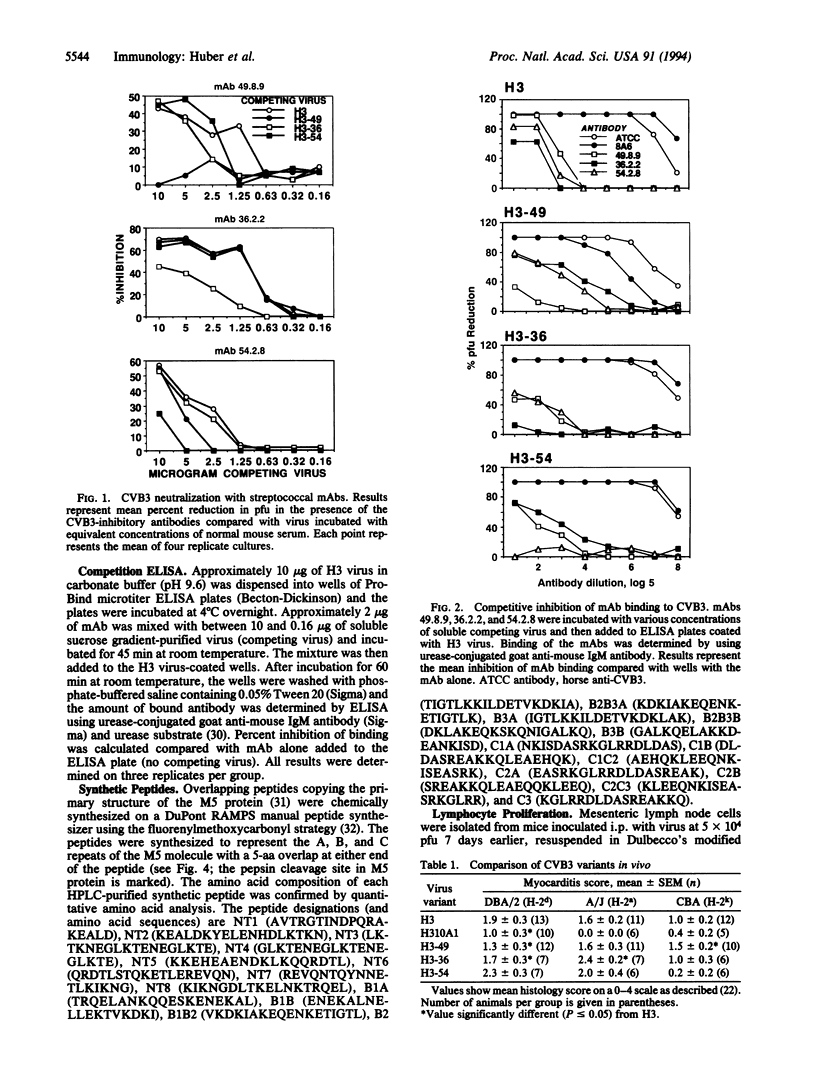
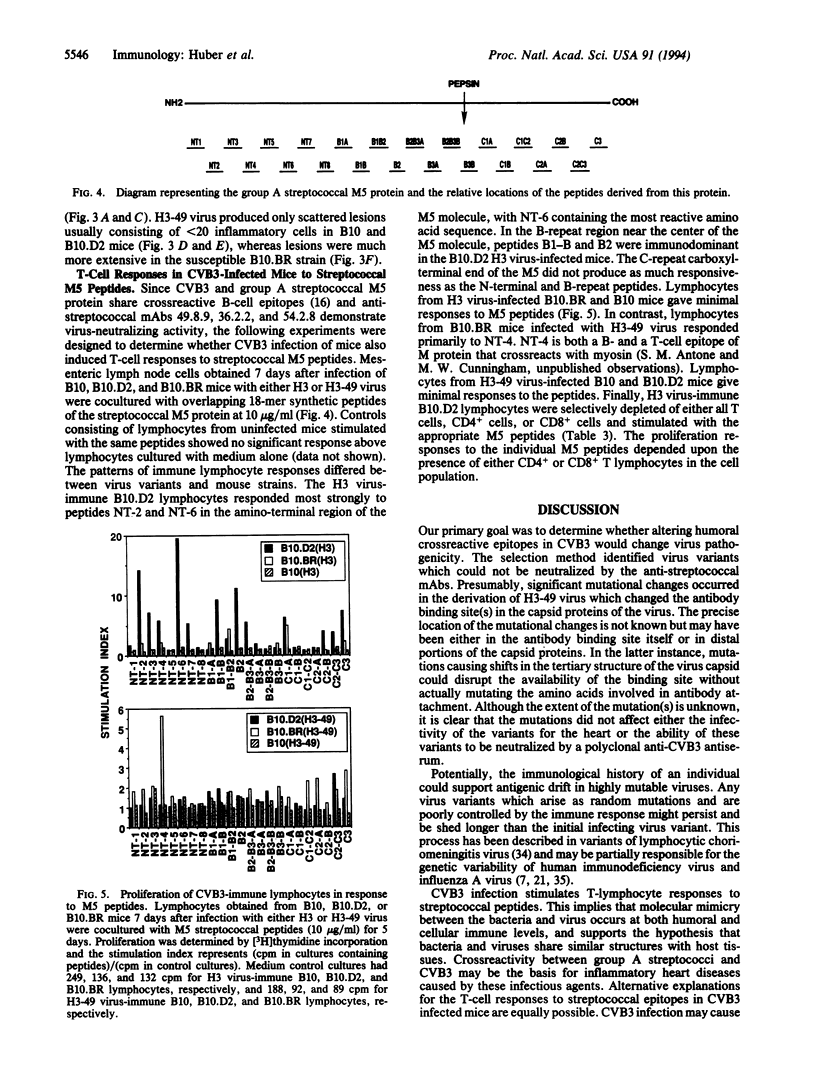
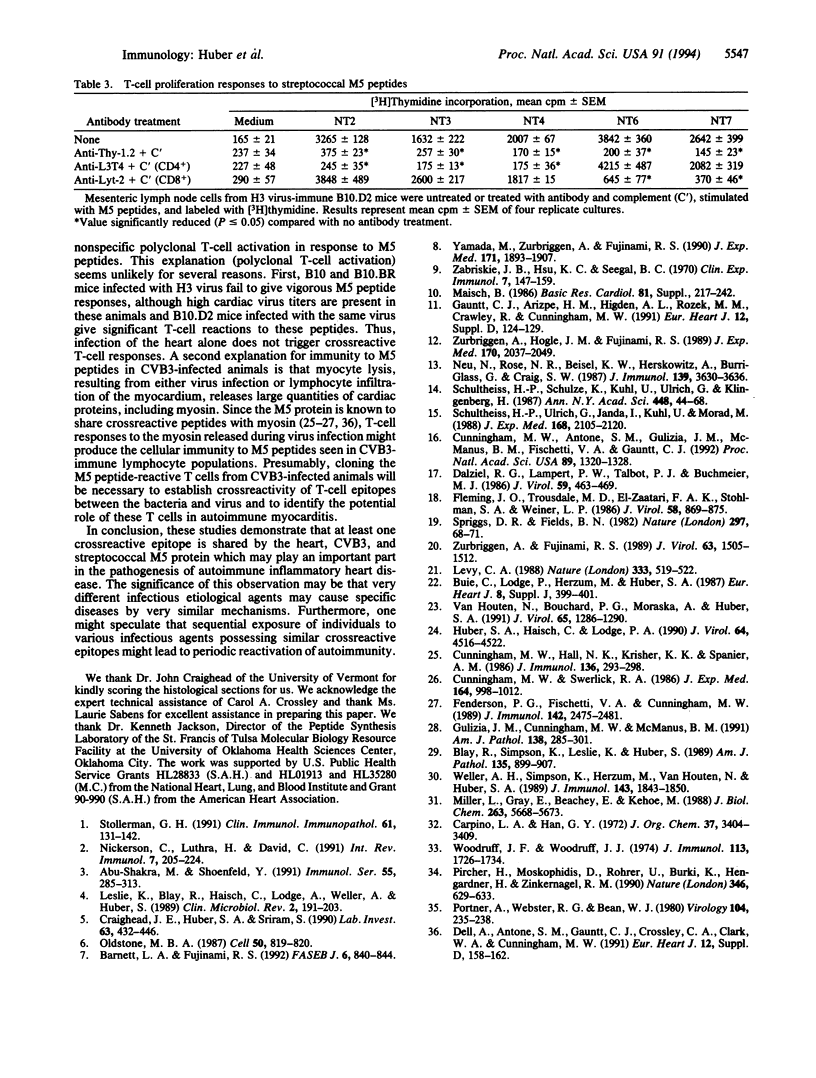
Abstract
Three monoclonal antibodies (mAbs), 49.8.9, 36.2.2, and 54.2.8, made to the group A streptococcus M5 serotype identify crossreactive epitopes in cardiac tissues and also neutralize a highly myocarditic variant of coxsackievirus B3 (H3). Mutants of H3 were selected with these mAbs and evaluated for pathogenicity compared with the wild-type virus. H3 and the mutant variants selected with mAbs 36.2.2 (H3-36) and 54.2.8 (H3-54) induced severe myocarditis in DBA/2 (H-2d) and A/J (H-2a) male mice, whereas CBA (H-2k) mice were disease resistant. The virus variant isolated with mAb 49.8.9 (H3-49) was strikingly different and caused disease in CBA and A/J mice but not in DBA/2 animals, suggesting that the major histocompatibility complex association of the disease had been altered. This hypothesis was confirmed by using B10 congenic mice. In addition, T lymphocytes from the H3 and H3-49 virus-infected mice responded to distinctly different peptides in the streptococcal M protein, suggesting that certain epitopes of infectious agents which are shared with host tissues may be critical in determining disease susceptibility in genetically distinct individuals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Shakra M., Shoenfeld Y. Chronic infections and autoimmunity. Immunol Ser. 1991;55:285–313. [PubMed] [Google Scholar]
- Barnett L. A., Fujinami R. S. Molecular mimicry: a mechanism for autoimmune injury. FASEB J. 1992 Feb 1;6(3):840–844. doi: 10.1096/fasebj.6.3.1740233. [DOI] [PubMed] [Google Scholar]
- Blay R., Simpson K., Leslie K., Huber S. Coxsackievirus-induced disease. CD4+ cells initiate both myocarditis and pancreatitis in DBA/2 mice. Am J Pathol. 1989 Nov;135(5):899–907. [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Huber S. A., Sriram S. Animal models of picornavirus-induced autoimmune disease: their possible relevance to human disease. Lab Invest. 1990 Oct;63(4):432–446. [PubMed] [Google Scholar]
- Cunningham M. W., Antone S. M., Gulizia J. M., McManus B. M., Fischetti V. A., Gauntt C. J. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M. W., Hall N. K., Krisher K. K., Spanier A. M. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J Immunol. 1986 Jan;136(1):293–298. [PubMed] [Google Scholar]
- Cunningham M. W., Swerlick R. A. Polyspecificity of antistreptococcal murine monoclonal antibodies and their implications in autoimmunity. J Exp Med. 1986 Oct 1;164(4):998–1012. doi: 10.1084/jem.164.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel R. G., Lampert P. W., Talbot P. J., Buchmeier M. J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986 Aug;59(2):463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell A., Antone S. M., Gauntt C. J., Crossley C. A., Clark W. A., Cunningham M. W. Autoimmune determinants of rheumatic carditis: localization of epitopes in human cardiac myosin. Eur Heart J. 1991 Aug;12 (Suppl 500):158–162. doi: 10.1093/eurheartj/12.suppl_d.158. [DOI] [PubMed] [Google Scholar]
- Fenderson P. G., Fischetti V. A., Cunningham M. W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989 Apr 1;142(7):2475–2481. [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., el-Zaatari F. A., Stohlman S. A., Weiner L. P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986 Jun;58(3):869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Arizpe H. M., Higdon A. L., Rozek M. M., Crawley R., Cunningham M. W. Anti-Coxsackievirus B3 neutralizing antibodies with pathological potential. Eur Heart J. 1991 Aug;12 (Suppl 500):124–129. doi: 10.1093/eurheartj/12.suppl_d.124. [DOI] [PubMed] [Google Scholar]
- Gulizia J. M., Cunningham M. W., McManus B. M. Immunoreactivity of anti-streptococcal monoclonal antibodies to human heart valves. Evidence for multiple cross-reactive epitopes. Am J Pathol. 1991 Feb;138(2):285–301. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Haisch C., Lodge P. A. Functional diversity in vascular endothelial cells: role in coxsackievirus tropism. J Virol. 1990 Sep;64(9):4516–4522. doi: 10.1128/jvi.64.9.4516-4522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie K., Blay R., Haisch C., Lodge A., Weller A., Huber S. Clinical and experimental aspects of viral myocarditis. Clin Microbiol Rev. 1989 Apr;2(2):191–203. doi: 10.1128/cmr.2.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Mysteries of HIV: challenges for therapy and prevention. Nature. 1988 Jun 9;333(6173):519–522. doi: 10.1038/333519a0. [DOI] [PubMed] [Google Scholar]
- Maisch B. Immunologic regulator and effector functions in perimyocarditis, postmyocarditic heart muscle disease and dilated cardiomyopathy. Basic Res Cardiol. 1986;81 (Suppl 1):217–241. doi: 10.1007/978-3-662-11374-5_21. [DOI] [PubMed] [Google Scholar]
- Miller L., Gray L., Beachey E., Kehoe M. Antigenic variation among group A streptococcal M proteins. Nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988 Apr 25;263(12):5668–5673. [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Nickerson C., Luthra H., David C. Antigenic mimicry and autoimmune diseases. Int Rev Immunol. 1991;7(3):205–224. doi: 10.3109/08830189109061775. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Pircher H., Moskophidis D., Rohrer U., Bürki K., Hengartner H., Zinkernagel R. M. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature. 1990 Aug 16;346(6285):629–633. doi: 10.1038/346629a0. [DOI] [PubMed] [Google Scholar]
- Portner A., Webster R. G., Bean W. J. Similar frequencies of antigenic variants in Sendai, vesicular stomatitis, and influenza A viruses. Virology. 1980 Jul 15;104(1):235–238. doi: 10.1016/0042-6822(80)90382-7. [DOI] [PubMed] [Google Scholar]
- Schultheiss H. P., Kühl U., Janda I., Melzner B., Ulrich G., Morad M. Antibody-mediated enhancement of calcium permeability in cardiac myocytes. J Exp Med. 1988 Dec 1;168(6):2105–2119. doi: 10.1084/jem.168.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs D. R., Fields B. N. Attenuated reovirus type 3 strains generated by selection of haemagglutinin antigenic variants. Nature. 1982 May 6;297(5861):68–70. doi: 10.1038/297068a0. [DOI] [PubMed] [Google Scholar]
- Stollerman G. H. Rheumatogenic streptococci and autoimmunity. Clin Immunol Immunopathol. 1991 Nov;61(2 Pt 1):131–142. doi: 10.1016/s0090-1229(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Van Houten N., Bouchard P. E., Moraska A., Huber S. A. Selection of an attenuated Coxsackievirus B3 variant, using a monoclonal antibody reactive to myocyte antigen. J Virol. 1991 Mar;65(3):1286–1290. doi: 10.1128/jvi.65.3.1286-1290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A. H., Simpson K., Herzum M., Van Houten N., Huber S. A. Coxsackievirus-B3-induced myocarditis: virus receptor antibodies modulate myocarditis. J Immunol. 1989 Sep 15;143(6):1843–1850. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]
- Yamada M., Zurbriggen A., Fujinami R. S. Monoclonal antibody to Theiler's murine encephalomyelitis virus defines a determinant on myelin and oligodendrocytes, and augments demyelination in experimental allergic encephalomyelitis. J Exp Med. 1990 Jun 1;171(6):1893–1907. doi: 10.1084/jem.171.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie J. B., Hsu K. C., Seegal B. C. Heart-reactive antibody associated with rheumatic fever: characterization and diagnostic significance. Clin Exp Immunol. 1970 Aug;7(2):147–159. [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A., Fujinami R. S. A neutralization-resistant Theiler's virus variant produces an altered disease pattern in the mouse central nervous system. J Virol. 1989 Apr;63(4):1505–1513. doi: 10.1128/jvi.63.4.1505-1513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A., Hogle J. M., Fujinami R. S. Alteration of amino acid 101 within capsid protein VP-1 changes the pathogenicity of Theiler's murine encephalomyelitis virus. J Exp Med. 1989 Dec 1;170(6):2037–2049. doi: 10.1084/jem.170.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]



