Abstract
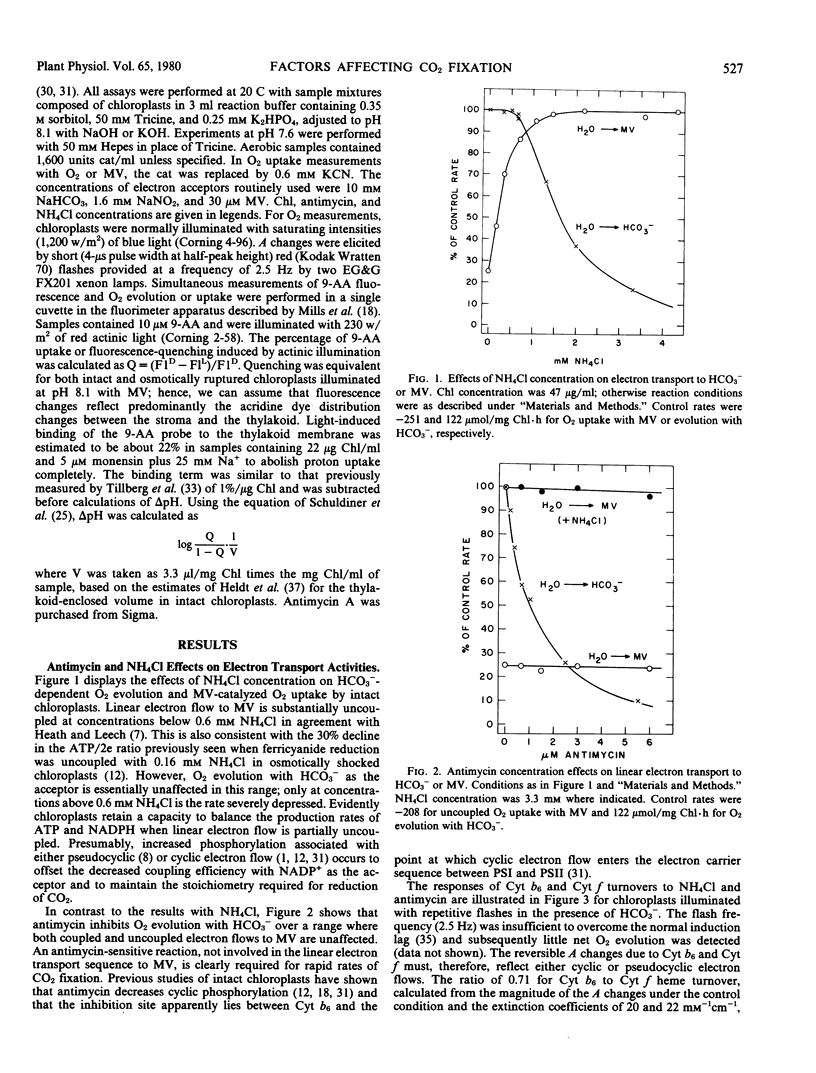
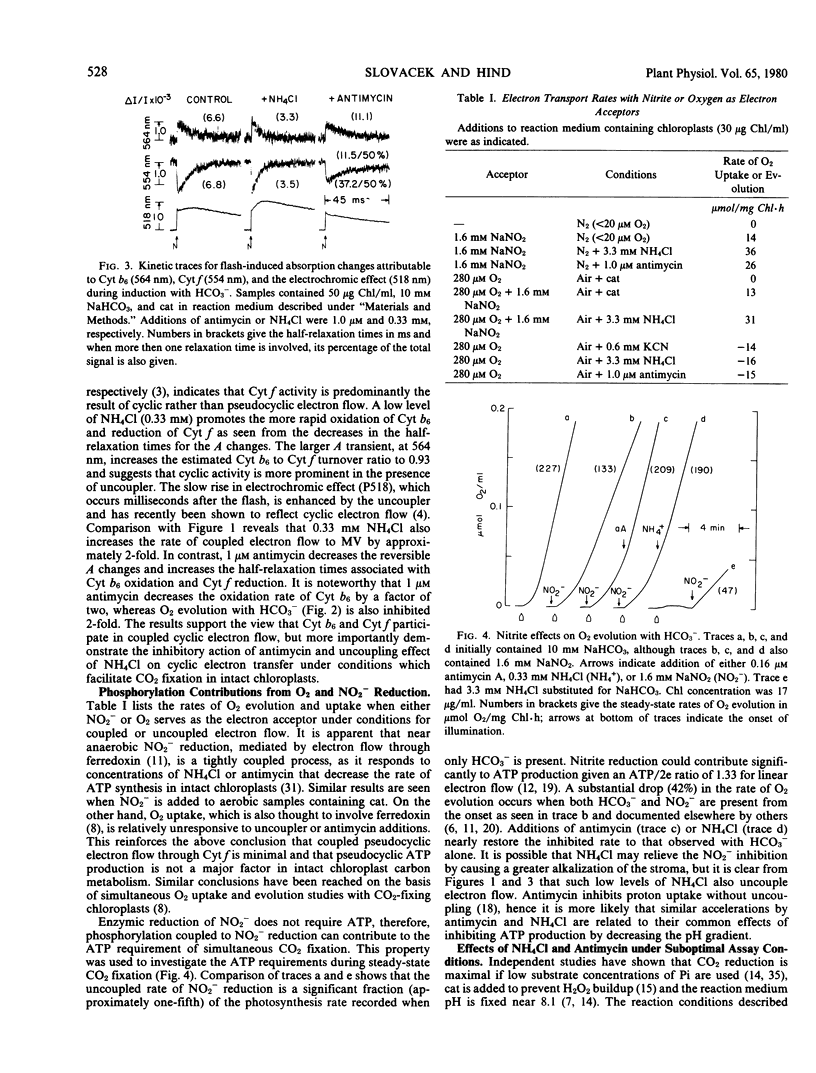
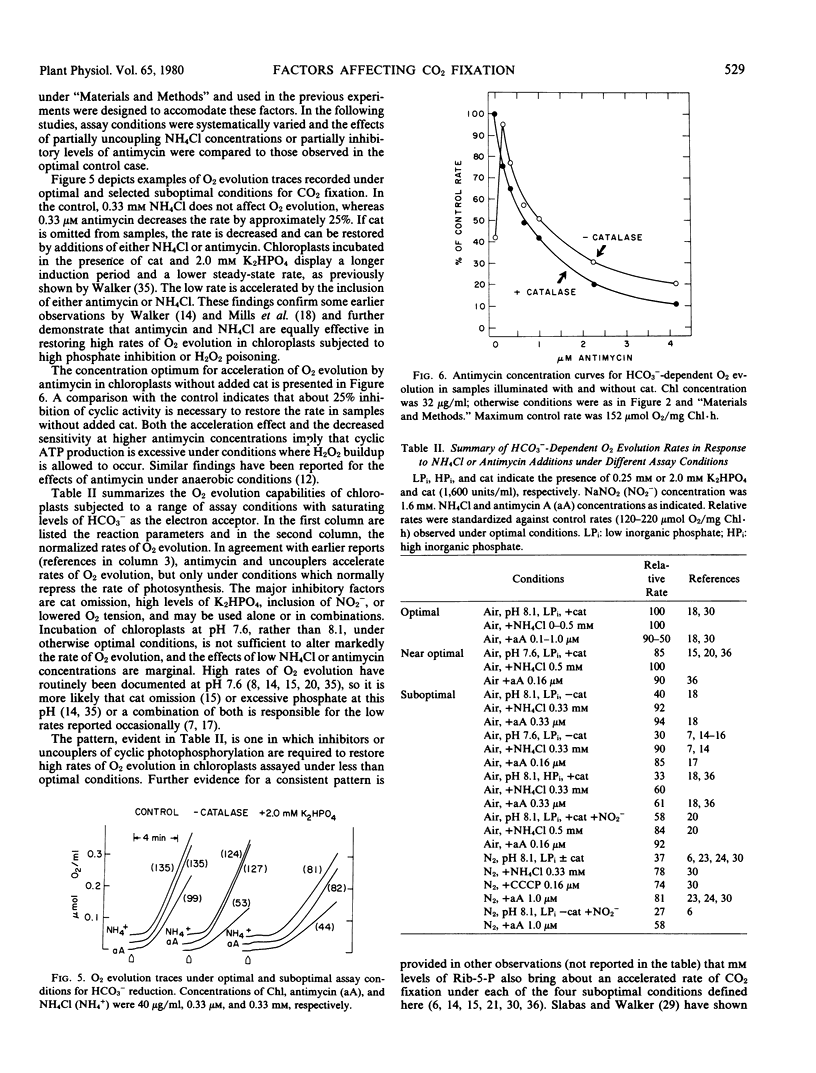
Light- and HCO3−-saturated (10 millimolar) rates of O2 evolution (120 to 220 micromoles O2 per milligram chlorophyll per hour), obtained with intact spinach chloroplasts, are decreased up to 3-fold by changes in assay conditions such as omission of catalase from the medium, the use of high (≥1 millimolar) inorganic phosphate, inclusion of NO2− as an electron acceptor, or bright illumination at low partial pressures of O2. These inhibitions may be reversed by addition of uncoupling levels of NH4Cl or of antimycin concentrations that partially block cyclic electron transfer between cytochrome b6 and cytochrome f. Measurements of the pH gradient across the thylakoid membrane with the fluorescent probe, 9-aminoacridine, indicate that changes in ΔpH are sufficient to account for both the inhibited and restored rates of electron transport. It follows that the rate of HCO3−-saturated photosynthesis may be restricted by a proton gradient back pressure under these conditions.
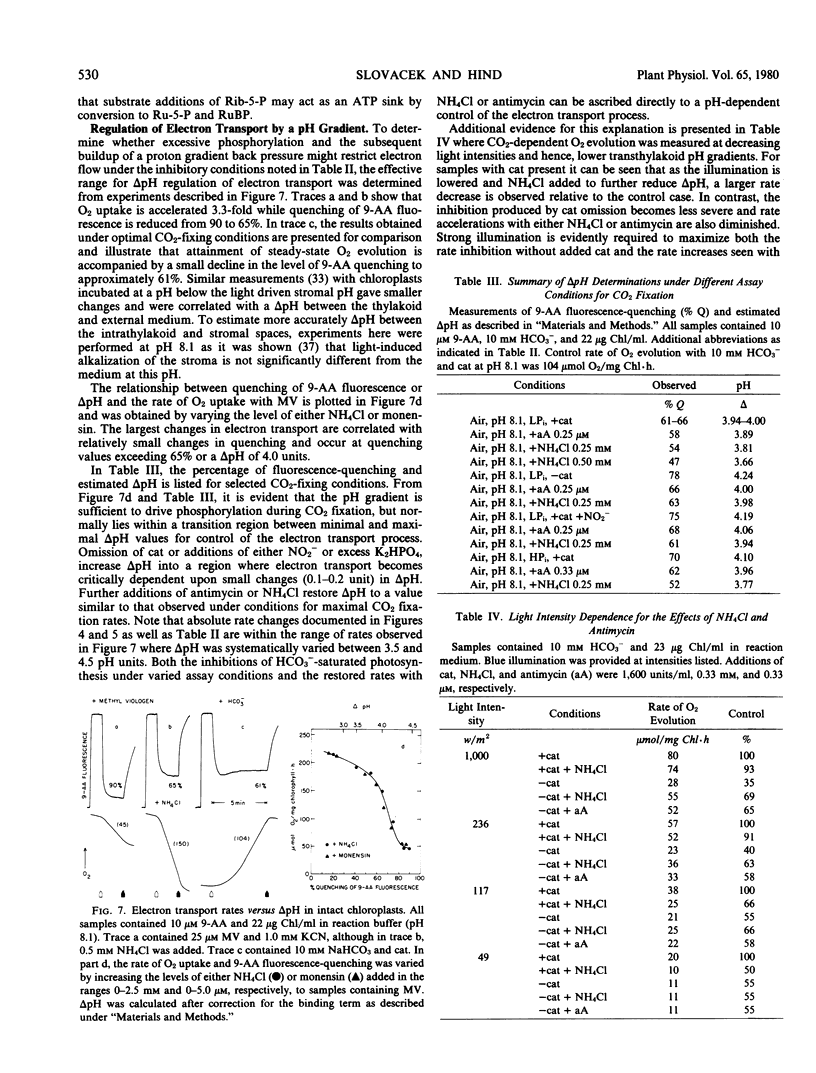
The rate of O2 evolution is also decreased 3-fold when ambient CO2 (0.63 millimolar HCO3− at pH 8.1) is used in place of saturating HCO3− and chloroplasts are illuminated aerobically with catalase and a low level (0.25 millimolar) of K2HPO4. Only inhibitory effects are observed with additions of antimycin or NH4Cl. Under these conditions, excessive photophosphorylation or a large pH gradient does not limit the rate of photosynthesis.
Full text
PDF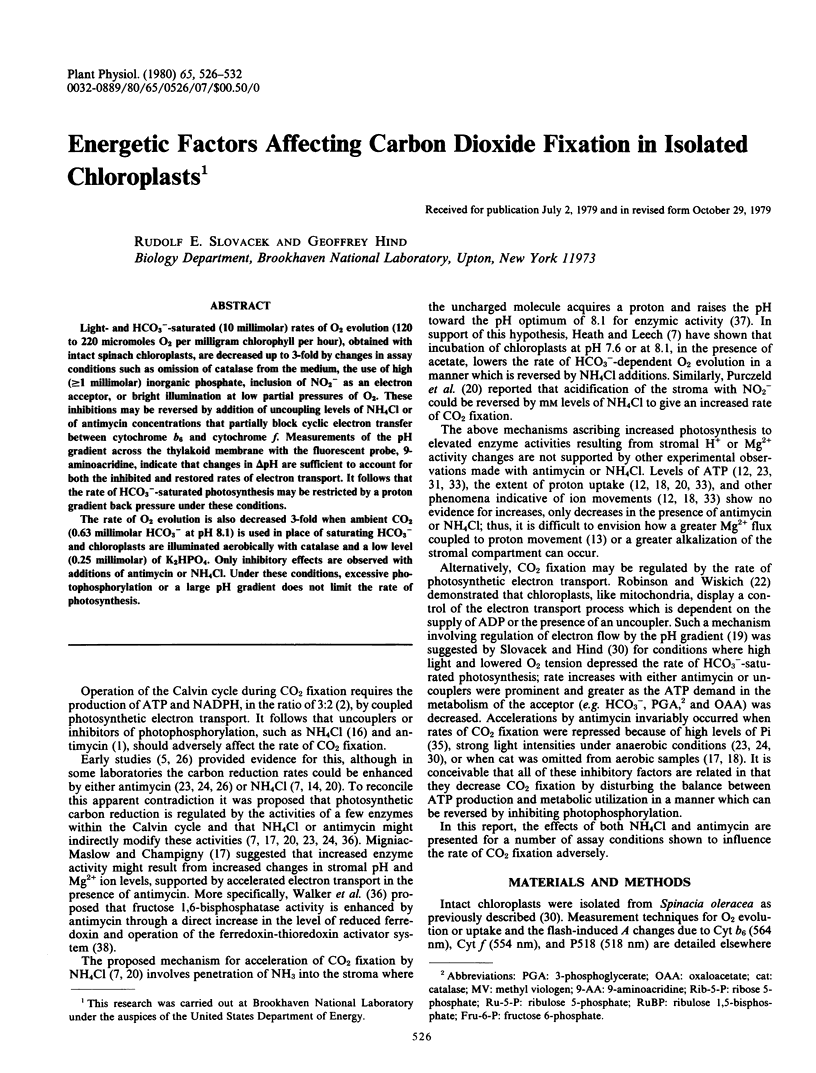


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. Role of ferredoxin in photosynthesis. Naturwissenschaften. 1969 Jun;56(6):295–305. doi: 10.1007/BF00602160. [DOI] [PubMed] [Google Scholar]
- Crowther D., Mills J. D., Hind G. Protonmotive cyclic electron flow around photosystem I in intact chloroplasts. FEBS Lett. 1979 Feb 15;98(2):386–390. doi: 10.1016/0014-5793(79)80223-9. [DOI] [PubMed] [Google Scholar]
- Gould E. S., Bassham J. A. Inhibitor studies on the photosynthetic carbon reduction cycle in Chlorella pyrenoidosa. Biochim Biophys Acta. 1965 May 25;102(1):9–19. doi: 10.1016/0926-6585(65)90199-8. [DOI] [PubMed] [Google Scholar]
- Heath R. L., Leech R. M. The stimulation of CO2-supported O2 evolution in intact spinach chloroplasts by ammonium ion. Arch Biochem Biophys. 1978 Sep;190(1):221–226. doi: 10.1016/0003-9861(78)90271-0. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind G., Nakatani H. Y., Izawa S. Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1484–1488. doi: 10.1073/pnas.71.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Effect of pH on chloroplast photosynthesis. Inhibition of O2 evolution by inorganic phosphate and magnesium. Biochim Biophys Acta. 1979 Jan 11;545(1):131–140. doi: 10.1016/0005-2728(79)90120-8. [DOI] [PubMed] [Google Scholar]
- Kaiser W. The effect of hydrogen peroxide on CO2 fixation of isolated intact chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):476–482. doi: 10.1016/0005-2728(76)90035-9. [DOI] [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miginiac-Maslow M., Champigny M. L. Relationship between the Level of Adenine Nucleotides and the Carboxylation Activity of Illuminated Isolated Spinach Chloroplasts: A Study with Antimycin A. Plant Physiol. 1974 Jun;53(6):856–862. doi: 10.1104/pp.53.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. D., Slovacek R. E., Hind G. Cyclic electron transport in isolated intact chloroplasts. Further studies with antimycin. Biochim Biophys Acta. 1978 Nov 9;504(2):298–309. doi: 10.1016/0005-2728(78)90178-0. [DOI] [PubMed] [Google Scholar]
- Purczeld P., Chon C. J., Portis A. R., Jr, Heldt H. W., Heber U. The mechanism of the control of carbon fixation by the pH in the chloroplast stroma. Studies with nitrite-mediated proton transfer across the envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):488–498. doi: 10.1016/0005-2728(78)90116-0. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Photosynthetic intermediates, the warburg effect, and glycolate synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Jun;53(6):790–797. doi: 10.1104/pp.53.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Wiskich J. T. Factors affecting the ADP/O ratio in isolated chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):131–146. doi: 10.1016/0005-2728(76)90119-5. [DOI] [PubMed] [Google Scholar]
- Schacter B. Z., Gibbs M., Champigny M. L. Effect of antimycin a on photosynthesis of intact spinach chloroplasts. Plant Physiol. 1971 Oct;48(4):443–446. doi: 10.1104/pp.48.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter B., Bassham J. A. Antimycin A Stimulation of Rate-limiting Steps of Photosynthesis in Isolated Spinach Chloroplasts. Plant Physiol. 1972 Mar;49(3):411–416. doi: 10.1104/pp.49.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P., Buchanan B. B., Arnon D. I. Role of cyclic photophosphorylation in photosynthetic carbon dioxide assimilation by isolated chloroplasts. Biochim Biophys Acta. 1972 Apr 20;267(1):111–124. doi: 10.1016/0005-2728(72)90143-0. [DOI] [PubMed] [Google Scholar]
- Shain Y., Gibbs M. Formation of glycolate by a reconstituted spinach chloroplast preparation. Plant Physiol. 1971 Sep;48(3):325–330. doi: 10.1104/pp.48.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabas A. R., Walker D. A. Transient inhibition by ribose 5-phosphate of photosynthetic O2 evolution in a reconstituted chloroplast system. Biochim Biophys Acta. 1976 Apr 9;430(1):154–164. doi: 10.1016/0005-2728(76)90231-0. [DOI] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Flash spectroscopic studies of cyclic electron flow in intact chloroplasts. Biochem Biophys Res Commun. 1978 Oct 30;84(4):901–906. doi: 10.1016/0006-291x(78)91668-6. [DOI] [PubMed] [Google Scholar]
- Slovacek R. E., Hind G. Influence of antimycin a and uncouplers on anaerobic photosynthesis in isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):538–542. doi: 10.1104/pp.60.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steup M., Peavey D. G., Gibbs M. The regulation of starch metabolism by inorganic phosphate. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1554–1561. doi: 10.1016/s0006-291x(76)80191-x. [DOI] [PubMed] [Google Scholar]
- Tillberg J. E., Giersch C., Heber U. CO2 reduction by intact chloroplasts under a diminished proton gradient. Biochim Biophys Acta. 1977 Jul 7;461(1):31–47. doi: 10.1016/0005-2728(77)90067-6. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Slabas A. R., Fitzgerald M. P. Photosynthesis in a reconstituted chloroplast system from spinach. Some factors affecting CO2-dependent oxygen evolution with fructose-1,6-bisphosphate as substrate. Biochim Biophys Acta. 1976 Jul 9;440(1):147–162. doi: 10.1016/0005-2728(76)90120-1. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


