Abstract
Background
It is increasingly evident that microbial colonization of the respiratory tract might have a role in the pathogenesis of asthma.
Objective
We sought to characterize and compare the microbiome of induced sputum in asthmatic and nonasthmatic adults.
Methods
Induced sputum samples were obtained from 10 nonasthmatic subjects and 10 patients with mild active asthma (8/10 were not using inhaled corticosteroids). Total DNA was extracted from sputum supernatants and amplified by using primers specific for the V6 hypervariable region of bacterial 16s rRNA. Samples were barcoded, and equimolar concentrations of 20 samples were pooled and sequenced with the 454 GS FLX sequencer. Sequences were assigned to bacterial taxa by comparing them with 16s rRNA sequences in the Ribosomal Database Project.
Results
All sputum samples contained 5 major bacterial phyla: Firmicutes, Proteobacteria, Actinobacteria, Fusobacterium, and Bacteroidetes, with the first 3 phyla accounting for more than 90% of the total sequences. Proteobacteria were present in higher proportions in asthmatic patients (37% vs 15%, P < .001).
In contrast, Firmicutes (47% vs 63%, P = .17) and Actinobacteria (10% vs 14%, P = .36) were found more frequently in samples from nonasthmatic subjects, although this was not statistically significant. Hierarchical clustering produced 2 significant clusters: one contained primarily asthmatic samples and the second contained primarily nonasthmatic samples. In addition, samples from asthmatic patients had greater bacterial diversity compared with samples from nonasthmatic subjects.
Conclusion
Patients with mild asthma have an altered microbial composition in the respiratory tract that is similar to that observed in patients with more severe asthma.
Keywords: Asthma, microbiome, Proteobacteria, metagenomics, sputum
Asthma is a chronic inflammatory disease of the airways, but the genetic and environmental factors that determine asthma are not well understood. Several lines of evidence suggest that microbial colonization in the airways might have a role in the chronic inflammatory process. Both Mycoplasma pneumoniae and Chlamydophila pneumoniae have been isolated from patients with acute asthma exacerbations.1,2 This suggests that macrolides and similar compounds might be used to treat such exacerbations, and a clinical trial with ketolides showed promising results.3 A report using a large database of patients in the state of Tennessee concluded that invasive pneumococcal pneumonia is at least twice as likely to occur in asthmatic patients as in control subjects.4 Bisgaard et al5 reported that neonates whose upper airways were colonized at 1month of agewith Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis or with a combination of these organisms are at increased risk for recurrent wheeze and asthma later in life. Most of these studies detected microbial agents either by using targeted PCR approaches or by culturing specific organisms, reflecting only a small fraction of existing taxa. Characterizing the entire microbial diversity in the airways will provide insight into the role of the microbiome in asthma pathogenesis.
Metagenomics enables the study of microbes in their native ecological states and provides culture-independent estimates of microbial diversity.6 Metagenomic approaches have been successfully used to demonstrate the effect of the microbiome on several aspects of human health, such as obesity, diabetes, and inflammatory bowel disease.7–10 In 2 recent studies metagenomic approaches were used to characterize the microbial communities in airways of asthmatic and nonasthmatic subjects that were present in bronchial samples. Using PCR and Sanger sequencing, Hilty et al11 showed that the airways of asthmatic and nonasthmatic subjects have approximately 120 distinct bacterial groups and that Proteobacteria are present more frequently in asthmatic patients, whereas Bacteroidetes are more frequent in bronchial samples from nonasthmatic subjects. A more recent study with a PhyloChip approach demonstrated a higher bacterial load in asthmatic patients compared with that seen in control subjects, with Proteobacteria again present in higher abundance in asthmatic patients.12 However, all the asthmatic patients sampled in both of these studies were treated with inhaled corticosteroids, and effects of corticosteroids on the microbiome could not be ascertained.
To our knowledge, no studies have comprehensively characterized bacterial communities in induced sputum obtained from subjects with and without asthma. We used high-throughput 454 sequencing on induced sputum samples from asthmatic and nonasthmatic subjects. Of 10 asthmatic participants, 8 were not currently using corticosteroids, and 2 reported infrequent corticosteroid use.
METHODS
Subjects and sputum samples
Induced sputum samples from 10 patients with physician-diagnosed active asthma and 10 nonasthmatic adults were obtained for this study. The samples were collected as part of the ongoing Tucson Children’s Respiratory Study, a nonselected birth cohort whose participants are now adults in their early 30s.13 Asthma, wheeze, bronchitis, and smoking information was collected from participant-completed questionnaires at age 26 years. Spirometry was completed according to American Thoracic Society standards, and none of the participants had used a bronchodilator within 6 hours of testing. A fixed dose of 2 puffs of albuterol (180 µg) was administered after baseline spirometry from a metered-dose inhaler and aerochamber holding device (Monaghan Medical Corp, Plattsburgh, NY), and postbronchodilator spirometry was repeated after 15 minutes. Methacholine challenges were performed with the protocol described by Yan et al14 and the dose-response slope calculated as described by Stein et al.15 Allergy skin prick testing to local aeroallergens was completed as previously described.16 Ethnicity/race was based on parental report at birth.
Collection and processing of induced sputum
Induced sputum from each subject was collected by means of inhalation of a nebulized solution of 3% saline over a 2-minute period. The subject then spits out the saliva, takes 2 deep inspirations of saline, and coughs sputum into a separate cup. The procedure is repeated 6 times for a total of 12 minutes. Peak flow is monitored throughout the procedure. The mouth is rinsed with saline water before sputum induction to minimize oral contamination. Within an hour of collection, cell counts are performed, 2 volumes of dithiothreitol are added to sputum, and the sample is mixed vigorously on a plate shaker for 15 to 20 minutes to solubilize mucus. The sputum is centrifuged, and the supernatant is collected and stored at −70°C. An aliquot of the supernatant was used for bacterial DNA extraction.
DNA extraction and sequencing
The total DNA from a 200-µL aliquot of the supernatant was extracted with the Qiagen DNA extraction kit (Qiagen, Hilden, Germany), according to the manufacturer’s recommendations. The bacterial DNAwas amplified with the 967F and 1046R primers specific for the V6 hypervariable region of bacterial 16s rRNA. Each forward primer had an adaptor sequence and a barcode sequence to help identify the samples after sequencing. Equimolar concentrations of 20 samples were pooled and sequenced with the 454 GS FLX sequencer using Titanium chemistry (454 Life Sciences, Branford, Conn), according to the manufacturer’s instructions.
Statistics: Identification of bacterial communities
After sequencing, the reads were preprocessed to remove any that did not match the barcode or the primer sequences. A single mismatch was allowed in both sequences. Briefly, after filtering for low-quality reads and eliminating reads that did not match barcode sequences, each read from an individual sample was compared with all the remaining reads from that sample to identify unique reads. Unique reads from each sample were clustered into a group of similar sequences, each representative of a 16s rRNA sequence from a bacterial species/genus, called operational taxonomic units (OTUs). All the analyses were performed with the 454 sequencing pipeline of the Ribosomal Database Project, Release 10 (Ribosomal Database Project, East Lansing, Mich).17 Sequences were assigned to bacterial taxa by comparing them with 16s rRNA sequences in the Ribosomal Database Project by using the RDP Classifier.18 Only sequences that could be assigned to a bacterial group with a confidence estimate of 50% or greater were used for further analysis. OTUs for each sample were derived by clustering sequences from individual samples at a genetic distance of 0.03, and the species richness estimate Chao19 was obtained for each sample by using Mothur.20 The proportion of bacterial genera in each sample was used to calculate the Shannon diversity index, a mathematic measure commonly used to characterize the species diversity in a community. The Shannon Index accounts for both the diversity and evenness of the bacterial species present in samples from asthmatic and nonasthmatic subjects.21 Hierarchical clustering was performed with pvclust22 in the R statistics package (www.r-project.org), and support for each branch was obtained by performing bootstrap analysis with 10,000 replicates (pvclust and bootstrapping are described in the Methods section in this article’s Online Repository at www.jacionline.org).
Ethics statement
The studies reported herein were approved by the Institutional Review Board of the University of Arizona, and written informed consent was obtained from each participant.
RESULTS
Characteristics of the study subjects are presented in Table I.15 All subjects were part of a longitudinal study of the natural history of asthma in a nonselected birth cohort,13 and therefore the previous history of asthma could be confirmed from available data. Inhaled medication use for asthma or wheeze during the past year is described in Table II. None of the participants reported using oral medications for asthma during the past year. The majority of the asthmatic patients had a methacholine dose-response slope that was less than the 10th percentile of the methacholine doseresponse slope for a nonatopic nonasthmatic healthy reference subgroup (6/7). Only a minority of the nonasthmatic subjects met this criterion (2/10, P = .008).
TABLE I.
Phenotypic characterization of the participants
| Sample | Sex | Current smoker |
No. of cigarettes/d |
Race/ ethnicity |
Age (y) | SPT* | Season† | % Predicted FEV1 before BD |
% Predicted FEV1 after BD |
Meth DRS‡ |
FEV1/FVC before BD |
FEV1/FVC after BD |
Bronchitis in past year |
Asthma attacks in past year |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | F | No | H-W | 27.6 | + | W | 103 | 107 | −0.72‖ | 81.2 | 86.6 | No | 1–3 | |
| A2 | F | No | NH-W | 26.5 | + | Sp | 95 | 93 | −0.81‖ | 87.2 | 88.3 | Yes | 1–3 | |
| A3 | F | No | NH-W | 26.1 | + | Sp | 86 | 91 | −1.40‖ | 77.4 | 83.9 | No | 1–3 | |
| A4 | F | No | H-A | 25.9 | + | S | 105 | 114 | ND | 78.1 | 84.6 | No | 1–3 | |
| A5 | F | Yes | 1 | NH-W | 26.1 | + | F | 98 | 98 | −0.54‖ | 89.2 | 89.4 | Yes | 4–8 |
| A6 | M | No | NH-W | 26.2 | + | Sp | 107 | 106 | ND | 80.9 | 80.5 | Yes | ≥13 | |
| A7 | F | Yes | 4 | H-W | 26.1 | − | W | 82 | 86 | −0.98‖ | 77.5 | 81.0 | No | 4–8 |
| A8 | M | No | H-W | 26.0 | + | S | 69 | ND | ND | 72.6 | No | 1–3 | ||
| A9¶ | F | No | NH-W | 26.0 | + | W | 73 | 74 | −0.52‖ | 81.4 | 84.6 | No | 1–3 | |
| A10 | F | No | NH-W§ | 25.6 | + | s | 98 | 104 | −0.43‖ | 78.0 | 81.8 | No | ≥13 | |
| C1 | M | No | NH-W | 26.8 | − | Sp | 90 | 97 | −0.47 | 74.1 | 79.0 | No | ||
| C2 | F | Yes | 10 | H-W | 26.8 | − | Sp | 104 | 108 | −0.26 | 82.0 | 83.0 | No | |
| C3 | F | No | NH-W | 26.4 | + | Sp | 118 | 122 | −0.20 | 91.1 | 94.5 | No | ||
| C4 | F | No | NH-W | 26.1 | + | Sp | 115 | 112 | −0.16 | 84.1 | 86.0 | No | ||
| C5 | F | No | H-W* | 25.9 | + | S | 109 | 108 | −0.71‖ | 86.7 | 88.6 | No | ||
| C6 | F | No | NH-W | 26.8 | + | S | 88 | 96 | −1.01‖ | 71.8 | 79.4 | No | ||
| C7 | F | Yes | 20 | NH-W | 25.9 | + | S | 106 | 107 | −0.61 | 82.8 | 86.6 | No | |
| C8 | F | No | NH-W | 26.1 | − | W | 96 | ND | −0.14 | 79.6 | No | |||
| C9 | M | No | NH-W | 25.9 | − | S | 91 | 96 | −0.23 | 85.8 | 91.7 | No | ||
| C10 | F | No | NH-W | 26.8 | + | Sp | 113 | 112 | −0.14 | 86.5 | 86.6 | No |
A, Asian; BD, bronchodilator; H, Hispanic; F, Female; FVC, forced vital capacity; M, male; ND, not determined; NH, non-Hispanic; W, white.
Positive allergy skin prick test response to 1 or more of 7 local aeroallergens at age 26 years.
Season of sputum induction defined as winter (W; December, January, and February), spring (Sp; March, April, and May), summer (S; June, July, and August), and fall (F; September, October, and November).
Lowest methacholine dose-response slope (DRS), as defined by Stein et al,15 from data at ages 16, 22, and 25 years.
Father’s ethnicity is missing.
Subject was defined as having bronchial hyperresponsiveness based on a methacholine dose-response slope value of less than the 10th percentile of the methacholine doseresponse slope for a nonatopic, nonasthmatic healthy reference subgroup.
Participant A9 shows methacholine hyperresponsiveness and reported asthma-like symptoms, with a spirometry consistent with a nonreversible, mild restrictive pattern.
TABLE II.
Inhaled medication use for asthma or wheeze during the past year: Frequency (occasionally, at least once per week, or daily) and number of months used
| Short-acting bronchodilator | Inhaled corticosteroids | Nedocromil | ||||
|---|---|---|---|---|---|---|
| Participant samples* | Frequency | No. of mo | Frequency | No. of mo | Frequency | No. of mo |
| A1 | Occasionally | NA | ||||
| A4 | Once/wk | 12 mo | Once/wk | 12 mo | ||
| A5 | Once/wk | 3 mo | ||||
| A6 | Daily | 12 mo | Daily | 12 mo | Once/wk | 12 mo |
| A9 | Occasionally | NA | Occasionally | NA | ||
| A10 | Occasionally | <1 mo | ||||
None of the participants reported using oral medications for asthma during the past year.
NA, Not available.
Participants A2, A3, A7, and A8 did not report medication use for asthma or wheeze during the year before sputum collection.
The average percentage of squamous cells in the sputum samples was 34.8% (SD, 25.4%). The sputum samples were barcoded, and equimolar concentrations of the 20 samples were pooled into a single sequencing run. After filtering for low-quality reads and eliminating reads that did not match barcode sequences, there were approximately 800,000 reads, with an average of 40,000 ± 11,245 (SD) total reads per sample (Table III). Comparing each read from an individual sample with all the remaining reads from that sample resulted in the identification of 3,919 to 10,608 unique reads per sample. Clustering of unique reads from individual samples at 97% identity indicated the presence of 1,500OTUs on average, with 2,264OTUs detected in at least 1 subject. Species richness estimates based on Chao indicate that between 75% and 80% of the estimated bacterial diversity in the sputum samples is captured in this study, and additional sequencing is required for capturing the complete diversity (see Fig E1 in this article’s Online Repository at www.jacionline.org).
TABLE III.
Sequence characteristics of samples from asthmatic and nonasthmatic subjects
| Sample | Total tags | Unique tags | OTUs |
|---|---|---|---|
| A1 | 41,189 | 8,514 | 1,719 |
| A2 | 38,998 | 7,899 | 1,718 |
| A3 | 46,826 | 8,258 | 1,653 |
| A4 | 64,098 | 10,007 | 1,836 |
| A5 | 29,721 | 5,953 | 1,301 |
| A6 | 23,662 | 3,919 | 822 |
| A7 | 54,900 | 10,608 | 2,264 |
| A8 | 40,477 | 7,124 | 1,404 |
| A9 | 31,342 | 5,845 | 1,302 |
| A10 | 39,457 | 5,717 | 1,110 |
| C1 | 26,354 | 5,873 | 1,248 |
| C2 | 38,175 | 8,462 | 2,062 |
| C3 | 44,158 | 7,473 | 1,492 |
| C4 | 42,038 | 7,717 | 1,576 |
| C5 | 34,708 | 6,629 | 1,400 |
| C6 | 63,044 | 9,922 | 2,027 |
| C7 | 31,592 | 6,224 | 1,315 |
| C8 | 21,717 | 4,251 | 918 |
| C9 | 48,450 | 8,814 | 1,611 |
| C10 | 39,457 | 7,784 | 1,778 |
A1–A10, Asthmatic patients; C1–C10, nonasthmatic subjects.
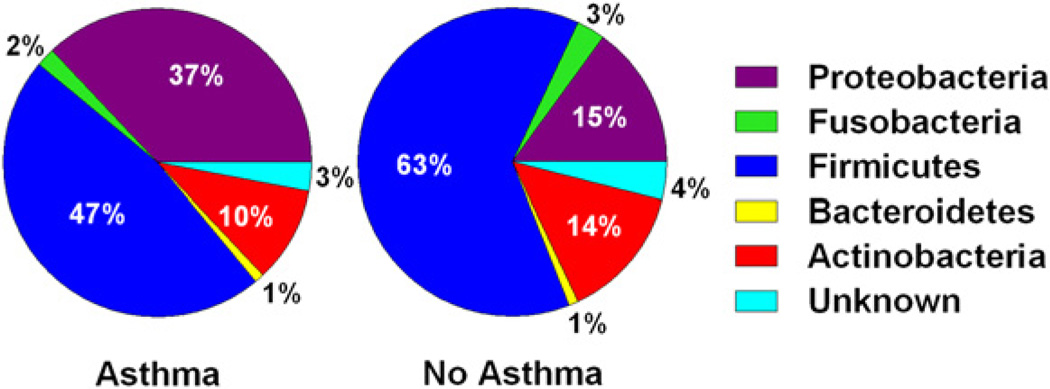
Bacterial lineages in each sample were identified by comparing the V6 sequences with the bacterial 16S rRNA sequences in the Ribosomal Database Project, Release 10. All sputum samples contained 5 major bacterial phyla: Firmicutes, Proteobacteria, Actinobacteria, Fusobacterium, and Bacteroidetes, with the first 3 phyla accounting for more than 90% of the total sequences in both asthmatic and nonasthmatic subjects (Fig 1). Relevant differences were found in Proteobacteria and Firmicutes between samples from asthmatic and nonasthmatic subjects. Proteobacteria were present in higher proportions in asthmatic patients (37% vs 15%, P < .001), especially Gammaproteobacteria (P < .05, Mann-Whitney U test). In contrast, Firmicutes (47% vs 63%, P = .17) and Actinobacteria (10% vs 14%, P = .36) were found more frequently in samples from nonasthmatic subjects, although these differences were not statistically significant. Firmicutes are gram-positive bacteria that predominantly form endospores and have some notable pathogenic bacteria, such as Bacillus, Staphylococcus, and Streptococcus species, whereas, Bacteroidetes are a group of gram-negative non–spore-forming bacteria widely present in the environment.
FIG 1.
Median percentage values of bacterial phyla in samples from asthmatic and nonasthmatic subjects found by using the RDP Classifier, version 2.1. Pie charts show the distribution of organisms for asthmatic and nonasthmatic subjects.
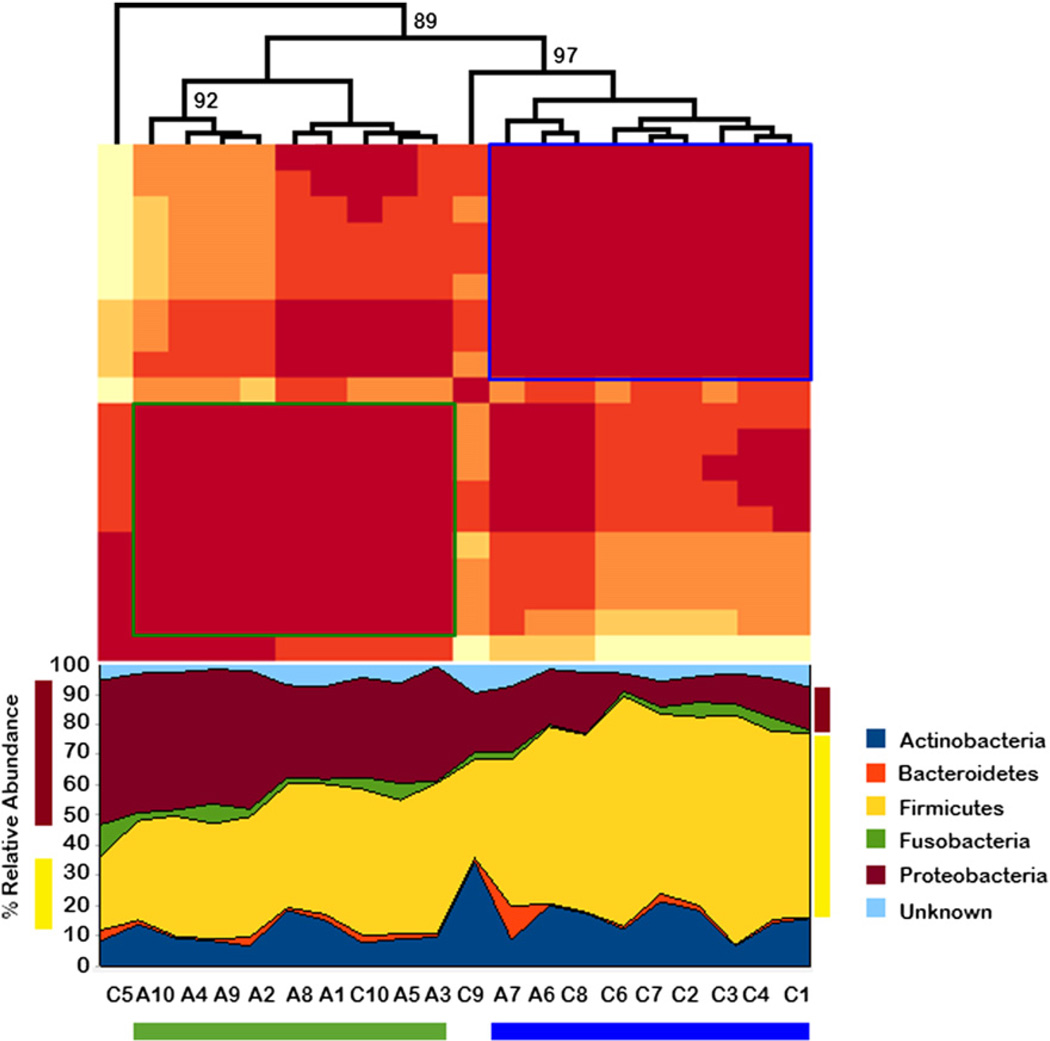
Hierarchical clustering (complete linkage with Euclidean distance) of all the samples based on pairwise R2 values of bacterial composition produced 2 significant clusters, one that contained most of the samples from asthmatic subjects (8 with asthma and 1 without asthma) and the second consisting predominantly of the samples from nonasthmatic subjects (2 with asthma and 7 without asthma, Fig 2). This further suggests the compositional similarity of samples associated with disease state. Two nonasthmatic samples were not included in either cluster. We repeated the analysis but by limiting inclusion to atopic subjects among both asthmatic and nonasthmatic participants (see Fig E2 in this article’s Online Repository at www.jacionline.org). Results were very similar to those depicted in Fig 2.
FIG 2.
Hierarchical clustering of samples from asthmatic and nonasthmatic subjects. The green and blue boxes indicate the asthmatic and nonasthmatic clusters, respectively. A1–A10, Samples from asthmatic patients; C1–C10, samples from nonasthmatic subjects. The numbers on the branches indicate the bootstrap support for the cluster.
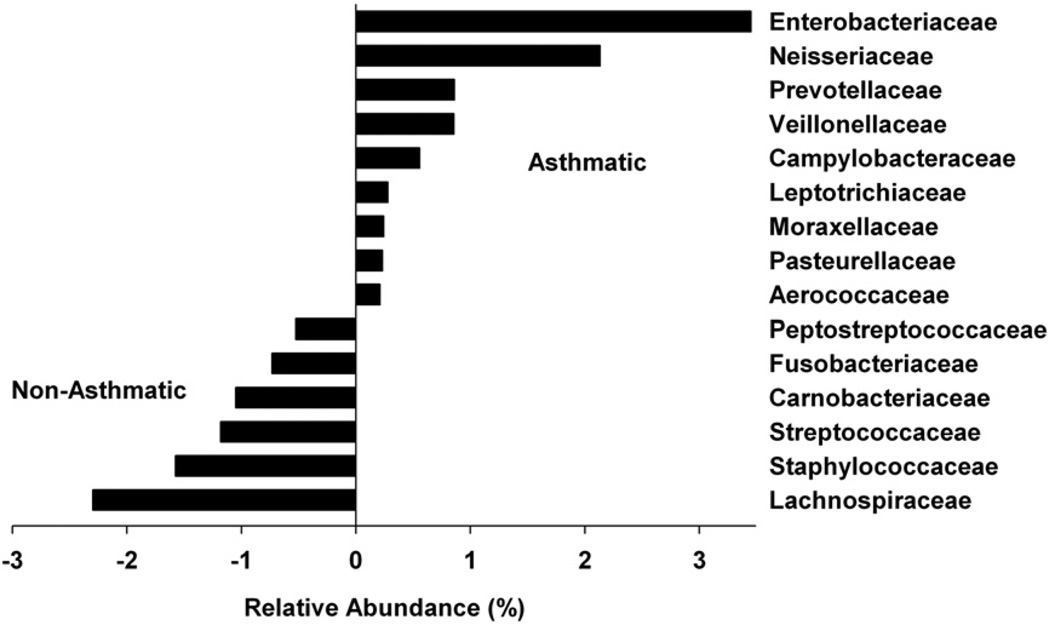
Only 60% of the reads could be assigned to a bacterial family with 50% or greater confidence. On the basis of these assignments, the sequences represented in 5 phyla were distributed into 107 bacterial families. Fifteen of the 107 bacterial families differed in their relative abundance in samples from asthmatic patients versus nonasthmatic subjects (Fig 3). Enterobacteriaceae and Neisseriaceae were more common in samples from asthmatic patients than from nonasthmatic subjects, although these differences were not statistically different. Moraxellaceae and Pasteurellaceae, to which Moraxella catarrhalis and Haemophilus influenzae belong, respectively, were also present in higher abundance in samples from asthmatic patients. Prevotellaceae, a bacterial family belonging to Bacteroidetes, were also more frequent in the asthmatic samples. However, this observation could be partially attributed to a 10-fold increase in the abundance of Bacteroidetes in a sample from a single asthmatic patient (no. A7).
FIG 3.
Relative abundance of 15 bacterial families in samples from asthmatic and nonasthmatic subjects. The value of zero indicates a similar percentage contribution of the bacterial family in both asthmatic and nonasthmatic individuals. A positive value for a bacterial family indicates its higher percentage abundance in asthmatics and a negative value indicates the higher abundance in nonasthmatics. For example, Enterobacteriaceae is ~4% more abundant in asthmatics.
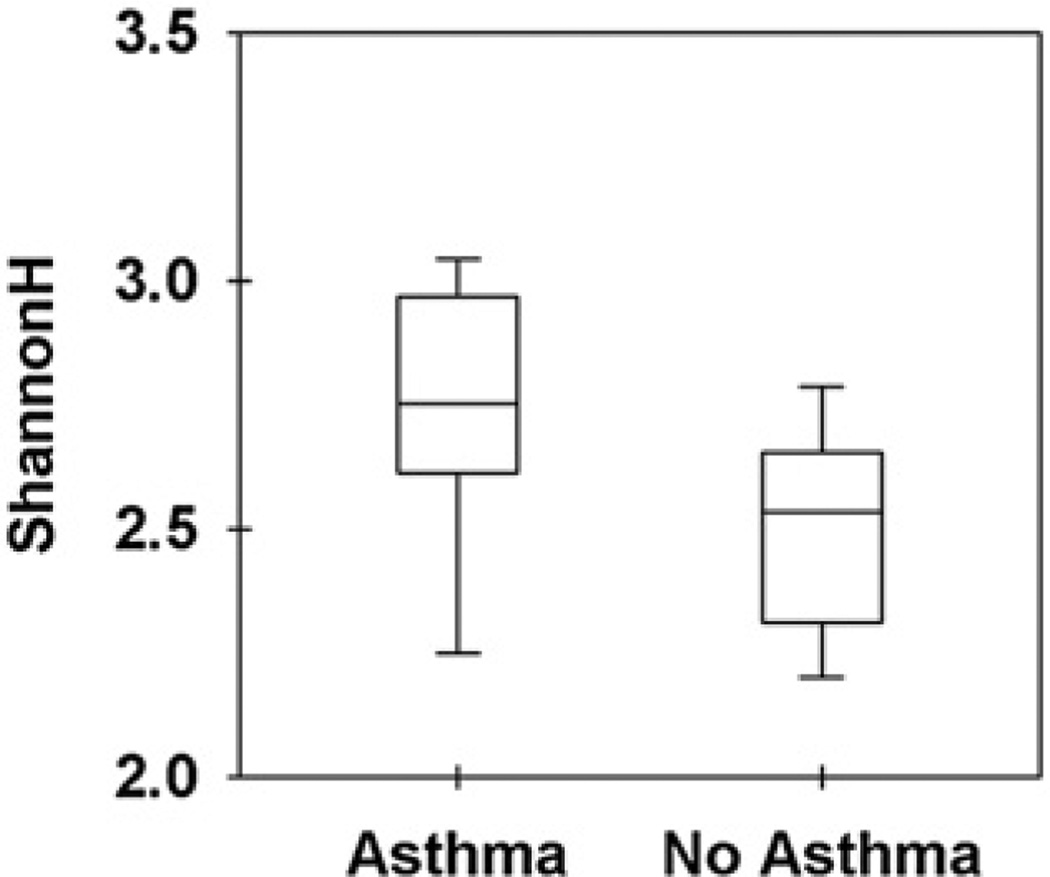
By comparing the clean sequence reads with the RDP database and considering only those that were assigned with a confidence of 50% or greater, we were able to assign about half of the sequences to a particular bacterial genera, resulting in the identification of 320 distinct bacterial genera. The Shannon diversity index based on the proportion of bacterial genera indicated that samples from asthmatic patients were associated with significantly greater bacterial diversity compared with samples from nonasthmatic subjects (P < .05, independent-samples t test; Fig 4).
Figure 4.
Diversity of samples from asthmatic and nonasthmatic subjects based on bacterial genera. The Shannon Index is a mathematic measure commonly used to characterize the species diversity in a community, and it accounts for both the diversity and evenness of the bacterial species present in samples from asthmatic and nonasthmatic subjects.
DISCUSSION
In this study, for the first time, we used the high-throughput 454 sequencing technology to obtain estimates of bacterial diversity in induced sputum. We demonstrate that, as was observed in 2 previous studies using invasive techniques to obtain airway samples, differences in bacterial community composition are associated with active asthma and Proteobacteria were more abundant in induced sputum of asthmatic than nonasthmatic subjects.
We found that samples from asthmatic patients have greater bacterial diversity based on the Shannon Index compared with samples from nonasthmatic subjects, supporting an earlier study that demonstrated an increased bacterial load and diversity in samples from asthmatic patients.12 However, our study indicates that more than 2000 different bacterial taxa were present, a significant increase in bacterial diversity in sputum compared with previous estimates based on airway samples from asthmatic patients. 11 We also observed a slightly higher number of bacterial families compared with a recent analysis of samples from asthmatic patients using a PhyloChip.12 The additional diversity in our study could either result from the more sensitive high-throughput 454 sequencing technology used or reflect the presence of additional microbial communities from the oral cavity. The fact that we were able to precisely identify the bacterial genera in only half of the sequences suggests that many bacteria found in sputum are novel (or uncommon) and have yet to be characterized. Additional sequencing efforts are warranted to completely characterize the microbes in sputum to understand their role in asthma.
In our study sputum samples from asthmatic patients had a significantly higher proportion of Proteobacteria. These results agree with previous microbiome studies using bronchial samples, which have also demonstrated an increase in the abundance of Proteobacteria in samples from asthmatic patients.11,12 The higher abundance of Moraxellaceae and Pasteurellaceae in asthmatic samples (to which Moraxella catarrhalis and Haemophilus influenza belong, respectively) supports the role of these bacteria in the development of asthma.5 However, the abundance of Firmicutes in samples from nonasthmatic subjects contrasts with the results of Hilty et al,11 who showed an abundance of Bacteroidetes in samples from nonasthmatic subjects. Bacteroidetes accounted for 10% to 20% of the total bacteria found in their study. However, in our study, with the exception of a single sample from an asthmatic patient, Bacteroidetes accounted for a mere 1% to 2% of the total bacterial composition. Because the human microbiome has been shown to vary between subjects and populations,8 this difference in the composition of Bacteroidetes could be attributable to differences in the populations from which samples were obtained. Although samples in our study were collected from a mixed population in the United States, the samples in the Hilty et al11 study were from an Irish population.
A major advantage in our study is the fact that our subjects were not selected from tertiary clinics that treat asthmatic patients with more severe disease, as was the case for the 2 previous studies of the airway microbiome in asthmatic patients.11,12 As a consequence, patients included in both those studies had been treated with medium to high doses of inhaled corticosteroids, in some cases for prolonged periods of time, whereas control subjects were steroid naive. Corticosteroids are known to have extensive immunosuppressive effects,23 and therefore the possibility that the observed differences could be attributable to the therapy the patients were receiving rather than their disease state could not be excluded in those studies. In contrast, patients and control subjects in our study were participants in a longitudinal observational study of asthma, which is based on an unselected population of newborns enrolled in the early 1980s.13 The history of asthma (or lack thereof) could thus be confirmed from existing data, and most subjects had mild disease for which they were not taking inhaled corticosteroids, even if they had been prescribed. In addition, results did not change markedly when we limited the analysis to atopic subjects, suggesting that differences in allergic sensitization between cases and control subjects cannot explain our findings.
Although a careful sampling procedure to obtain induced sputum was followed and oral rinses were performed before inducing sputum, we acknowledge that our samples could have been affected by oral microbes. However, we anticipate that this effect is likely to be common across participants with and without asthma. This conclusion is supported by the finding that bacterial composition of induced sputum obtained in this study is quite distinct compared with that of saliva (roughly equal proportions of Bacteroidetes, Firmicutes, and Proteobacteria24), the oropharynx (dominated by Firmicutes), and the nostrils (which have roughly equal proportion of Actinobacteria and Firmicutes25). Moreover, although efforts to avoid contamination were made in the 2 previous studies of airway microbiota using invasive techniques,11,12 it is still possible that the airway microbial communities identified in those studies might have inadvertently originated, at least in part, in the nasal/oral/pharyngeal cavities. Interestingly, it was recently reported that the healthy lung does not harbor a distinct microbiome but instead contains low levels of bacterial sequences largely indistinguishable from upper respiratory tract flora.26 It is thus possible that the consistent changes in microbial communities of sputum and lower respiratory tract samples observed in asthmatic patients and in previous studies might reflect changes occurring in the whole respiratory tract and not in any specific compartment.
In summary, the overabundance of Proteobacteria and increased microbial diversity we observed in the sputum of asthmatic patients is in agreement with results recently reported in studies from samples obtained from the lower airways.11,12 Moreover, our results suggest that a disordered microbial composition of the respiratory tract is not attributable to inhaled corticosteroid therapy or atopic status, is not only present in patients with severe asthma but also in those with more mild disease, and could be a hallmark of asthma. Our data do not allow us to determine whether this disordered microbial composition is the consequence of the disease process or is somehow involved in its pathogenesis.
Supplementary Material
Clinical implications.
Young adults with mild asthma show changes in the composition of induced sputum microbiota compared with nonasthmatic subjects, and these changes might have implications for asthma pathogenesis and management.
Acknowledgments
We thank Penelope Graves and Sujatha Panyala for helping with the preparation of DNA samples for sequencing.
Supported by Southwest Environmental Health Sciences (SWEHSC) training grant ES007901 (to P.R.M.). Part of this study was supported by National Heart, Lung, and Blood Institute grants HL 56177 and HL 14136 (to F.D.M. and A.L.W.) and SWEHSC grant ES006694 (to D.B.).
D. A. Stern has been supported by one or more grants from and/or has one or more grants pending with the National Institutes of Health (NIH). A. L. Wright has been supported by one or more grants from and/or has one or more grants pending with NIH, has received one or more payments for lecturing from or is on the speakers’ bureau for the Association of American Medical Colleges and the University of Vermont, and has received one or more payments for the development of educational presentations for Association of American Medical Colleges. F. D. Martinez has been supported by one or more grants from CRS (HL56177), has consultancy arrangements with MedImmune, has received one or more grants from or has one or more grants pending with the NIH, has received one or more payments for lecturing from or is on the speakers’ bureau for Abbott and Merck, and has received one or more payments for travel/accommodations/meeting expenses from Abbott and Merck.
Abbreviation used
- OTU
Operational taxonomic unit
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Martin RJ. Infections and asthma. Clin Chest Med. 2006;27:87–98. vi. doi: 10.1016/j.ccm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Maffey AF, Barrero PR, Venialgo C, Fernandez F, Fuse VA, Saia M, et al. Viruses and atypical bacteria associated with asthma exacerbations in hospitalized children. Pediatr Pulmonol. 2010;45:619–625. doi: 10.1002/ppul.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston SL, Blasi F, Black PN, Martin RJ, Farrell DJ, Nieman RB. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 4.Talbot TR, Hartert TV, Mitchel E, Halasa NB, Arbogast PG, Poehling KA, et al. Asthma as a risk factor for invasive pneumococcal disease. N Engl J Med. 2005;352:2082–2090. doi: 10.1056/NEJMoa044113. [DOI] [PubMed] [Google Scholar]
- 5.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 6.Hugenholtz P, Tyson GW. Microbiology: metagenomics. Nature. 2008;455:481–483. doi: 10.1038/455481a. [DOI] [PubMed] [Google Scholar]
- 7.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854. e1. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381. e1–e3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–676. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 14.Yan K, Salome C, Woolcock AJ. Rapid method for measurement of bronchial responsiveness. Thorax. 1983;38:760–765. doi: 10.1136/thx.38.10.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax. 1997;52:946–952. doi: 10.1136/thx.52.11.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern DA, Lohman IC, Wright AL, Taussig LM, Martinez FD, Halonen M. Dynamic changes in sensitization to specific aeroallergens in children raised in a desert environment. Clin Exp Allergy. 2004;34:1563–1669. doi: 10.1111/j.1365-2222.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 17.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao A. Nonparametric estimation of the number of classes in a population. Scand J Stat. 1984;11:265–270. [Google Scholar]
- 20.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao A, Shen TJ. Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environ Ecol Stat. 2003;10:429–443. [Google Scholar]
- 22.Suzuki R, Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 23.Gedalia A, Shetty AK. Chronic steroid and immunosuppressant therapy in children. Pediatr Rev. 2004;25:425–434. [PubMed] [Google Scholar]
- 24.Lazarevic V, Whiteson K, Huse S, Hernandez D, Farinelli L, Osteras M, et al. Metagenomic study of the oral microbiota by Illumina high-throughput sequencing. J Microbiol Methods. 2009;79:266–271. doi: 10.1016/j.mimet.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemon KP, Klepac-Ceraj V, Schiffer HK, Brodie EL, Lynch SV, Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. MBio. 2010;1 doi: 10.1128/mBio.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.