Abstract
Caloric restriction has consistently been shown to extend life span and ameliorate aging-related diseases. These effects may be due to diet-induced reactive oxygen species acting to up-regulate sirtuins and related protective pathways, which research suggests may be partially inhibited by dietary anti-oxidant supplementation. Because caloric restriction is not sustainable long term for most humans, we investigated an alternative dietary approach, intermittent fasting (IF), which is proposed to act on similar biological pathways. We hypothesized that a modified IF diet, where participants maintain overall energy balance by alternating between days of fasting (25% of normal caloric intake) and feasting (175% of normal), would increase expression of genes associated with aging and reduce oxidative stress and that these effects would be suppressed by anti-oxidant supplementation. To assess the tolerability of the diet and to explore effects on biological mechanisms related to aging and metabolism, we recruited a cohort of 24 healthy individuals in a double-crossover, double-blinded, randomized clinical trial. Study participants underwent two 3-week treatment periods—IF and IF with anti-oxidant (vitamins C and E) supplementation. We found strict adherence to study-provided diets and that participants found the diet tolerable, with no adverse clinical findings or weight change. We detected a marginal increase (2.7%) in SIRT3 expression due to the IF diet, but no change in expression of other genes or oxidative stress markers analyzed. We also found that IF decreased plasma insulin levels (1.01 μU/mL). Although our study suggests that the IF dieting paradigm is acceptable in healthy individuals, additional research is needed to further assess the potential benefits and risks.
Introduction
Decades of research have demonstrated that sustained under-consumption of daily calorie intake (60%–80% of normal) has significant health benefits1,2 and delays the onset of a number of age-related diseases.3 A growing body of literature suggests that caloric restriction (CR) transiently increases production of cellular reactive oxygen species (ROS), which in turn initiates a protective, adaptive response. This protective response appears to be an important mechanism underlying the beneficial effects of CR.3–5 In particular, SIRT1, a conserved histone deacetylase with widespread effects on cellular metabolism and aging, has been found to regulate genes that have direct effects on aging and increases expression of genes that act as endogenous ROS scavengers to protect cells from further ROS insult.6,7 Mitochondria are believed to play an important role in these protective responses, as mitochondria are the primary site of endogenous ROS production and mitochondrial transcription factors are up-regulated with CR.3,7,8 Thus, CR promotes a transient state of oxidative stress, leading to adaptive protective responses, which in the long run protect cells from future ROS insults.
Some vitamins act as anti-oxidants and quench levels of damaging ROS species, which would be expected to provide significant health benefits. However, several large clinical trials and epidemiological studies have assessed the effect of anti-oxidative vitamins (e.g., vitamin C and E) supplementation and have not found an association of vitamin supplementation with decreased mortality or morbidity,9–14 whereas others have observed protective effects.15–19 Thus, the potential value of supplementation with vitamins is a topic of current scientific debate. It has been proposed that the observed lack of benefit is because anti-oxidant supplementation actually quenches ROS to such an extent that ROS-mediated induction of protective pathways is prevented.3,5,20 Thus, it is important to identify alternative means of inducing these protective pathways, and dietary restriction has emerged as one candidate approach.
While CR research has yielded much valuable information, it is largely unknown whether the positive benefits of CR would exist in the absence of weight loss in humans. In addition, CR appears to be an unsustainable prophylactic measure for most individuals.21 Of the alternative dietary intervention paradigms, intermittent fasting (IF) has emerged as an alternative dietary regimen that may produce effects on specific biological pathways comparable to CR.22–26 Preclinical findings suggest that the transient periods of fasting induce benefits on aging similar to extended CR.22–26 A previous study in humans compared intermittent energy restriction to continuous energy restriction and found that the intermittent paradigm induced similar benefits as the continuous paradigm.26 An alternate-day fasting paradigm has been shown to reduce markers of oxidative stress and improve clinical findings.22,27,28 However, in these studies, the participants ate ad libitum on feasting days (which resulted in overall negative energy balance and weight loss), conflating the effects of IF and energy restriction on the parameters measured. As such, these IF regimens have been examined in recent clinical trials for their potential to promote weight loss.26,29,30 However, IF diets can also be designed to maintain weight, with individuals alternating between fasting and feasting.
There is a paucity of clinical studies exploring the effect of IF on protective cellular responses and examining the practicality of IF in human subjects. Namely, it is unknown if a protective ROS response occurs through mechanisms similar to those induced by CR. On the basis of previous studies,22–26 we hypothesized that the IF dieting paradigm would be well tolerated and could have beneficial effects on cellular oxidative stress and expression of genes related to aging and metabolism, and that anti-oxidant supplementation would negate the effect of the IF diet on these markers. To address this question, we performed a proof-of-principle clinical study in healthy human subjects to assess the safety and feasibility of an IF dieting regimen. Our IF dieting regimen maintains overall caloric intake, allowing us to disentangle the effects of decreased caloric intake from the effects of intermittent periods of fasting. We further study the effects of this diet on key biological mechanisms related to aging, oxidative stress, and metabolism.
Methods
Overview
We recruited a cohort of healthy individuals in a 10-week double-crossover, double-blinded, randomized clinical trial. Participants were initiated on an IF dieting paradigm, which was designed to promote weight stability, thus allowing us to assess the effect of IF in the absence of weight loss. We assessed expression of genes related to aging and protective responses as well as oxidative stress markers in the participants. In addition, we administered satisfaction surveys and collected extensive compliance data to assess the practicality of the IF dieting paradigm. The study was approved by the Institutional Review Board of the University of Florida and registered with ClinicalTrials.Gov (ID: NCT02132091).
Participant recruitment
Study participants were recruited from Alachua County, FL, from March to August of 2011. Briefly, eligibility requirements included body mass index (BMI) in the range of 20.0–30.0 kg/m2, age between 19 and 30, stable weight (less than 10% change for 3 months prior to the study), and no history of metabolic disorders, cardiovascular disease, or thyroid dysfunction. Additional eligibility and exclusion criteria are listed in Supplementary Methods (Supplementary Data are available at www.liebertonline.com/rej/). All study participants provided written informed consent.
Trial design
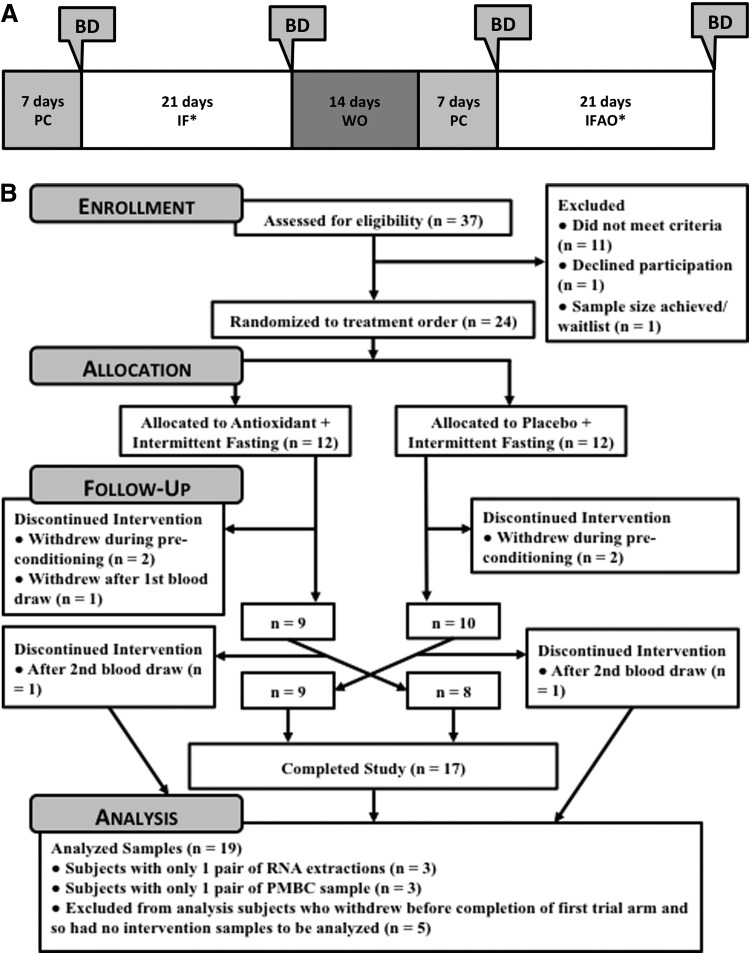
The study was designed as a 10-week double-crossover trial, consisting of two 3-week treatment periods—IF and IF with anti-oxidant supplementation (IFAO) (Fig. 1A). Each 3-week treatment period was preceded by a 1-week pre-conditioning period used to establish baseline caloric intake and to acclimate the participants to study-provided food. The first treatment period and the second pre-conditioning period were separated by a 2-week washout during which participants were instructed to resume their normal diets. Each participant was assigned to complete both the IF and IFAO treatment periods, but the order in which they completed the treatment periods was randomized. Venous blood samples were collected from each participant at the beginning and end of each 3-week treatment period. Participants were instructed to maintain an overnight fast prior to the blood draw, and blood draw times for each participant were kept consistent throughout the study. Blood sampling and clinical measurements were performed at the University of Florida's Clinical Research Center.
FIG. 1.
Trial design and enrollment. (A) The trial consisted of two 3-week trial sequences—intermittent fasting (IF) and IF with anti-oxidant supplementation (IFAO). Each 3-week sequence was preceded by a 1-week pre-conditioning period (PC). The two trial periods were separated by a 2-week washout (WO) period. During the IF and IFAO trial sequences, participants alternated between 25% and 175% daily caloric intake. During the PC periods, participants consumed 100% of daily caloric intake. During the IFAO sequences, participants supplemented their diet with vitamin C (1000 mg) and vitamin E (400 IU) each day orally, whereas during the IF sequences, participants supplemented their diet with a placebo. Each participant completed both the IF and IFAO trial sequences, but in randomized order. Blood draws (BD) were performed at the beginning and end of each three-week trial period. Additional details are provided in the Methods section. (B) Enrollment included 24 subjects who were randomly allocated to each treatment order. Five participants withdrew before completion of the first trial sequence and were not included in the sample analysis. Analysis of samples included two subjects who withdrew during the 2-week washout period and the remaining 17 subjects who completed the trial. PBMC, peripheral blood mononuclear cells.
A total of 24 participants were enrolled in the trial (power calculations provided in Supplementary Methods). The use of a cross-over study design allowed us to make the primary experimental comparison within each individual on the impact of anti-oxidant supplementation during IF, with secondary observational comparisons of the effect of the IF or IFAO treatments relative to normal (i.e., energy-balanced) diets. Due to the nature of the design, participants who withdrew, but remained in the study long enough to collect both pre- and post-treatment blood samples (n=2), were analyzed under the respective secondary comparison. The analysis of all other completers (n=17) included primary and secondary comparisons.
IF diet and anti-oxidant supplementation
During each trial sequence, participants were placed on an IF scheme in which they alternated between consuming 175% (feasting) of normal caloric intake and 25% (fasting) of normal caloric intake. Normal caloric intake (defined as the daily caloric intake needed for weight maintenance) levels were established by an experienced registered dietitian (M.N.S) using Mifflin–St. Jeor equations for resting energy expenditure and individualized activity factors.31 These estimates were adjusted on the basis of minor weight changes observed during the pre-conditioning periods. Menus were developed and dietary intake data were analyzed using Nutrition Data System for Research software version 2010, developed by the Nutrition Coordinating Center (NCC) at the University of Minnesota, Minneapolis, MN. The 2010 Dietary Guidelines for Americans was used to guide macronutrient composition in the prepared meals, with only small variations allowed to tailor meals according to participant preferences.32 One exception was the sodium content, which was maintained at average US consumption rather than dietary guideline levels. These compositions were maintained across the fasting, feasting, and pre-conditioning diets. Meals were prepared by the metabolic kitchen at the University of Florida Clinical Research Center.
Study participants were assigned to treatment sequence order by an independent statistician using a computer-generated simple randomization list. This order was provided to University of Florida's Investigational Pharmacy for supplement and placebo dispensing, but was not shared with the participants, Clinical Research Center staff, or study investigators (i.e., double blinded). During the IFAO trial arms, participants were supplied pill bottles and instructed to consume oral doses of vitamin C (500 mg twice each day; morning and evening) and vitamin E (400 IU once each day; morning). During the IF trial arms, participants received identical instructions and were supplied with placebo capsules matched for appearance and taste with the vitamin C and E pills.
Measurement of gene expression
Total RNA was extracted from whole blood using the PAXgene Blood RNA Kit (Qiagen). First-strand cDNA synthesis was performed using the GoScript Reverse Transciption System (Promega) with random hexamers. qPCR amplification of 18S rRNA (housekeeping gene) and a priori specified outcome genes SOD2, TFAM, SIRT1, and SIRT3 was performed using the GoTaq qPCR Master Mix (Promega). Detection was performed on an ABI PRISM 7000 (Applied Biosystems). qPCR primer sequences are provided in Supplementary Methods. Gene expression levels were characterized using a modification of the ΔΔCt method, which examined relative percent change over the treatment period.33
Measurement of RNA/DNA oxidation
Peripheral blood mononuclear cells (PBMCs) were extracted from 10 mL of blood (see Supplementary Methods). High-performance liquid chromatography coupled to electrochemical detection (HPLC-ECD) was then used to quantify a priori-specified RNA and DNA oxidation products 8-oxo-7,8-dihydroguanosine (8-oxo-G) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) as previously described.34 Ratios were calculated by normalizing nucleotide oxidation levels against non-oxidized nucleotide levels.
Insulin measurements
Insulin levels were measured from plasma using the Human Insulin ELISA Kit (EMD Millipore) according to manufacturer's specifications.
Satisfaction survey
Participants completed a follow-up survey to assess satisfaction with the diet and the impact it had on quality of life. One version of the survey (see Survey A, in Supplementary Methods) was provided to participants who completed the study; a modified version was provided to participants who withdrew (see Survey B, in Supplementary Methods). All participants who began the intervention completed the survey (17 who completed the study and 7 who withdrew during the study).
Statistical analyses
Analyses were intent-to-treat, pre-specified, and used parametric t-tests to examine the effect of each treatment relative to baseline and differences in effect between treatments. For the HPLC data, ratios were log transformed to achieve normality, with differences computed between log-transformed values. In addition, several outliers, defined as values more than 1.5 times the interquartile range from the 25th or 75th percentile in the log-transformed data, were removed from the analyses. A threshold of α <0.05 was used to assess significance. Confidence intervals (95%) were computed, and log-transformed means and upper and lower limits were back-transformed into fold-change metric. Analyses were performed using SAS version 9.3 (SAS Institute), MATLAB version 2011b (MathWorks), and R version 3.0.1 (CRAN).
Results
Participant characteristics
A total of 24 participants were enrolled and 12 were randomized to each treatment order (Fig. 1B). Seven participants withdrew from the study for various reasons (Table S1), resulting in 17 participants who completed both treatment phases of the study. However, two of the participants who withdrew completed one set of pre- and post-treatment measurements, which allowed for analysis of the completed treatment periods.
Characteristics of the participants enrolled in the study are shown in Table 1, Table S2, and Table S3. Male participants had an average BMI of 25.3 kg/m2 (range 21.7–31.6), whereas the average BMI for females was 22.8 kg/m2 (19.2–28.2). On average, participants were 24 years old and reported engaging in 1.5 hr/week of exercise. There were no significant differences in the characteristics of the participants by treatment sequence.
Table 1.
Baseline Characteristics of Study Participants
| Mean | Range | |
|---|---|---|
| Age (years) | ||
| Males | 24.82 | 22.1–30.5 |
| Females | 24.05 | 20.5–30.8 |
| Body mass index (kg/m2) | ||
| Males | 25.32 | 21.78–31.64 |
| Females | 22.77 | 19.25–28.27 |
| Amount of exercise (min/week) | ||
| Males | 82.50 | 0–135 |
| Females | 97.50 | 0–225 |
Note that males composed 32% of study participants.
Diet compliance
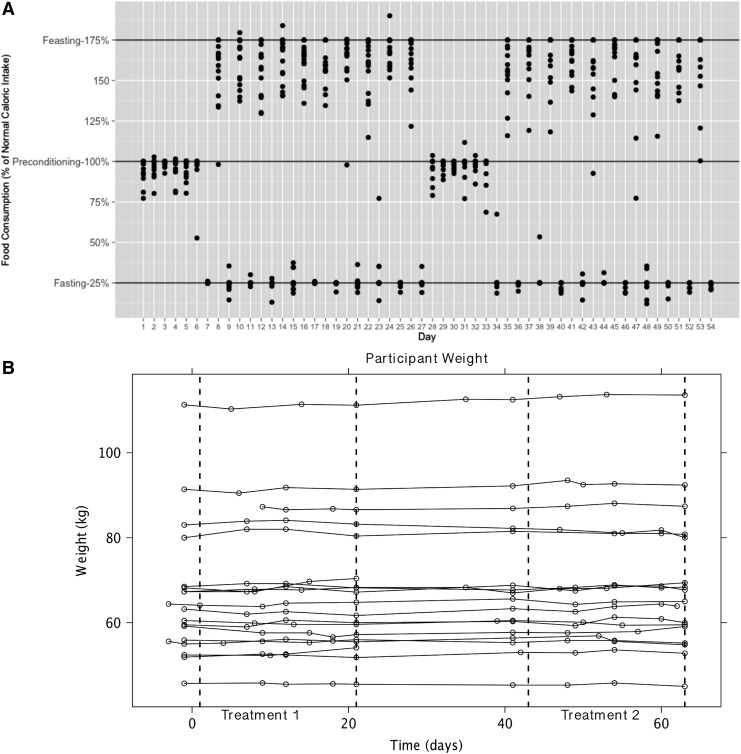
We assessed compliance to the diet by measuring the amount of unconsumed food and also by diet logs, where participants were asked to record any food they consumed outside of the study-provided meals. Figure 2A shows the percent of normal caloric intake for all participants on each day of the study. There was no apparent trend of increased or decreased compliance over time for either the pre-conditioning, fasting, or feasting days. However, participants appeared to experience consistent difficulty consuming the full 175% of normal caloric intake on the feasting days. Distributions of daily caloric intake during pre-conditioning, fasting, and feasting arms of the study are shown in Fig. S1. Participants consumed between 95% and 105% (100%±5%) of normal caloric intake on 81.7% of pre-conditioning days and consumed between 20% and 30% (25%±5%) of normal caloric intake on 91.4% of fasting days. In contrast, participants consumed between 170% and 180% (175%±5%) of normal caloric intake on only 49.0% of feasting days. However, during 33.0% of days, participants consumed between 150% and 170% of normal caloric intake, indicating that a majority of non-compliance on feasting days was due to slight under-consumption of calories. Importantly, participants' weights remained stable over the entire trial period (Fig. 2B).
FIG. 2.
Participant compliance with diet and weight change. (A) The plot displays the percent of normal caloric intake consumed by each participant on a given day of the study. Each point on the plot represents the number of calories consumed by a given person on that day. Days 1–6 and 28–33 are pre-conditioning days, during which participants were provided 100% of normal caloric intake. On days 7–27 and 34–54, participants alternated between fasting (25% of normal) and feasting (175% of normal). (B) The plot shows the weight of each participant over the course of the study. Each line is the weight of a given participant and each circle represents a weight measurement. Note that part A represents day of study provided food, whereas part B represents overall days over the course of the study.
Compliance to anti-oxidant supplementation was assessed by counting pill bottles, which participants returned at the end of each treatment period. On the basis of the pill counts, participants had greater than 90% adherence to both vitamin C and vitamin E supplementation (93% and 95%, respectively) (Table S4).
Expression of genes reflecting aging and oxidative stress
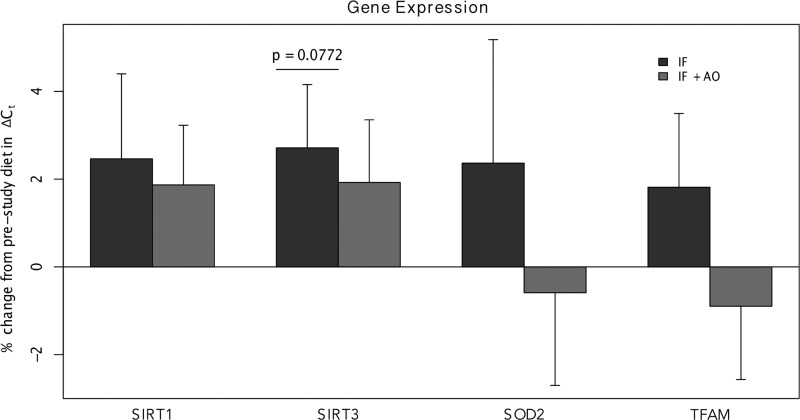
We assessed expression of SIRT1, SIRT3, SOD2, and TFAM, as these genes have been implicated from previous literature in mediating diet-induced benefits on aging.4,7 Additionally, these genes were chosen because they are expressed in PBMCs. Increased gene expression was observed for all selected genes following IF with placebo: SIRT1 (+2.46%; 95% CI [−1.66%, 6.59%]; n=16), SIRT3 (+2.71%; 95% CI [−0.33%, 5.76%]; n=18), SOD2 (+2.36%; 95% CI [−3.57%, 8.30%]; n=18), TFAM (+1.82%; 95% CI [−1.73%, 5.36%]; n=18). None of these genes reached statistical significance, but SIRT3 trended toward significance (p=0.0772). For IFAO, expression changes in SIRT1 (+1.87%; 95% CI −1.05%, 4.78%]; n=15) and SIRT3 (+1.92%; 95% CI −1.10%, 4.95%]; n=17) showed a trend toward increasing values, whereas SOD2 (−0.59%; 95% CI −5.07%, 3.89%]; n=17) and TFAM (−0.90%; 95% CI −4.44%, 2.65%]; n=17) appeared to be unaffected (Fig. 3). Finally, while each increase in expression following IF is qualitatively larger than the post-treatment change for IFAO, paired t-tests revealed no statistically significant differences by target gene.
FIG. 3.
Gene expression changes. Expression of SIRT1, SIRT3, SOD2, and TFAM from PBMCs was assayed by qPCR. The height of the bars represents the average percent change from pre-study diet during the intermittent fasting (IF) (dark grey) or IF with anti-oxidant supplementation (IF+AO) (light grey) periods. The bars represent an average across the participants and error bars are the standard errors of the mean.
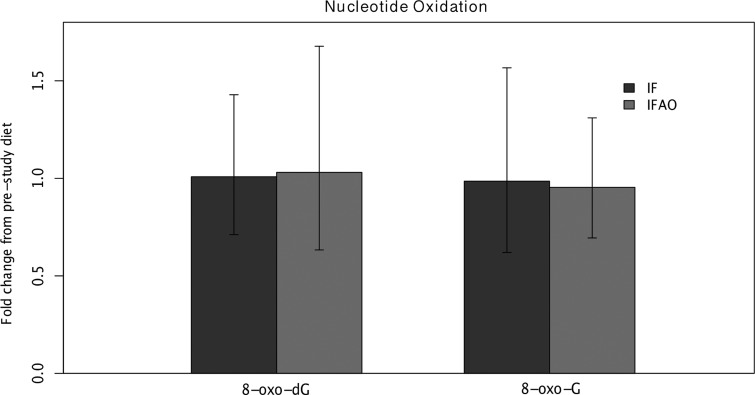
We also examined whether the interventions affected levels of oxidative stress, as previous studies of alternate-day fasting have found decreased oxidative stress.27 We chose to measure RNA (8-oxo-G) and DNA (8-oxo-dG) because they are integrative measures of overall oxidative stress and are perturbed by metabolic changes.34 Levels of RNA oxidation relative to pre-study diets remained unchanged for both IF (0.99; 95% CI [0.62, 1.57]; n=15) and IFAO (0.95; 95% CI [0.69, 1.31]; n=14). Similarly, DNA oxidation measurements demonstrated no change for IF (1.01; 95% CI [0.71, 1.43]; n=16) and IFAO (1.03; 95% CI [0.63, 1.68]; n=14) (Fig. 4). Differences in oxidation levels between the two treatments (i.e., the differential effects due to anti-oxidant supplementation) were also not significant.
FIG. 4.
Nucleotide oxidation changes. Nucleotide oxidation products 8-oxo-7,8-dihydroguanosine (8-oxo-G) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG) were measured by high-performance liquid chromatography coupled to electrochemical detection (HPLC-ECD), normalized by non-oxidative nucleotide levels, and log-transformed before computing mean differences and confidence intervals. The height of the bars represents the (back-transformed) fold change from pre-study diet during the intermittent fasting (IF) (dark grey) or IF with anti-oxidant supplementation (IFAO) (light grey) periods. The bars represent an (back-transformed) average across the participants and error bars are the (back-transformed) 95% confidence intervals.
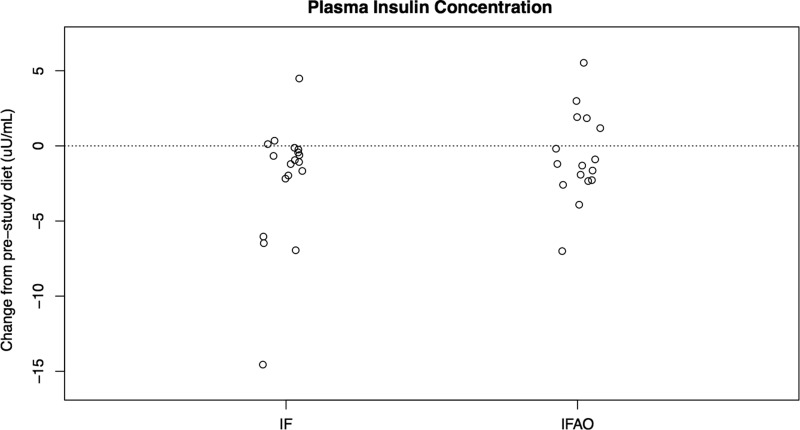
We also examined whether plasma insulin levels were altered as a result of IF or IFAO. We found that IF decreased plasma insulin by 1.01 μU/mL (p=0.0023, Mann–Whitney U-test), whereas IFAO did not significantly impact insulin levels (p=0.33) (Fig. 5).
FIG. 5.
Plasma insulin levels. Changes in plasma insulin levels were evaluated for intermittent fasting (IF) and IF with anti-oxidant supplementation (IFAO). Each point represents a study participant. Plotted are the insulin concentration changes in μU/mL.
Dietary satisfaction survey
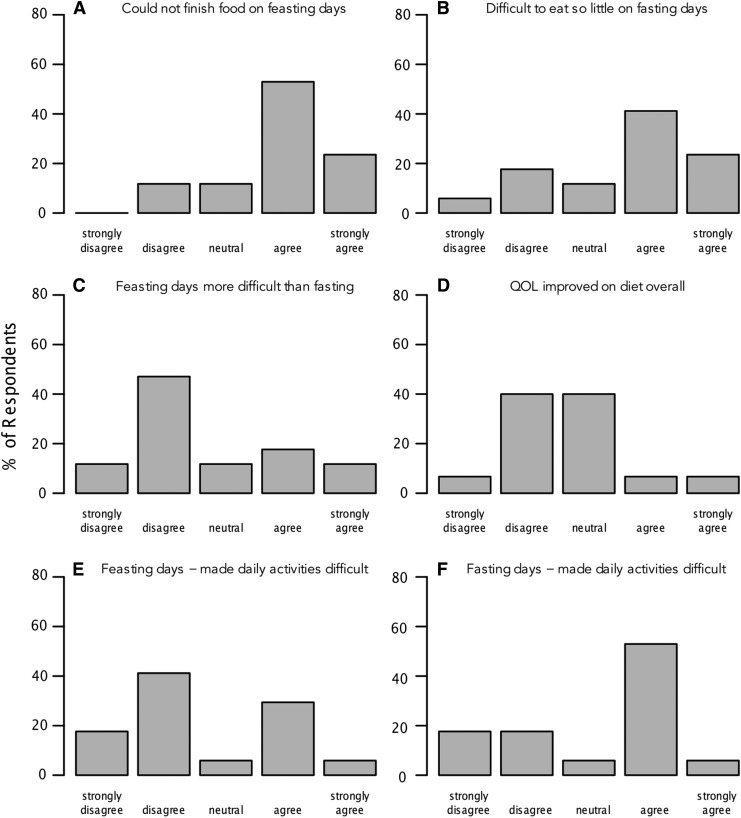
To assess satisfaction with the IF dieting paradigm, we administered a survey to participants following completion of the study (see Supplementary Methods). Here, we summarize responses to six of the survey questions (Fig. 6; full survey results are available in Table S5). A majority of participants expressed difficulty with finishing food on feasting days (76% agreed; Fig. 6A) and with eating so little on fasting days (65% agreed; Fig. 6B). In comparing fasting and feasting days, the majority of participants disagreed that feasting was more difficult than fasting (55% disagreed; Fig. 6C). Few participants rated the diet as improving quality of life overall (35% agreed; Fig. 6D). Importantly, a majority of participants rated the fasting days as making daily activities more difficult (Fig. 6F), but less than half of the participants rated the feasting days as making daily activities more difficult (Fig. 6E). Although participants would recommend the diet to a friend (71% agreed), most described the diet as more difficult than previously attempted diets (71% agreed), and only 18% would follow the diet if prescribed by a physician.
FIG. 6.
Dietary satisfaction survey results. Participants were administered a dietary satisfaction survey, which was graded on a five-point scale (strongly disagree, disagree, neutral, agree, and strongly agree). Results for six questions from the survey are shown. The height of the bars represents the percentage of respondents providing a given rating.
Discussion
In this study, we have assessed the practicality of an IF diet in healthy non-obese young adults and the diet's effect on expression of genes implicated in biological mechanisms related to aging and metabolism. Participants were generally adherent to study diets, with most non-adherence occurring when participants consumed slightly fewer calories than prescribed on feasting days. Dietary satisfaction surveys indicated that the diet was generally acceptable.
We found suggestive evidence that IF (without anti-oxidant supplementation) mildly increases SIRT3 expression (+2.71%). The results are also suggestive of small increases in all genes examined, with the effect on SOD2 and TFAM expression potentially abrogated by anti-oxidant supplementation. These findings suggest that IF may mediate some protective responses by increasing SIRT3 expression, but these responses are not seemingly abrogated by further supplementation with anti-oxidants. This might suggest that SIRT3 is not induced as a hormetic response to oxidative stress. We also found that plasma insulin levels decreased as a result of the IF diet (−1.01 μU/mL), but not IFAO. This suggests that the IF diet may have a beneficial effect on glucose metabolism by lower insulin levels and perhaps have an anti-diabetic effect. Furthermore, the finding that IFAO did not lower insulin levels suggests that the insulin-lowering effects might be stimulated by IF-induced oxidative stress, which is quenched by the further anti-oxidant supplementation. Importantly, these findings are attributable to the IF diet in the absence of weight loss, thus removing weight change as a confounding variable. Future studies will examine additional metabolic markers and hormones to disentangle the effects of IF and IFAO on metabolism.
We detected mild elevation (2.71%) of SIRT3 as a result of the IF diet, which trended toward statistical significance (p=0.0772). Elevation of SIRT3 is consistent with literature indicating that SIRT3 mediates many of the benefits of CR.35,36 SIRT3 is a member of the sirtuin family of histone deacetylases, and recent studies indicate that SIRT3 may be key in the mechanism underlying the beneficial effects of CR.37,38 SIRT3 is primarily localized to the mitochondria and is believed to be the primary mitochondrial protein deacetylase in the mitochondria.36,39 Several studies have suggested a role for SIRT3 in protecting against oxidative stress.37,38 However, these studies were largely limited to animal models and cell culture systems. Our results, while not definitive, suggest that SIRT3 elevation can occur with intermittent CR in humans. Future human clinical trial trials are needed to definitively establish a role for SIRT3 in vivo.
The results of our satisfaction survey indicate that participants found the diet acceptable, suggesting that IF could be practical for humans (Fig. 6; Table S5). Overall compliance was excellent, with participants consuming between±5% of the prescribed food on most (80% or more) pre-conditioning and fasting days. However, we found that adherence decreased substantially on feasting days; participants had difficulty consuming the full 175% of normal caloric intake (Fig. 2A). If no stipulations for consumption were established on feasting days, it would seem that most individuals would not meet their net caloric requirements, and thus consistent, repeated days of IF would be expected to produce weight loss. These compliance and satisfaction data provide important results that could inform the design of dietary schemes and future clinical trials.
There are several limitations to our study. First, we had a small sample size. However, computed confidence intervals were precise, especially for gene expression, having a range between a 6% to 11% change from baseline values. Also, our sample size allowed for the detection of approximately 60% increase or 35% decrease in PBMC nucleotide oxidation levels. Second, IF and IFAO treatments were only 3 weeks long, which may be too little time for changes to be observed. However, previous studies of IF have detected effects in as few as 2 weeks.22,27 Third, we only collected peripheral blood from the participants and measured oxidation levels and gene expression changes in the isolated PBMCs. Unlike tissues such as muscle or adipose, PBMCs do not have a clear role in metabolism and are likely a poor proxy for the metabolic and oxidative changes occurring in tissues more relevant to metabolism. Blood samples, however, have been used in previous trials of IF40 and are more feasible for future larger-scale studies. Fourth, we did not objectively assess circulating anti-oxidant levels. Although good adherence to study-provided anti-oxidants was assessed via pill counts (Table S4), it is possible that participants misrepresented the number of pills taken. Previous studies have also indicated poor bioavailability for orally administered vitamins C and E.41,42 Last, only a single blood sample was collected prior to and after each intervention period. Questions remain whether this single measurement has appropriate reliability for the markers of interest. Discerning this potential caveat will require more frequent measurement in future trials.
We note that our IF approach, where participants alternate between 25% and 175% of normal caloric intake, is an intense dietary regimen chosen to maximize effect size given a limited sample size and short treatment duration. Thus, the dietary scheme was designed as a “proof-of-principle” that IF with caloric maintenance could have metabolic benefits. We also assessed compliance and satisfaction with the diet. While compliance was strong, a majority of participants found that fasting days made activities of daily living more difficult and only 18% of participants would adhere to the diet if prescribed by a physician. These data suggest that modifications to the dieting paradigm are needed to maximize potential effectiveness and patient satisfaction. Such refinements might involve spacing out fasting days (e.g., one to two times per week) or increasing caloric intake on fasting days while decreasing or eliminating extra caloric intake on other days. For example, studies have shown that isolated fasting days during the Ramadan holiday can have metabolic benefits.43 Another caveat of our IF approach is that participants were instructed to consume 175% of normal caloric intake on feasting days. This overeating on feasting days could induce oxidative stress in and of itself, obscuring a potential effect of IF. Our approach contrasts with alternate-day fasting diet schemes where participants eat ad libitum on feasting days. However, it is known that individuals on this dieting paradigm and who are allowed to eat ad libitum on feasting days will not eat enough calories to fully compensate for the restricted caloric intake on fasting days.22,27,28 Consistently, we found that participants had difficulty consuming the full 175% of normal caloric intake on feasting days (Fig. 2A). We chose this approach to maintain overall caloric intake/energy balance to try to disentangle the effects of decreased caloric intake from IF. Nonetheless, additional research is needed to identify the optimal dieting schemes.
The findings of our study suggest that IF may have some benefits on biological pathways that directly affect metabolism and potentially longevity, even in healthy individuals. The results will inform the design of future studies of IF diets. However, additional research is needed to better assess the effects of the IF dietary regimen. These studies will likely include larger participant cohorts treated for extended periods of time. Metabolic tissues such as adipose or muscle will need to be collected to better understand the metabolic effects. Moreover, additional studies will also need to assess how oxidative stress and anti-oxidants interplay with dietary restriction to mediate the potential health effects.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants for their time and contributions to this project. In addition, we would like to thank Jonathan Shuster, PhD, for his statistical advisement during the study design and Brian Bouverat for his assistance with the insulin assay and technical advice. We are also grateful to the University of Florida MD-PhD program and Skip Harris for their support and encouragement.
This work was supported in part by the National Institutes of Health Clinical and Translational Science Awards to the University of Florida (TL1 TR000066 and UL1 TR000064) and the National Institute on Aging award to the University of Florida Institute on Aging and Claude D. Pepper Older Americans Independence Center (1 P30AG028740). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Cruzen C, Colman RJ. Effects of caloric restriction on cardiovascular aging in non-human primates and humans. Clin Geriatr Med 2009;25:733–743, ix–x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anton S, Leeuwenburgh C. Fasting or caloric restriction for healthy aging. Exp Gerontol 2013;48:1003–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 2010;45:410–418 [DOI] [PubMed] [Google Scholar]
- 4.Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: An emerging link. Aging (Albany NY) 2013;5:144–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med 2011;51:327–336 [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Curr Opin Clin Nutr Metab Care 2009;12:431–437 [DOI] [PubMed] [Google Scholar]
- 7.Haigis MC, Yankner BA. The aging stress response. Mol Cell 2010;40:333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013;155:1624–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians' Health Study II randomized controlled trial. JAMA 2008;300:2123–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the women's antioxidant cardiovascular study. Arch Intern Med 2007;167:1610–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kataja-Tuomola M, Sundell JR, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia 2008;51:47–53 [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE, Manson JE. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J Natl Cancer Inst 2009;101:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: A randomized controlled trial. Am J Clin Nutr 2009;90:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selman C, McLaren JS, Meyer C, Duncan JS, Redman P, Collins AR, Duthie GG, Speakman JR. Life-long vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech Ageing Dev 2006;127:897–904 [DOI] [PubMed] [Google Scholar]
- 15.Gaziano JM, Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schvartz M, Manson JE, Glynn RJ, Buring JE. Multivitamins in the prevention of cancer in men: The physicians' health study II randomized controlled trial. JAMA 2012;308:1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Q, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen H. Multivitamin use and telomere length in women. Am J Clin Nutr 2009;89:1857–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chew EY, Clemons TE, Agron E, Sperduto RD, Sangiovanni JP, Kurinij N, Davis MD, Age-Related Eye Disease Study Research Group. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 2013;120:1604–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarris J, Cox KH, Camfield DA, Scholey A, Stough C, Fogg E, Kras M, White DJ, Sali A, Pipingas A. Participant experiences from chronic administration of a multivitamin versus placebo on subjective health and wellbeing: A double-blind qualitative analysis of a randomised controlled trial. Nutr J 2012;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juraschek SP, Guallar E, Appel LJ, Miller ER., 3rd Effects of vitamin C supplementation on blood pressure: A meta-analysis of randomized controlled trials. Am J Clin Nutr 2012;95:1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 2008;87:142–149 [DOI] [PubMed] [Google Scholar]
- 21.McCaffree J. What you should know about calorie restriction. J Am Diet Assoc 2004;104:1524., 1526. [DOI] [PubMed] [Google Scholar]
- 22.Varady KA, Bhutani S, Church EC, Klempel MC. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am J Clin Nutr 2009;90:1138–1143 [DOI] [PubMed] [Google Scholar]
- 23.Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res 2005;13:574–581 [DOI] [PubMed] [Google Scholar]
- 24.Halberg N, Henriksen M, Soderhamn N, Stallknecht B, Ploug T, Schjerling P, Dela F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J Appl Physiol 2005;99:2128–2136 [DOI] [PubMed] [Google Scholar]
- 25.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK, Longo DL, Mattson MP. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr 2007;85:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM, Flyvbjerg A, Howell A. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 2007;42:665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varady KA, Bhutani S, Klempel MC, Kroeger CM, Trepanowski JF, Haus JM, Hoddy KK, Calvo Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr J 2013;12:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collier R. Intermittent fasting: The next big weight loss fad. CMAJ 2013;185:E321–E322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA. Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J 2012;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–247 [DOI] [PubMed] [Google Scholar]
- 32.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Available at www.health.gov/dietaryguidelines/2010.asp
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 34.Hofer T, Seo AY, Prudencio M, Leeuwenburgh C. A method to determine RNA and DNA oxidation simultaneously by HPLC-ECD: Greater RNA than DNA oxidation in rat liver after doxorubicin administration. Biol Chem 2006;387:103–111 [DOI] [PubMed] [Google Scholar]
- 35.Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci 2013;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman JC, He W, Verdin E. Mitochondrial protein acylation and intermediary metabolism: Regulation by sirtuins and implications for metabolic disease. J Biol Chem 2012;287:42436–42443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 2010;12:662–667 [DOI] [PubMed] [Google Scholar]
- 38.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010;143:802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 2007;27:8807–8814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elliott RM, de Roos B, Duthie SJ, Bouwman FG, Rubio-Aliaga I, Crosley LK, Mayer C, Polley AC, Heim C, Coort SL, Evelo CT, Mulholland F, Daniel H, Mariman EC, Johnson IT. Transcriptome analysis of peripheral blood mononuclear cells in human subjects following a 36 h fast provides evidence of effects on genes regulating inflammation, apoptosis and energy metabolism. Genes Nutr 2014;9:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr 2010;103:1251–1259 [DOI] [PubMed] [Google Scholar]
- 42.Borel P, Preveraud D, Desmarchelier C. Bioavailability of vitamin E in humans: An update. Nutr Rev 2013;71:319–331 [DOI] [PubMed] [Google Scholar]
- 43.Faris MA, Hussein RN, Al-Kurd RA, Al-Fararjeh MA, Bustanji YK, Mohammad MK. Impact of ramadan intermittent fasting on oxidative stress measured by urinary 15-f(2t)-isoprostane. J Nutr Metab 2012;2012:802924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.