Abstract
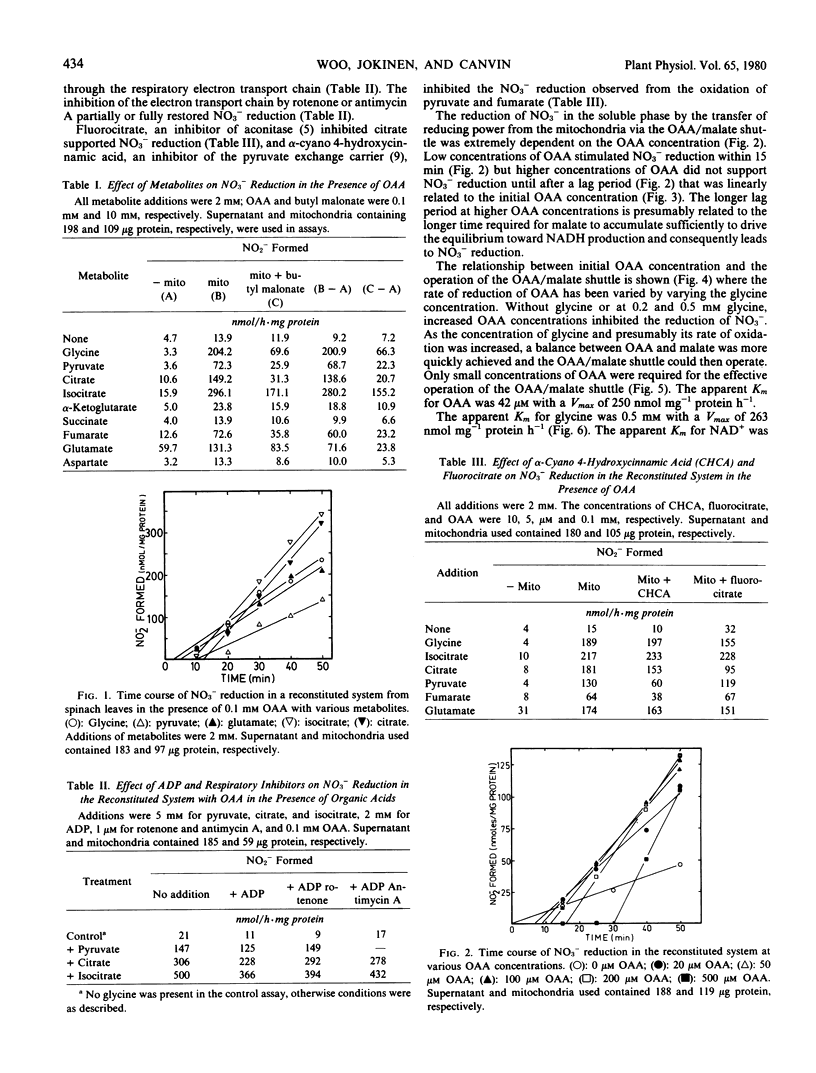
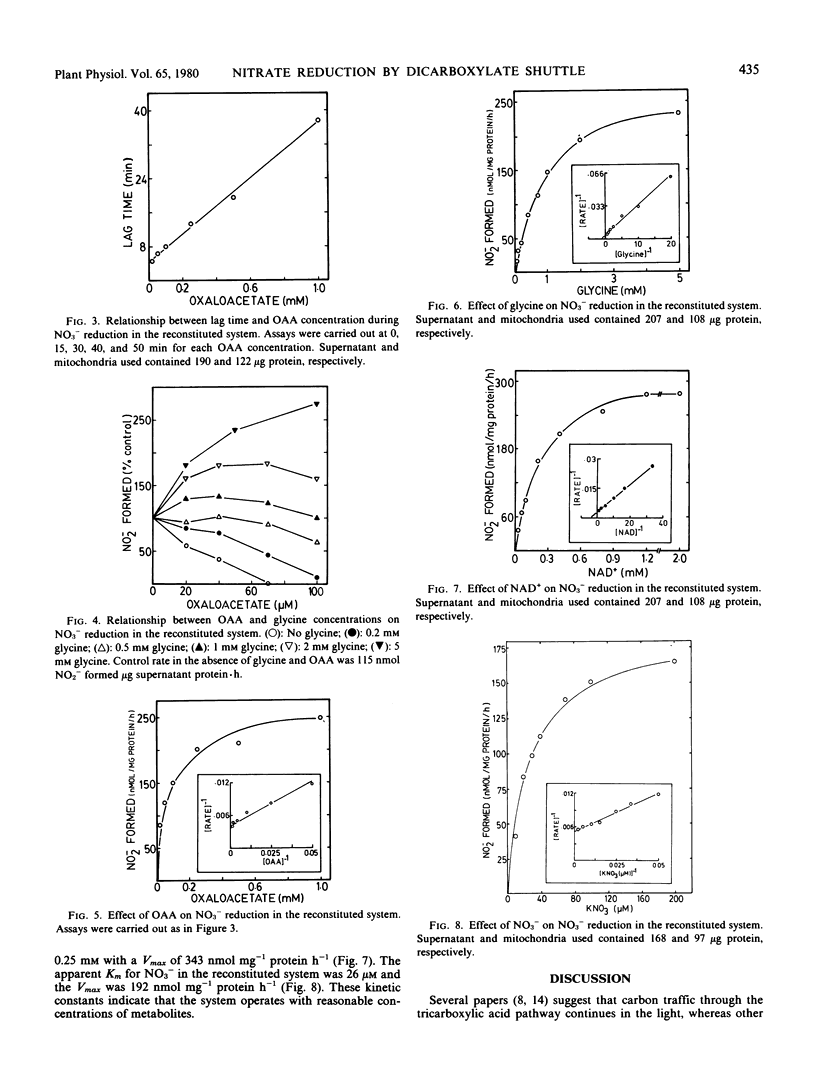
Substantial rates of nitrate reduction could be achieved with a reconstituted system from spinach leaves containing supernatant, mitochondria, NAD+, oxaloacetate (OAA), and an oxidizable substrate. Appropriate substrates were glycine, pyruvate, citrate, isocitrate, fumarate, or glutamate. The reduction of NO3− with any of the substrates could be inhibited by n-butyl malonate, showing that the transfer of reducing power from the mitochondria to the supernatant involved the malate exchange carrier. The addition of ADP to the reconstituted system decreased NO3− reduction and this decrease could be reversed by the addition of rotenone or antimycin A. The operation of the OAA/malate shuttle was achieved most quickly in the system when low concentrations (≤0.1 millimolar) of OAA were added. A corresponding increase in the lag time for the operation of the OAA/malate shuttle was observed when the OAA concentration was increased. Concentrations for half-maximal activity of OAA, glycine, NAD+, and NO3− in the reconstituted system were 42 micromolar, 0.5 millimolar, 0.25 millimolar, and 26 micromolar, respectively. The transfer of reducing power from the mitochondria to the soluble phase via the OAA/malate shuttle can not only provide NADH for cytoplasmic reduction but can also sustain oxidation of tricarboxylic cycle acids and the generation of α-ketoglutarate independently of the respiratory electron transport chain.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird I. F., Cornelius M. J., Keys A. J., Whittingham C. P. Adenosine triphosphate synthesis and the natural electron acceptor for synthesis of serine from glycine in leaves. Biochem J. 1972 Jun;128(1):191–192. doi: 10.1042/bj1280191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. D., Evans S. M., Mendes-Mourão J., Chappell J. B. Fluorocitrate inhibition of aconitate hydratase and the tricarboxylate carrier of rat liver mitochondria. Biochem J. 1973 May;134(1):217–224. doi: 10.1042/bj1340217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. A., Graham D. The Effect of Light on the Tricarboxylic Acid Cycle in Green Leaves: II. Intermediary Metabolism and the Location of Control Points. Plant Physiol. 1974 Jun;53(6):886–892. doi: 10.1104/pp.53.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Hanson J. B. Pyruvate and malate transport and oxidation in corn mitochondria. Plant Physiol. 1977 Apr;59(4):630–635. doi: 10.1104/pp.59.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Chappell J. B. The inhibition of malate, tricarboxylate and oxoglutarate entry into mitochondria by 2-n-butylmalonate. Biochem Biophys Res Commun. 1967 Jul 21;28(2):249–255. doi: 10.1016/0006-291x(67)90437-8. [DOI] [PubMed] [Google Scholar]
- Rocha V., Ting I. P. Malate dehydrogenases of leaf tissue from Spinacia oleracea: properties of three isoenzymes. Arch Biochem Biophys. 1971 Nov;147(1):114–122. doi: 10.1016/0003-9861(71)90316-x. [DOI] [PubMed] [Google Scholar]
- Sawhney S. K., Naik M. S., Nicholas D. J. Regulation of NADH supply for nitrate reduction in green plants via photosynthesis and mitochondrial respiration. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1209–1216. doi: 10.1016/0006-291x(78)91265-2. [DOI] [PubMed] [Google Scholar]
- Woo K. C. Properties and intramitochondrial localization of serine hydroxymethyltransferase in leaves of higher plants. Plant Physiol. 1979 Apr;63(4):783–787. doi: 10.1104/pp.63.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]


