Abstract
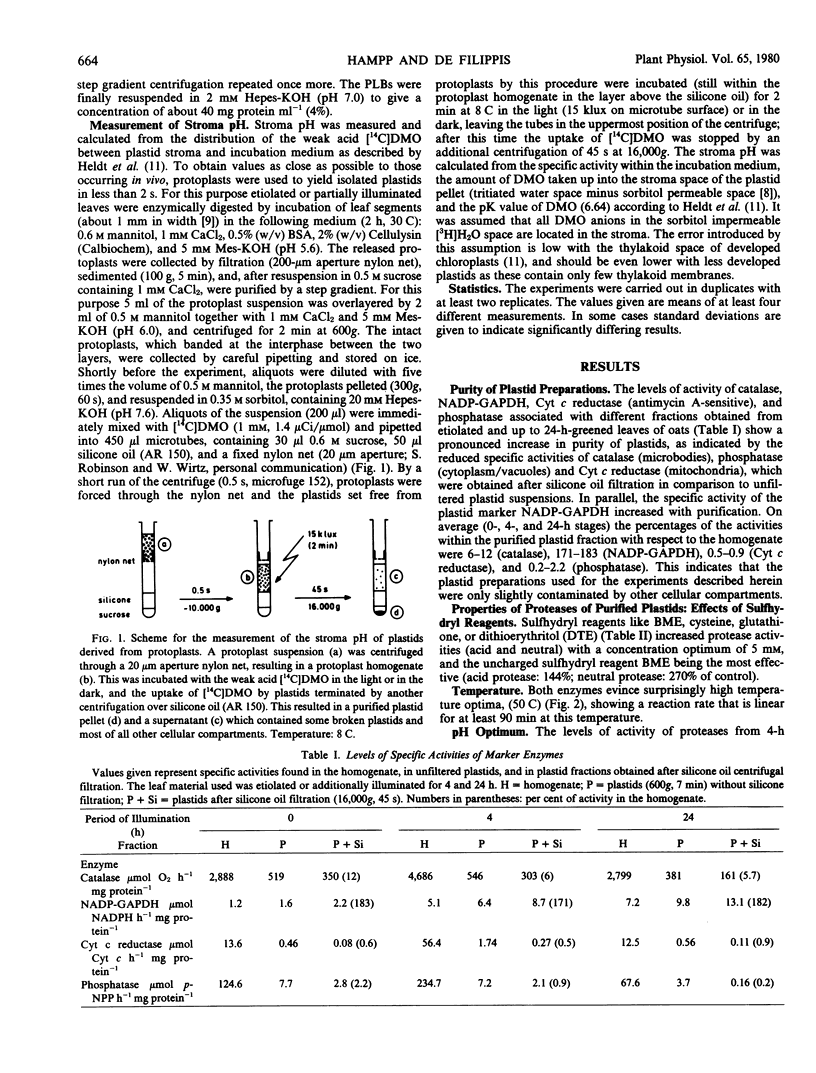
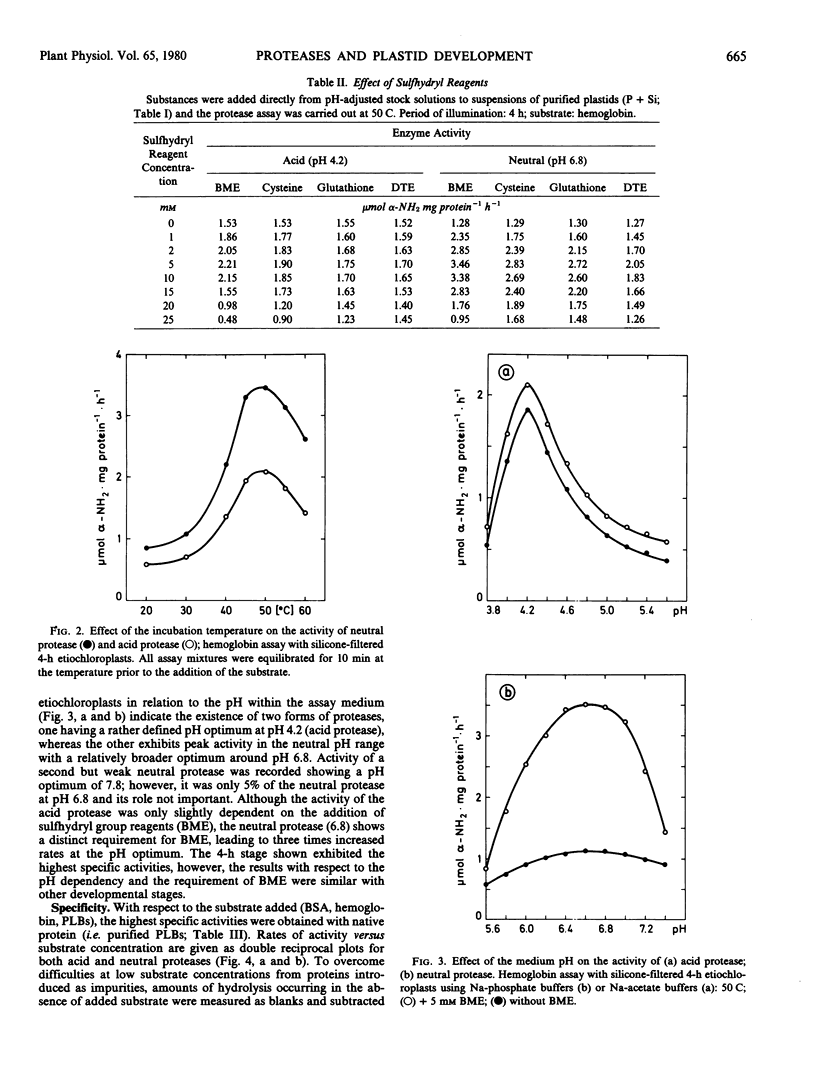
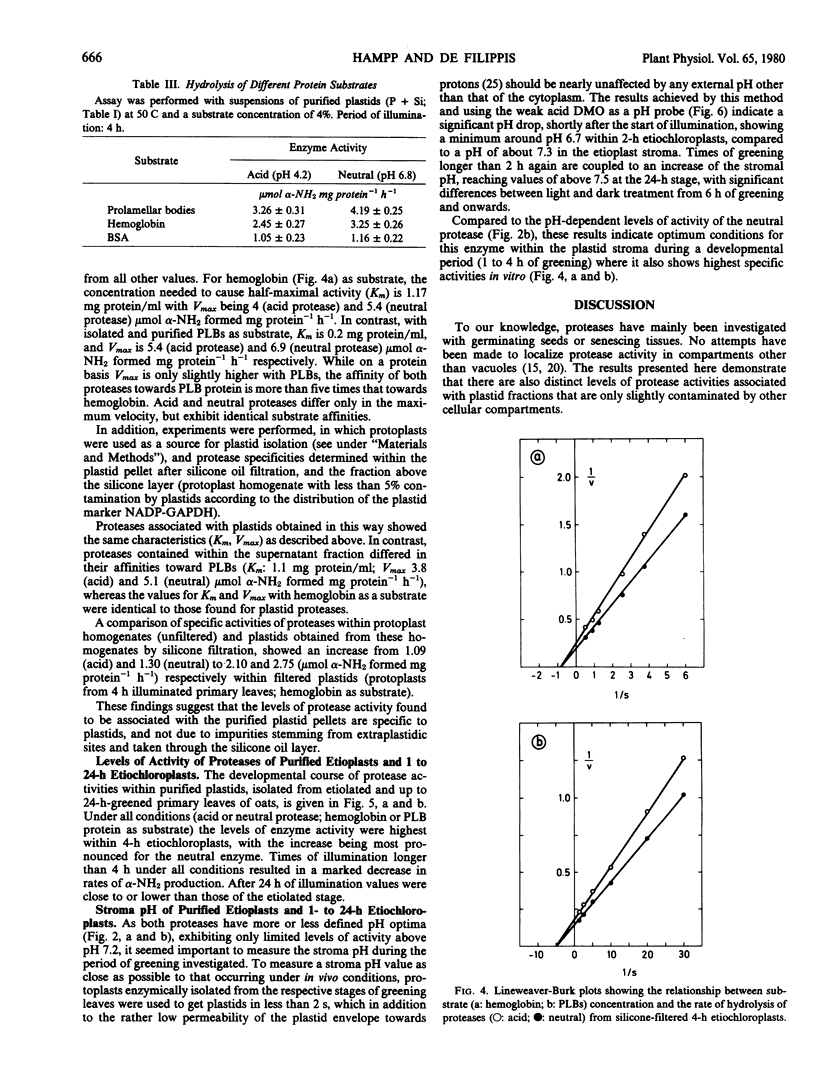
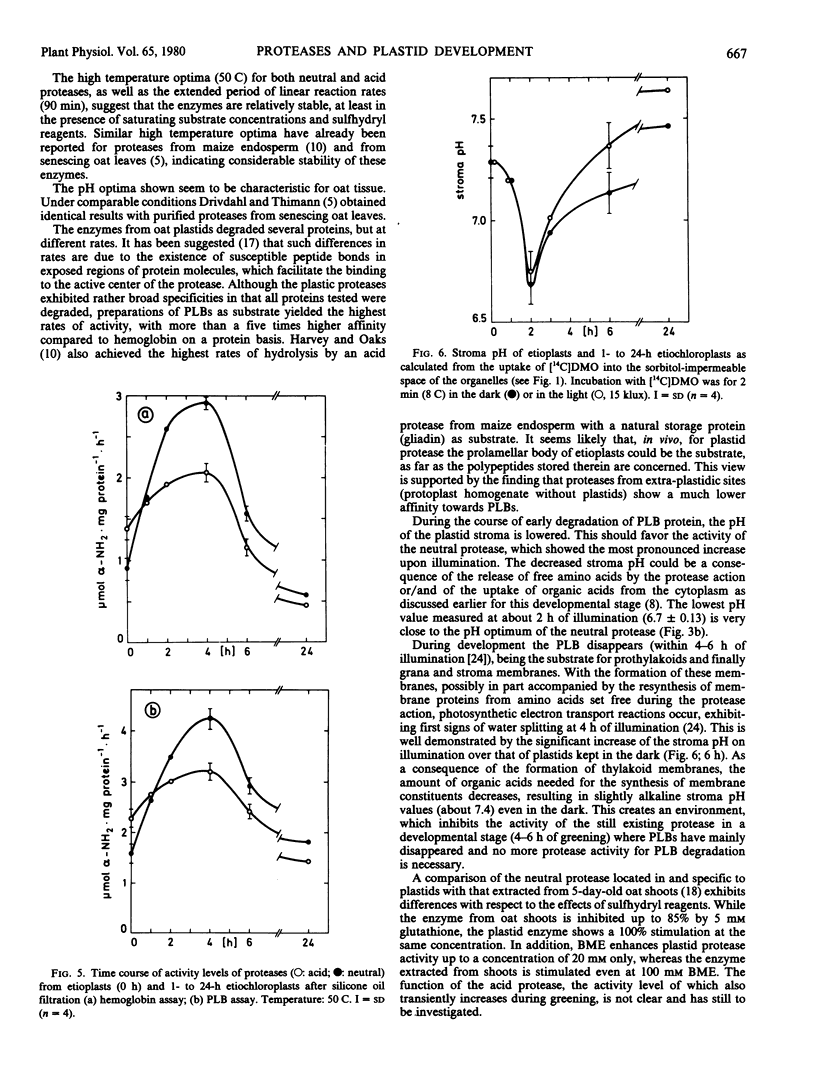
Two proteases active in and specific to oat etioplasts and up to 24-hour etiochloroplasts, only very slightly contaminated by other cellular compartments are described. The enzyme showed pH optima of 4.2 (acid) and 6.8 (neutral), temperature optima of 50 C and the highest level of enzyme activity was with prolamellar bodies (PLBs) as substrate. Both enzymes showed evidence of a sulfhydryl reagent requirement, particularly for the neutral enzyme. Levels of both proteases increased up to 4 hours of illumination of leaves, and then sharply decreased with the largest differences exhibited by the neutral protease. The pH values in the plastid stroma indicated that the neutral enzyme was likely to be the most important in PLB transformation. A comparison between plastid-associated and extra-plastidic protease activities showed similar properties, except the affinity toward PLBs, which was much higher for plastid proteases (Km: 0.2 and 1.1 milligrams protein per milliliter, respectively).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Drivdahl R. H., Thimann K. V. Proteases of Senescing Oat Leaves: II. Reaction to Substrates and Inhibitors. Plant Physiol. 1978 Apr;61(4):501–505. doi: 10.1104/pp.61.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drivdahl R. H., Thimann K. V. Proteases of senescing oat leaves: I. Purification and general properties. Plant Physiol. 1977 Jun;59(6):1059–1063. doi: 10.1104/pp.59.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslin L., Spyridakis A., Labeyrie F. A study of several bonds hypersensitive to proteases in a complex flavohemoenzyme, yeast cytochrome b 2 . Modification of their reactivity with ligand-induced conformational transitions. Eur J Biochem. 1973 Apr;34(2):268–283. doi: 10.1111/j.1432-1033.1973.tb02756.x. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. Partial Purification and Characterization of a Phytochrome-degrading Neutral Protease from Etiolated Oat Shoots. Plant Physiol. 1972 Apr;49(4):521–530. doi: 10.1104/pp.49.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman M. D., Gibbs M. D-glyceraldehyde 3-phosphate dehydrogenases of higher plants. Plant Physiol. 1968 Nov;43(11):1805–1812. doi: 10.1104/pp.43.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn A. R., Hampp R. Appearance of photochemical function in prothylakoids during plastid development. Biochim Biophys Acta. 1979 Aug 14;547(2):380–397. doi: 10.1016/0005-2728(79)90019-7. [DOI] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]


