Abstract
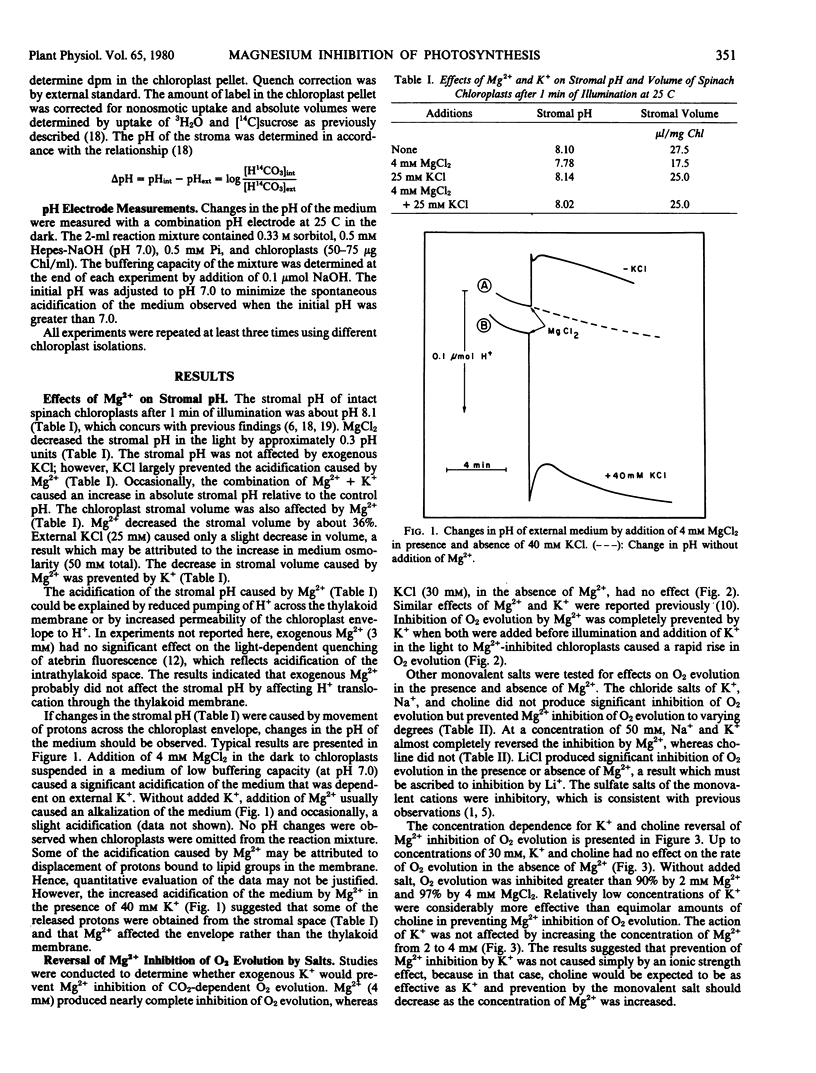
Exogenous Mg2+ (2 millimolar) altered the stromal pH of intact spinach chloroplasts. Without added KCl in the medium, Mg2+ decreased the stromal pH in the light by approximately 0.3 pH unit. External KCl (25 millimolar) largely prevented the acidification caused by Mg2+. Effects on the stromal pH were not caused by changes in H+ pumping across the thylakoid membrane because Mg2+ had no effect on the light-induced quenching of atebrin fluorescence by intact chloroplasts. However, Mg2+ affected H+ fluxes across the envelope. Addition of Mg2+ to intact chloroplasts in the dark caused a significant acidification of the medium that was dependent on the presence of K+.
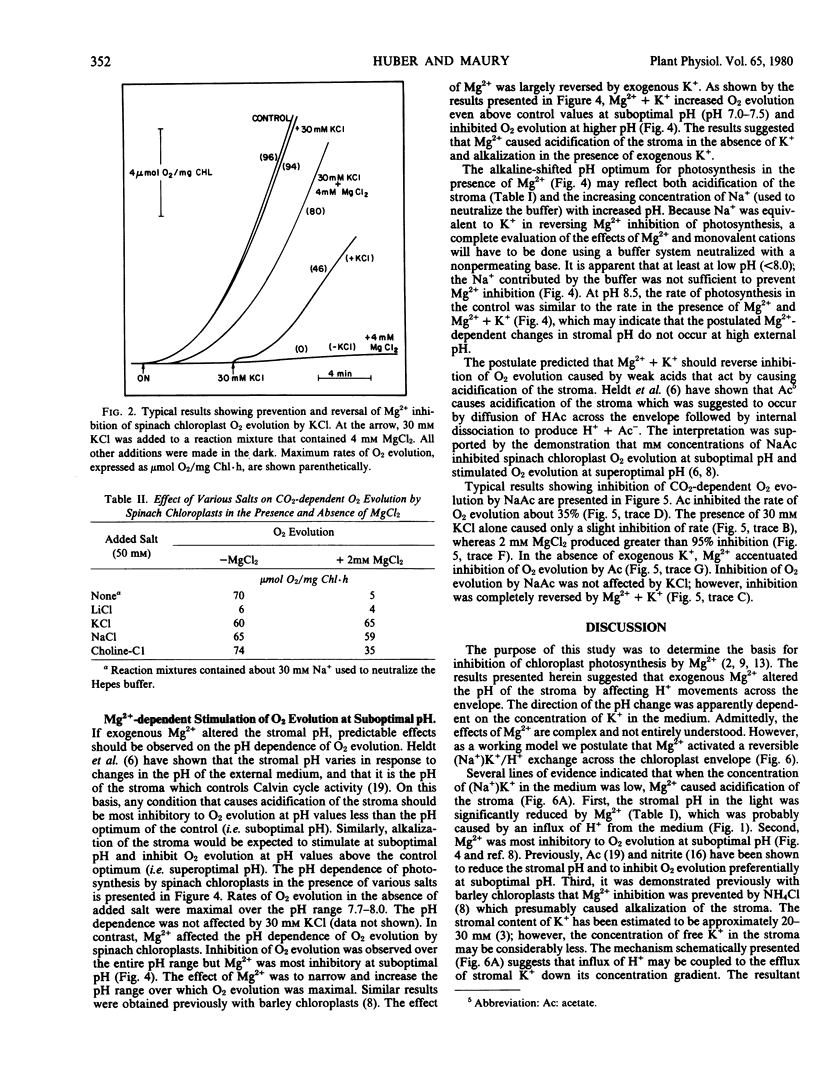
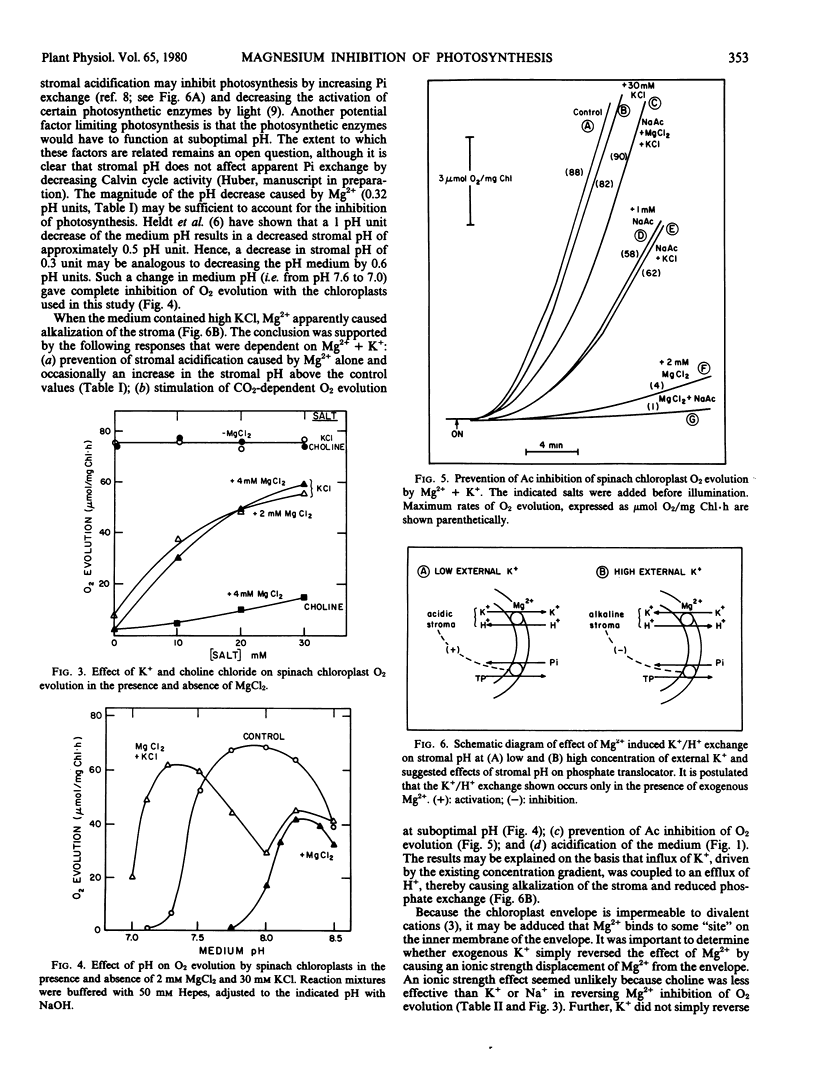
External K+ or Na+ also prevented the inhibition of CO2-dependent O2 evolution by Mg2+, whereas choline chloride was less effective. The combination of Mg2+ and K+ stimulated O2 evolution at suboptimal pH, inhibited O2 evolution at optimal and superoptimal pH, and prevented the inhibition of photosynthesis caused by acetate. In the absence of added K+, Mg2+ was most inhibitory to O2 evolution at suboptimal pH.
The results suggested that Mg2+ activated a reversible (Na+)K+/H+ exchange across the chloroplast envelope. It is postulated that changes in the stromal pH may explain the inhibition of photosynthesis caused by the presence of exogenous Mg2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldry C. W., Cockburn W., Walker D. A. Inhibition, by sulphate, of the oxygen evolution associated with photosynthetic carbon assimilation. Biochim Biophys Acta. 1968 Feb 12;153(2):476–483. doi: 10.1016/0005-2728(68)90089-3. [DOI] [PubMed] [Google Scholar]
- Bamberger E. S., Avron M. Site of action of inhibitors of carbon dioxide assimilation by whole lettuce chloroplasts. Plant Physiol. 1975 Oct;56(4):481–485. doi: 10.1104/pp.56.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Hensley J. R., Hanson J. B. The action of valinomycin in uncoupling corn mitochondria. Plant Physiol. 1975 Jul;56(1):13–18. doi: 10.1104/pp.56.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Effect of pH on chloroplast photosynthesis. Inhibition of O2 evolution by inorganic phosphate and magnesium. Biochim Biophys Acta. 1979 Jan 11;545(1):131–140. doi: 10.1016/0005-2728(79)90120-8. [DOI] [PubMed] [Google Scholar]
- Huber S. C. Effect of photosynthetic intermediates on the magnesium inhibition of oxygen evolution by barley chloroplasts. Plant Physiol. 1979 Apr;63(4):754–757. doi: 10.1104/pp.63.4.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Regulation of chloroplast photosynthetic activity by exogenous magnesium. Plant Physiol. 1978 Sep;62(3):321–325. doi: 10.1104/pp.62.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause G. H., Thorne S. W., Lorimer G. H. Glycolate synthesis by intact chloroplasts. Studies with inhibitors of photophosphorylation. Arch Biochem Biophys. 1977 Oct;183(2):471–479. doi: 10.1016/0003-9861(77)90382-4. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Schwenn J. D., Walker D. A. Inorganic pyrophosphatase and photosynthesis by isolated chloroplasts. II. The controlling influence of orthophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):596–604. doi: 10.1016/0005-2728(73)90219-3. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Purczeld P., Chon C. J., Portis A. R., Jr, Heldt H. W., Heber U. The mechanism of the control of carbon fixation by the pH in the chloroplast stroma. Studies with nitrite-mediated proton transfer across the envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):488–498. doi: 10.1016/0005-2728(78)90116-0. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W. Accumulation of bicarbonate in intact chloroplasts following a pH gradient. Biochim Biophys Acta. 1972 Dec 14;283(3):430–441. doi: 10.1016/0005-2728(72)90260-5. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


