ABSTRACT
The B cell-activating factor (BAFF) is critical for B cell development and humoral immunity in mice and humans. While the role of BAFF in B cells has been widely described, its role in innate immunity remains unknown. Using BAFF receptor (BAFFR)-deficient mice, we characterized BAFFR-related innate and adaptive immune functions following infection with vesicular stomatitis virus (VSV) and lymphocytic choriomeningitis virus (LCMV). We identified a critical role for BAFFR signaling in the generation and maintenance of the CD169+ macrophage compartment. Consequently, Baffr−/− mice exhibited limited induction of innate type I interferon production after viral infection. Lack of BAFFR signaling reduced virus amplification and presentation following viral infection, resulting in highly reduced antiviral adaptive immune responses. As a consequence, BAFFR-deficient mice showed exacerbated and fatal disease after viral infection. Mechanistically, transient lack of B cells in Baffr−/− animals resulted in limited lymphotoxin expression, which is critical for maintenance of CD169+ cells. In conclusion, BAFFR signaling affects both innate and adaptive immune activation during viral infections.
IMPORTANCE Viruses cause acute and chronic infections in humans resulting in millions of deaths every year. Innate immunity is critical for the outcome of a viral infection. Innate type I interferon production can limit viral replication, while adaptive immune priming by innate immune cells induces pathogen-specific immunity with long-term protection. Here, we show that BAFFR deficiency not only perturbed B cells, but also resulted in limited CD169+ macrophages. These macrophages are critical in amplifying viral particles to trigger type I interferon production and initiate adaptive immune priming. Consequently, BAFFR deficiency resulted in reduced enforced viral replication, limited type I interferon production, and reduced adaptive immunity compared to BAFFR-competent controls. As a result, BAFFR-deficient mice were predisposed to fatal viral infections. Thus, BAFFR expression is critical for innate immune activation and antiviral immunity.
INTRODUCTION
The B cell-activating factor (BAFF) receptor (BAFFR) is critical for B cell development (1, 2). Patients lacking the BAFFR have been identified within cohorts with common variable immunodeficiency, the most prevalent symptomatic primary immunodeficiency in adult patients. BAFFR-deficient humans exhibit severe B cell lymphopenia and impaired immunoglobulin production (3). Similarly, the lack of BAFF signaling in Baffr−/− mice is also associated with severe B cell lymphopenia (4). BAFF binds to three receptors, the BAFFR, transmembrane activator and CAML interactor (TACI), and B cell maturation antigen (BCMA) (5). While TACI and BCMA engage both BAFF and a proliferation-inducing ligand (APRIL), BAFFR binds BAFF exclusively. The BAFF-BAFFR association leads to recruitment and degradation of TRAF3 (6). TRAF3 negatively regulates NF-κB-inducing kinase (NIK), the upstream kinase for NF-κB2 activation (7). In the presence of BAFF, degradation of TRAF3 leads to stabilization of NIK and activation of NF-κB2, which triggers B cell survival (8–11). Furthermore, BAFF triggers activation of Akt signaling pathways, which increase the metabolic activity of B cells (12). Additionally, BAFF transmits survival signals via Erk activation, which triggers phosphorylation and degradation of the proapoptotic molecule Bim (13–15). Together, these signaling pathways promote BAFF-mediated survival of B cells. Whether innate immunity may be abnormal in Baffr−/− mice has not yet been investigated.
Successful defense against viral infections relies on effective innate and adaptive immunity. During infection, viral particles are captured in secondary lymphoid organs, such as the lymph node or the spleen (16). In mice, macrophages in the marginal sinus in the splenic white pulp or macrophages located in the subcapsular space of the lymph node filter pathogens from the blood and the lymph, respectively (16–18). Metallophilic macrophages and subcapsular sinus macrophages are characterized by expression of the C-type lectin CD169 (Siglec-1) (19). CD169+ cells are critical for innate cytokine production and viral antigen presentation to B cells and represent an important link between innate and adaptive immunity (18). In particular, Usp18-driven suppression of antiviral type I interferon signaling allows viral replication to occur in CD169+ cells in close proximity to marginal-zone B cells (20, 21). Consequently, large quantities of viral antigen are made available in order to induce rapid and robust adaptive immunity (22–25). The rapid induction of adaptive immune responses as a result of early viral replication in the spleen guarantees virus elimination and survival of the virus-infected host (20, 21).
B cells are important for the generation of the splenic architecture, including maintenance of the marginal zone (26, 27). Following a recent report indicating that BAFF is produced by neutrophils in the marginal zone of the spleen (28), we chose to investigate the impact of BAFFR signaling on innate immune responses. We found that absence of BAFFR resulted in reduced lymphotoxin expression, decreased presence of CD169+ cells, delayed and impaired innate and adaptive immune activation, and, consequently, promotion of fatal disease development after viral infection.
MATERIALS AND METHODS
Mice.
Mice were infected intravenously with vesicular stomatitis virus (VSV) or lymphocytic choriomeningitis virus (LCMV) at the indicated doses. Baffr−/− mice were bred on a C57BL/6 genetic background (4). CD45.1+ mice were purchased from Jackson Laboratory. Ltbfl/fl × CD19-Cre+ and Ifnar1−/− mice were previously described (29, 30). All experiments were performed in single ventilated cages. During survival experiments, the health status of the mice was checked twice daily. Upon the appearance of clinical signs of VSV replication in the central nervous system (CNS), such as paralysis, mice were removed from the experiment. Animal experiments were carried out with the authorization of the Veterinäramt of Nordrhein Westfalen, Recklinghausen, Germany, in accordance with the German law for animal protection and the institutional guidelines of the Ontario Cancer Institute.
Viruses.
VSV, Indiana strain (VSV-IND, Mudd-Summers isolate), was originally obtained from D. Kolakofsky (University of Geneva, Geneva, Switzerland). The virus was propagated on BHK-21 cells at a multiplicity of infection (MOI) of 0.01 and was then plaqued onto Vero cells. LCMV was used and virus titers were determined as previously described (21). Viruses were administered to mice through intravenous injection.
Poly(I·C) and recombinant interferon.
Poly(I·C) (GE Healthcare Life Sciences) and mouse recombinant interferon alpha (IFN-α) A (PBL Biosciences) were administered to mice through intravenous injection at the indicated doses.
Neutralizing antibodies were determined by a plaque reduction neutralization test (PRNT) as previously described (21). Serum was prediluted (1:40), and the complement system was inactivated (56°C for 30 min). The serum was titrated 1:2 over 12 steps and incubated with 1,000 PFU of VSV. After 90 min of incubation, the virus-serum mixture was plaqued on Vero cells. An overlay was added after 1 h. Plaques were counted 24 h later by crystal violet staining. The cutoff was 50% reduction of plaques compared to serum-free controls.
ELISA.
Interferon alpha enzyme-linked immunosorbent assay (ELISA) (PBL Biosciences) was performed as instructed by the manufacturer.
Purification of B cells.
For B cell purification, single-cell suspensions of splenocytes were enriched following the manufacturer's instructions with a CD45R (B220) MicroBeads mouse kit (Miltenyi).
RT-PCR analyses.
RNA purification and real-time (RT)-PCR analyses were performed as previously described according to the manufacturer's instructions (Qiagen) (31). Gene expression of Isg15, Mx1, Ifit2, Lta, Ltb, and Gapdh was performed using kits from Applied Biosystems. For analysis, the expression levels of all target genes were normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression levels (ΔCT). Gene expression values were then calculated based on the ΔΔCT method relative to naive wild-type (WT) controls. Relative quantities (RQ) were determined using the following equation: RQ = 2−ΔΔCT.
Histology.
Histological analyses were performed on snap-frozen tissue as described previously by using anti-VSV-G monoclonal antibody (clone Vi10) (21). CD90.2, CD4, CD8, and B220 antibodies were purchased from eBioscience (San Diego, CA). CD169 (clone MOMA-1) antibody was obtained from Abcam (Cambridge, MA). Hematoxylin and eosin (H&E) staining was described previously (32).
Flow cytometry.
Flow cytometry analyses were performed as previously described for LCMV tetramer staining and intracellular-cytokine staining (33). Different spleen immune populations were identified using anti-B220 (RA3-6B2), anti-major histocompatibility complex class II (MHC-II) (M5/114.15.2), anti-CD11c (N418), anti-GR-1 (RB6-8C5), anti-F4/80 (BM8), anti-CD3 (145-2C11), anti-CD8 (52-6.7), and anti-CD4 (GK1.5) antibodies (clone). Stem cell analyses were performed as previously described (34). The lineage (Lin) antibody mixture used for progenitor analysis contained CD3, CD11b (M1/70), Ly6C, Ter119 (TER-119), CD19, CD11c, MHC-II, interleukin 7 receptor (IL-7R) (A7R34), and NK1.1. Common myeloid progenitors (CMP) and granulocyte and macrophage progenitors (GMP) were determined using anti-CD34 (RAM34), anti-CD16/32 (93), anti-Sca1 (D7), and anti-CD117 (2B8) antibodies. All antibodies were obtained from eBioscience (San Diego, CA), except anti-CD169 (3D6.112), which was obtained from AbD Serotec (Dusseldorf, Germany).
Statistical analysis.
Data are expressed as means and standard errors of the mean (SEM). Student's t test was used to detect statistically significant differences between two groups. Significant differences between several groups were detected by one-way analysis of variance (ANOVA) with Bonferroni or Dunnett post hoc tests or as mentioned specifically in the figure legends. The level of statistical significance was set at a P value of <0.05.
RESULTS
BAFFR is critical in overcoming viral infection.
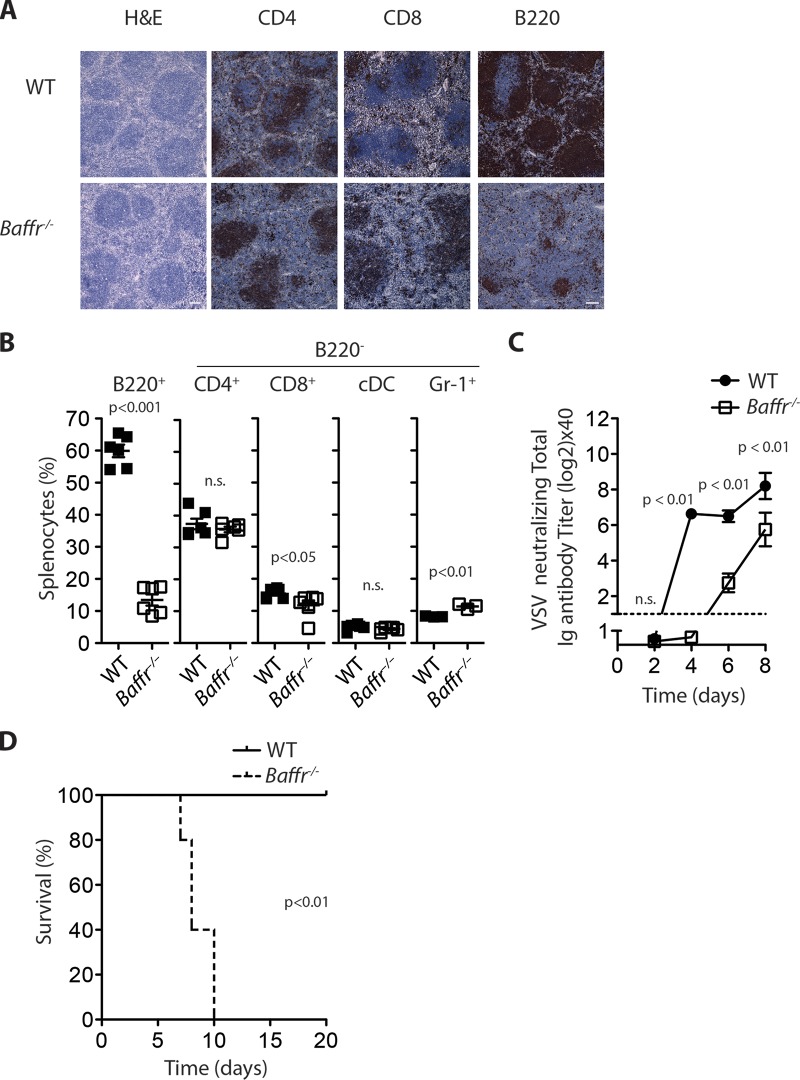
Murine BAFFR deficiency resulted in severe B cell lymphopenia but did not have a major impact on T cell, dendritic cell, or neutrophil numbers (Fig. 1A and B). As expected, Baffr−/− mice exhibited delayed virus-neutralizing antibody production after infection with the cytolytic VSV (Fig. 1C). Consistent with a requirement for rapid neutralizing antibody formation for elimination of VSV (21), Baffr−/− mice succumbed to VSV infection, while WT animals overcame the infection (Fig. 1D). When neuronal tissue was harvested from the sick animals (days 7 to 10), the presence of VSV could be detected (data not shown). These data suggest that absence of BAFFR resulted in fatal disease development during infection with VSV.
FIG 1.
BAFFR is essential for antiviral immunity. (A) Sections from snap-frozen spleen tissues of WT and Baffr−/− mice were stained with H&E (scale bar = 100 μm) or stained with anti-CD4, anti-CD8, and anti-B220 antibodies (scale bar = 50 μm). One representative out of 5 is shown. (B) Single-cell suspensions from splenocytes derived from WT and BAFFR-deficient mice were analyzed for immune cell populations with monoclonal antibodies specific for the indicated markers (n = 6). n.s., not significant. Except for B220+ cells, the percentages are related to B220− splenocytes). (C and D) WT and Baffr−/− mice were infected with 105 PFU of VSV. (C) Serum was taken at the indicated time points and analyzed for antiviral neutralizing antibodies by PRNT assay (n [initial] = 8). (D) Survival was monitored over the indicated period (n [initial] = 5). The error bars show SEM; n.s., not significant.
BAFFR mediates enforced viral replication during viral infection.
Despite detection of VSV replication in the CNS in the later phase of infection, VSV titers were below the detection limit in spleen tissues of Baffr−/− animals 8 h and 24 h after infection with VSV, while virus was readily detectable in WT controls (Fig. 2A). Overcoming VSV infection is not only dependent on production of neutralizing antibodies, but also on innate type I interferon production (30, 35, 36). Consistent with decreased replication of VSV in spleen tissue, Baffr−/− mice had reduced type I interferon levels in the serum compared to infected WT mice (Fig. 2B). Moreover, the increase of interferon-regulated genes (IRGs), such as Isg15, Mx1, and Ifit2, 24 h after VSV infection was reduced in brain tissue harvested from BAFFR-deficient animals compared to their corresponding controls (Fig. 2C). However, competent production of type I interferon was detectable in Baffr−/− mice following injection of the Toll-like receptor 3 (TLR3) agonist poly(I·C) in vivo (Fig. 2D). Furthermore, IRG expression levels in the brain tissues from Baffr−/− mice and their WT controls following treatment with poly(I·C) were similar (Fig. 2E). This indicates that the capacity to induce type I interferon was normal in Baffr−/− mice. Moreover, when animals were treated with mouse recombinant IFN-α, similar brain IRG expression levels were observed in BAFFR-deficient and WT mice (Fig. 2E). Collectively, reduced type I interferon levels in Baffr−/− mice following virus infection was likely explainable by a different mechanism than defective pathogen recognition receptor (PRR) signaling.
FIG 2.
BAFFR mediates enforced viral replication during viral infection. (A to C) WT and Baffr−/− mice were infected with 105 PFU of VSV. (A) Virus titers were measured in the spleen 8 h (left) and 24 h (right) after infection (p.i.) (n = 6). The dashed line indicates the detection limit. (B) IFN-α concentrations were measured 12 h and 24 h after infection with 105 PFU of VSV in the sera of WT and BAFFR-deficient mice (n = 6). (C) Isg15, Ifit2, and Mx1 mRNA expression was determined from brain tissues of infected WT and Baffr−/− mice 24 h p.i. (n = 5). (D) IFN-α concentrations were examined in the sera of WT and Baffr−/− mice 3 h after challenge with 25 μg or 100 μg poly(I·C) (n = 6). (E) Isg15, Ifit2, and Mx1 mRNA expression was determined from brain tissues of WT and Baffr−/− mice 6 h after treatment with 10,000 units of mouse recombinant IFN-α or 100 μg poly(I·C) (n = 6). The error bars show SEM; n.s., not significant.
BAFF signaling is required for maintenance of metallophilic macrophages in the spleen.
We have recently demonstrated that early virus replication in the spleen depends on CD169+ metallophilic macrophages and is triggered by Usp18-mediated resistance to type I interferon in these cells (21). In mice, CD169+ cells in the spleen are in direct contact with the bloodstream and remove virus particles and apoptotic cells from circulation (21, 37). Lack of CD169+ macrophages blocks virus replication early after infection, causing limited antigen amplification and reduced virus-induced immune activation (21, 38). Since BAFFR-deficient animals exhibited lower viral replication during early infection, we investigated potential mechanisms by which BAFFR signaling might control enforced viral replication. Myeloid progenitor populations in the bone marrow, such as the CMP (Lin−, Sca1−, IL-7R−, CD117+, CD34+, and CD16/32−) or the GMP (Lin−, Sca1−, IL-7R−, CD117+, CD34+, and CD16/32+) did not differ between Baffr−/− and WT mice (Fig. 3A). However, CD169+ metallophilic macrophages, which have a critical role during early viral replication (21), were highly reduced in spleen tissue of Baffr−/− mice compared to WT animals (Fig. 3B), while red-pulp macrophages were present at similar frequencies (Fig. 3C). Taken together, the data show that BAFFR appears to be required for maintenance of CD169+ cells.
FIG 3.
BAFFR signals are critical for maintenance of CD169+ cells and viral replication in spleen tissue early after infection. (A) CMP and GMP in the bone marrow of WT and Baffr−/− mice were analyzed by flow cytometry (n = 6; n.s., not significant). (B) (Left) Sections of snap-frozen spleen tissues of WT and Baffr−/− mice were stained with anti-CD169 and analyzed by fluorescence microscopy (clone MOMA-1; 1 representative out of 6 is shown; scale bar = 100 μm). (Right) CD169+ cells were measured in splenocytes of WT versus Baffr−/− mice by flow cytometry (n = 6). (C) F4/80+ cells were analyzed in spleen tissues from WT and BAFFR-deficient animals by flow cytometry (n = 6). (D) Snap-frozen spleen sections were stained with an anti-CD169 antibody 0, 3, 5, and 7 h after VSV infection of WT versus BAFFR-deficient mice (1 representative out of 6 is shown; scale bars = 100 μm). (E) Sections from snap-frozen spleen tissues obtained from WT and Baffr−/− mice after the indicated time periods following VSV infection were stained with anti-CD169 and anti-VSV-G protein (clone Vi10) (1 representative out of 6 is shown; scale bars = 100 μm).
Next, we investigated the presence of CD169+ cells following infection. CD169+ cells are present at reduced numbers in BAFFR-deficient animals, but a residual population is still detectable in the naive state (Fig. 3D and E). However, shortly after infection with VSV, CD169+ cells rapidly disappear in Baffr−/− mice, in contrast to WT animals (Fig. 3D). As a consequence of reduced CD169+ macrophages, VSV protein (detected by the VSV-specific monoclonal antibody Vi10) was reduced in Baffr−/− mice in spleen sections following VSV infection, while it was readily detectable in spleen sections from WT animals (Fig. 3E). Next, we wondered whether type I interferon directly affected CD169+ cell survival. However, the distributions of CD169+ cells in spleen tissues harvested from Ifnar1−/− mice and WT animals were similar (Fig. 4). Taken together, these data indicate that lack of BAFFR expression is associated with a decreased presence of splenic CD169+ cells after virus infection, which consequently diminishes early viral replication.
FIG 4.
CD169+ cell survival following VSV infection in Ifnar1−/− mice. Sections from snap-frozen spleen tissues obtained from WT and Ifnar1−/− mice after the indicated periods following VSV infection were stained with anti-CD169 and anti-VSV-G protein (clone Vi10) (1 representative out of 6 is shown; scale bars = 100 μm).
BAFFR deficiency results in reduced B cell-mediated maintenance of CD169+ cells.
Next, we addressed whether the reduction of CD169+ cells in Baffr−/− mice occurred due to severe B cell lymphopenia (39, 40). We transferred WT B cells into Baffr−/− mice and monitored CD169+ cells in spleen tissue after 40 days. Interestingly, CD169+ cells were readily detectable in spleen tissue of BAFFR-deficient mice that were supplemented with B cells (Fig. 5A). Furthermore, type I interferon production following VSV infection could be partially rescued by the transfer of WT B cells into Baffr−/− mice (Fig. 5B). Moreover, neutralizing antibody titers of the Baffr−/− animals that received WT B cells was increased compared to Baffr−/− animals (Fig. 5C), and the animals could overcome the VSV infection (Fig. 5D). These data indicate that B cells mediate maintenance of CD169+ cells and consequently contribute to innate immunity during infection.
FIG 5.
B cell-dependent maintenance of CD169+ cells. (A) Baffr−/− mice were reconstituted with sorted WT B cells; 40 days later, spleen tissue was harvested and compared to that of WT and Baffr−/− mice. CD169+ (top row) and transferred B cells (CD45.1; middle row) are shown in WT and Baffr−/− mice and Baffr−/− mice with transferred WT B cells (n = 5; one representative is shown; scale bars = 100 μm). (B to D) Baffr−/− mice were reconstituted with sorted WT B cells; 40 days later, the animals were infected with 105 PFU of VSV and compared to WT and Baffr−/− mice. (B) The IFN-α concentration was measured 24 h after infection in WT and Baffr−/− mice and Baffr−/− mice with transferred WT B cells. *, P < 0.05; ****, P < 0.0001; the Holm-Sidak test was used for post hoc testing. (C) Neutralizing Ig titers were determined at the indicated time points after infection (n = 4 or 5). ***, P < 0.001 between WT and Baffr−/− mice, and ##, P < 0.01 between WT and Baffr−/− mice after WT B cell transfer; §§§, P < 0.001 between Baffr−/− mice and Baffr−/− mice with transferred B cells. (D) Survival was monitored in WT and Baffr−/− mice and Baffr−/− mice 40 days after WT B cell transfer following infection with 105 PFU of VSV (n = 7 or 8). The error bars show SEM; n.s., not significant.
Lymphotoxin signaling is critical for CD169+ cell development in spleen and lymph node tissues (32, 38, 39, 41). Moreover, it has been shown that lymphotoxins are derived from B cells, which are important for maintenance of CD169+ cells (29, 39). Consistently, BAFFR-deficient animals exhibited lower lymphotoxin alpha (Ltα) and lymphotoxin beta (Ltβ) expression levels than their corresponding controls (Fig. 6A). These data suggest that impaired B cell numbers in Baffr−/− mice may contribute to insufficient lymphotoxin expression to maintain normal levels of CD169+ cells (29, 38, 41, 42). To investigate the role of lymphotoxin beta during enforced viral replication, we infected Ltbfl/fl × CD19-Cre+ animals and compared them to their corresponding controls. As expected, these animals exhibited fewer CD169+ cells than the WT controls (Fig. 6B) (29, 42). Consistent with previous reports and our data obtained in Baffr−/− mice, we observed reduced type I interferon production shortly after infection in Ltbfl/fl × CD19-Cre+ mice compared to Ltbfl/fl × CD19-Cre− animals (Fig. 6C) (39). Furthermore, low-dose infection resulted in reduced production of neutralizing antibodies (Fig. 6D). These data indicate that lack of B cells or B cell-derived expression of Ltβ results in limited innate immune activation and delayed adaptive immune priming.
FIG 6.
B cell-dependent maintenance of CD169+ cells depends on lymphotoxin beta expression. (A) Lymphotoxin alpha (left) and beta (right) mRNA expression levels were analyzed from spleen tissue harvested from WT and Baffr−/− mice (n = 5 to 7). (B) Snap-frozen spleen sections from Ltbfl/fl × CD19-Cre+ mice and control animals were stained with anti-CD169 and anti-F4/80 antibody (1 representative out of 3 is shown). (C) The IFN-α concentration was determined 24 h after infection with 105 PFU of VSV from Ltbfl/fl × CD19-Cre+ and control animals (n = 4 to 6). (D) Neutralizing antibody titers were measured in sera harvested from Ltbfl/fl × CD19-Cre+ and control animals at the indicated time points after infection (n = 4 to 6). The error bars show SEM; n.s., not significant.
BAFFR deficiency results in limited innate immune activation following LCMV infection.
To further analyze the importance of BAFFR in viral replication and the induction of antiviral immunity, we examined Baffr−/− mice following LCMV infection. Consistent with the VSV infection, we observed reduced LCMV replication in the spleen tissue of Baffr−/− mice in comparison to WT controls at 72 h after infection (Fig. 7A). Furthermore, type I interferon levels were highly reduced in the sera of Baffr−/− animals compared to WT controls (Fig. 7B). Tetramer-positive LCMV-specific T cells were highly reduced following LCMV infection of Baffr−/− mice compared to WT mice (Fig. 7C). Moreover, IFN-γ production after in vitro restimulation with LCMV peptides was reduced in both CD8+ and CD4+ T cells harvested from Baffr−/− mice in comparison to WT controls (Fig. 7D). When viral titers were determined 20 days after infection, WT animals had eliminated the virus from all organs tested (Fig. 7E). In sharp contrast, LCMV-infected Baffr−/− mice displayed high virus titers in all organs tested (Fig. 7E). Collectively, these data suggest that the absence of BAFFR signaling causes impaired generation of the marginal-zone compartment and impaired induction of innate and adaptive immune responses during viral infection.
FIG 7.
Impaired innate and adaptive immunity in BAFFR-deficient mice during LCMV infection. WT and Baffr−/− mice were infected with 200 PFU of LCMV-Docile. (A) Virus titers were measured in spleen, liver, lung, kidney, brain, and spinal cord tissues 72 h after infection (n = 5 or 6). (B) IFN-α concentrations were determined in the sera of infected animals 72 h following infection (n = 5 or 6). (C) Twenty days after infection, virus-specific CD8+ T cells were examined by tetramer (tet) staining in spleen tissues of mice (n = 5 or 6). (D) IFN-γ production of T cells as assessed by intracellular-cytokine staining and flow cytometric analysis was measured after in vitro restimulation with the MHC-I peptides gp33 and np396 (left) and the MHC-II peptide gp61 (right) (n = 5 or 6). (E) Virus titers were measured in spleen, liver, lung, kidney, brain, and spinal cord tissues 20 days after infection (n = 5 or 6). The error bars show SEM; n.s., not significant; the dashed line indicates the detection limit.
DISCUSSION
In this study, we have identified a critical role for BAFFR in the maintenance of CD169+ macrophages. Baffr−/− mice showed limited innate immune activation and reduced adaptive immune priming associated with fatal disease outcome. Mechanistically, impaired B cell development in Baffr−/− mice resulted in limited lymphotoxin expression and, likely as a consequence, reduced presence of CD169+ cells.
BAFF can be produced by a variety of immune cells, including dendritic cells, macrophages, and neutrophils (5). Interestingly, a recent report indicated BAFF-producing neutrophils were located in the marginal zone of the spleen (28). These neutrophil B helper cells contribute to marginal-zone B cell activation and antibody production against pathogens (28). Based on our results, BAFF production by neutrophil B helper cells, by promoting B cell-mediated lymphotoxin production, may also affect CD169+ cell survival and subsequently enforce antigen amplification and presentation. Furthermore, BAFF overexpression has been linked to a variety of autoimmune diseases, such as rheumatoid arthritis, lupus erythematosus, and Sjögren syndrome (5, 43–45). A clinically used BAFF-blocking antibody, belimumab, is effective in treating some lupus patients (46), and potentially, some clinical efficacy of BAFF neutralization in lupus patients may be due to effects on CD169+ macrophages.
Viral infections are potent activators of the immune system and can trigger autoimmunity (47) through several mechanisms, including molecular mimicry and bystander activation (48). Increased BAFF levels may affect not only B cell-mediated autoimmunity, but also B cell-mediated effects on CD169+ macrophages to increase bystander activation. Furthermore, replication of low-affinity antigens in CD169+ macrophages may contribute to development of virus-mediated autoimmunity induced by molecular mimicry (49). Considering our data, altered BAFF expression levels may lead to increased immune responses during viral infections. These mechanisms could also contribute to induction of autoantibodies observed during viral infections (50, 51).
As we show here, defects in BAFFR expression may limit innate immunity during infection. This may be triggered by reduced lymphotoxin beta production by B cells, as lack of lymphotoxin beta resulted in reduced presence of CD169+ macrophages (29, 38, 39). Furthermore, lymphotoxins trigger innate type I interferon production during viral infection (52, 53). This may be in part triggered by enforced viral replication in CD169+ cells, which is also critical for induction of adaptive immune priming (21, 49). Considering our data, deletions in BAFFR may not only affect B cell-driven immunity, but also trigger defects in innate immunity. Future studies may analyze the role of BAFFR deficiency during innate immunity in human patients.
In conclusion, we have determined that BAFFR deficiency mediates reduced presence of B cells, impacting the maintenance of CD169+ macrophages and innate immunity.
ACKNOWLEDGMENTS
We are grateful for the technical assistance of Eugene Bäcker, Konstanze Schättel, and Stefanie Münch.
M.R. holds a professorship from the Swiss National Science Foundation (PP00P3_144863). P.S.O. holds a Canada Research Chair in Autoimmunity and Tumor Immunity. This research was funded in part by the Ontario Ministry of Health and Long Term Care (OMOHLTC). This study was also supported by a CIHR grant to P.S.O. (CIHR-MOP-106529), by a grant to J.G. (CIHR-MOP-67157), by a Russian Scientific Fund grant to S.A.N. (14-50-00060), and by the Alexander von Humboldt Foundation (SKA2008 and SKA2010) and the German Research Council (CRC974 and LA2558/3-1/4-1/5-1).
The views expressed do not necessarily reflect those of the OMOHLTC.
REFERENCES
- 1.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. 2001. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. 2001. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 3.Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J, Kienzler AK, Pan-Hammarstrom Q, Hammarstrom L, Rakhmanov M, Schlesier M, Grimbacher B, Peter HH, Eibel H. 2009. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci U S A 106:13945–13950. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. 2004. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol 173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 5.Mackay F, Schneider P. 2009. Cracking the BAFF code. Nat Rev Immunol 9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 6.Gardam S, Sierro F, Basten A, Mackay F, Brink R. 2008. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity 28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Liao G, Zhang M, Harhaj EW, Sun SC. 2004. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem 279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 8.Claudio E, Brown K, Park S, Wang H, Siebenlist U. 2002. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol 3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. 2006. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity 24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzawa A, Tseng PH, Vallabhapurapu S, Luo JL, Zhang W, Wang H, Vignali DA, Gallagher E, Karin M. 2008. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science 321:663–668. doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. 2008. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol 9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. 2006. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med 203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craxton A, Draves KE, Gruppi A, Clark EA. 2005. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med 202:1363–1374. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otipoby KL, Sasaki Y, Schmidt-Supprian M, Patke A, Gareus R, Pasparakis M, Tarakhovsky A, Rajewsky K. 2008. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proc Natl Acad Sci U S A 105:12435–12438. doi: 10.1073/pnas.0805460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N, Tybulewicz VL. 2013. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity 38:475–488. doi: 10.1016/j.immuni.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junt T, Scandella E, Ludewig B. 2008. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat Rev Immunol 8:764–775. doi: 10.1038/nri2414. [DOI] [PubMed] [Google Scholar]
- 17.Mebius RE, Kraal G. 2005. Structure and function of the spleen. Nat Rev Immunol 5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 18.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, van Rooijen N, Mempel TR, Whelan SP, von Andrian UH. 2007. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 19.Oetke C, Kraal G, Crocker PR. 2006. The antigen recognized by MOMA-I is sialoadhesin. Immunol Lett 106:96–98. doi: 10.1016/j.imlet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Muller S, Hunziker L, Enzler S, Buhler-Jungo M, Di Santo JP, Zinkernagel RM, Mueller C. 2002. Role of an intact splenic microarchitecture in early lymphocytic choriomeningitis virus production. J Virol 76:2375–2383. doi: 10.1128/jvi.76.5.2375-2383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M, Klingel K, Sauter M, Kandolf R, Gailus N, van Rooijen N, Burkart C, Baldus SE, Grusdat M, Lohning M, Hengel H, Pfeffer K, Tanaka M, Haussinger D, Recher M, Lang PA, Lang KS. 2012. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol 13:51–57. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
- 22.Aichele P, Brduscha-Riem K, Zinkernagel RM, Hengartner H, Pircher H. 1995. T cell priming versus T cell tolerance induced by synthetic peptides. J Exp Med 182:261–266. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iezzi G, Karjalainen K, Lanzavecchia A. 1998. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8:89–95. doi: 10.1016/S1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 24.Zinkernagel RM. 2000. Localization dose and time of antigens determine immune reactivity. Semin Immunol 12:163–171, 257–344. doi: 10.1006/smim.2000.0253. [DOI] [PubMed] [Google Scholar]
- 25.Lanzavecchia A, Sallusto F. 2001. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat Immunol 2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. 2003. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med 198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You Y, Myers RC, Freeberg L, Foote J, Kearney JF, Justement LB, Carter RH. 2011. Marginal zone B cells regulate antigen capture by marginal zone macrophages. J Immunol 186:2172–2181. doi: 10.4049/jimmunol.1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. 2012. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, Drutskaya L, Stewart C, Chervonsky A, Nedospasov S. 2002. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity 17:239–250. doi: 10.1016/S1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 30.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 31.Lang PA, Xu HC, Grusdat M, McIlwain DR, Pandyra AA, Harris IS, Shaabani N, Honke N, Kumar Maney S, Lang E, Pozdeev VI, Recher M, Odermatt B, Brenner D, Haussinger D, Ohashi PS, Hengartner H, Zinkernagel RM, Mak TW, Lang KS. 2013. Reactive oxygen species delay control of lymphocytic choriomeningitis virus. Cell Death Differ 20:649–658. doi: 10.1038/cdd.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu YX, Huang G, Matsumoto M, Molina H, Chaplin DD. 1997. Independent signals regulate development of primary and secondary follicle structure in spleen and mesenteric lymph node. Proc Natl Acad Sci U S A 94:5739–5743. doi: 10.1073/pnas.94.11.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, Deenen R, Kohrer K, Rahbar R, Diefenbach A, Gibbert K, Lohning M, Hocker L, Waibler Z, Haussinger D, Mak TW, Ohashi PS, Lang KS, Lang PA. 2014. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity 40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. 2006. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 35.Fensterl V, Wetzel JL, Ramachandran S, Ogino T, Stohlman SA, Bergmann CC, Diamond MS, Virgin HW, Sen GC. 2012. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS Pathog 8:e1002712. doi: 10.1371/journal.ppat.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair S, Michaelsen-Preusse K, Finsterbusch K, Stegemann-Koniszewski S, Bruder D, Grashoff M, Korte M, Koster M, Kalinke U, Hauser H, Kroger A. 2014. Interferon regulatory factor-1 protects from fatal neurotropic infection with vesicular stomatitis virus by specific inhibition of viral replication in neurons. PLoS Pathog 10:e1003999. doi: 10.1371/journal.ppat.1003999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. 2007. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest 117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9:59–70. doi: 10.1016/S1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 39.Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A, Fu YX, Hacohen N, von Andrian UH. 2012. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity 36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nolte MA, Arens R, Kraus M, van Oers MH, Kraal G, van Lier RA, Mebius RE. 2004. B cells are crucial for both development and maintenance of the splenic marginal zone. J Immunol 172:3620–3627. doi: 10.4049/jimmunol.172.6.3620. [DOI] [PubMed] [Google Scholar]
- 41.Hiemstra IH, Beijer MR, Veninga H, Vrijland K, Borg EGF, Olivier BJ, Mebius RE, Kraal G, den Haan JMM. 2014. The identification and developmental requirements of colonic CD169+ macrophages. Immunology 142:269–278. doi: 10.1111/imm.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Junt T, Tumanov AV, Harris N, Heikenwalder M, Zeller N, Kuprash DV, Aguzzi A, Ludewig B, Nedospasov SA, Zinkernagel RM. 2006. Expression of lymphotoxin beta governs immunity at two distinct levels. Eur J Immunol 36:2061–2075. doi: 10.1002/eji.200626255. [DOI] [PubMed] [Google Scholar]
- 43.Mackay F, Silveira PA, Brink R. 2007. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr Opin Immunol 19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Cancro MP, D'Cruz DP, Khamashta MA. 2009. The role of B lymphocyte stimulator (BLyS) in systemic lupus erythematosus. J Clin Invest 119:1066–1073. doi: 10.1172/JCI38010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy KD, Pei Y, Novak L, Lee JY, Julian BA, Novak J, Ranger A, Gommerman JL, Browning JL. 2011. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121:3991–4002. doi: 10.1172/JCI45563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA. 2011. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 47.Getts DR, Chastain EM, Terry RL, Miller SD. 2013. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev 255:197–209. doi: 10.1111/imr.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munz C, Lunemann JD, Getts MT, Miller SD. 2009. Antiviral immune responses: triggers of or triggered by autoimmunity? Nat Rev Immunol 9:246–258. doi: 10.1038/nri2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honke N, Shaabani N, Zhang DE, Iliakis G, Xu HC, Haussinger D, Recher M, Lohning M, Lang PA, Lang KS. 2013. Usp18 driven enforced viral replication in dendritic cells contributes to break of immunological tolerance in autoimmune diabetes. PLoS Pathog 9:e1003650. doi: 10.1371/journal.ppat.1003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunziker L, Recher M, Macpherson AJ, Ciurea A, Freigang S, Hengartner H, Zinkernagel RM. 2003. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat Immunol 4:343–349. doi: 10.1038/ni911. [DOI] [PubMed] [Google Scholar]
- 51.Barzilai O, Ram M, Shoenfeld Y. 2007. Viral infection can induce the production of autoantibodies. Curr Opin Rheumatol 19:636–643. doi: 10.1097/BOR.0b013e3282f0ad25. [DOI] [PubMed] [Google Scholar]
- 52.Benedict CA, Banks TA, Senderowicz L, Ko M, Britt WJ, Angulo A, Ghazal P, Ware CF. 2001. Lymphotoxins and cytomegalovirus cooperatively induce interferon-beta, establishing host-virus detente. Immunity 15:617–626. doi: 10.1016/S1074-7613(01)00222-9. [DOI] [PubMed] [Google Scholar]
- 53.Schneider K, Loewendorf A, De Trez C, Fulton J, Rhode A, Shumway H, Ha S, Patterson G, Pfeffer K, Nedospasov SA, Ware CF, Benedict CA. 2008. Lymphotoxin-mediated crosstalk between B cells and splenic stroma promotes the initial type I interferon response to cytomegalovirus. Cell Host Microbe 3:67–76. doi: 10.1016/j.chom.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]