Abstract
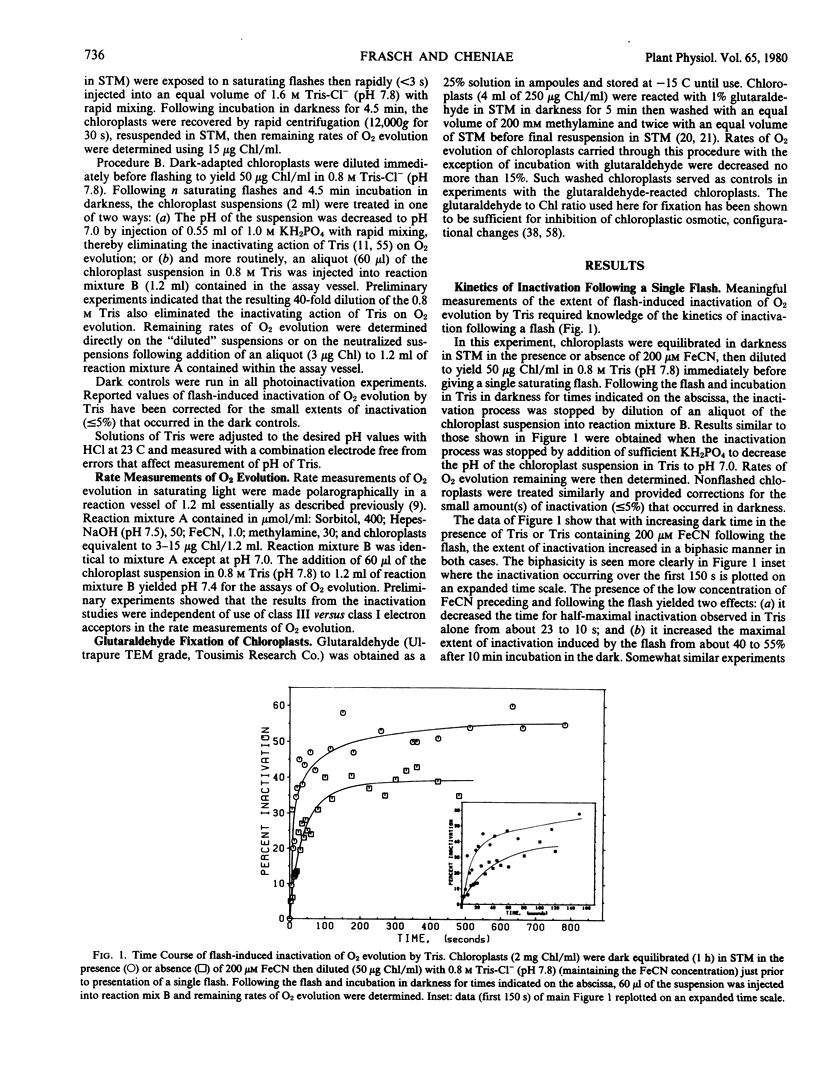
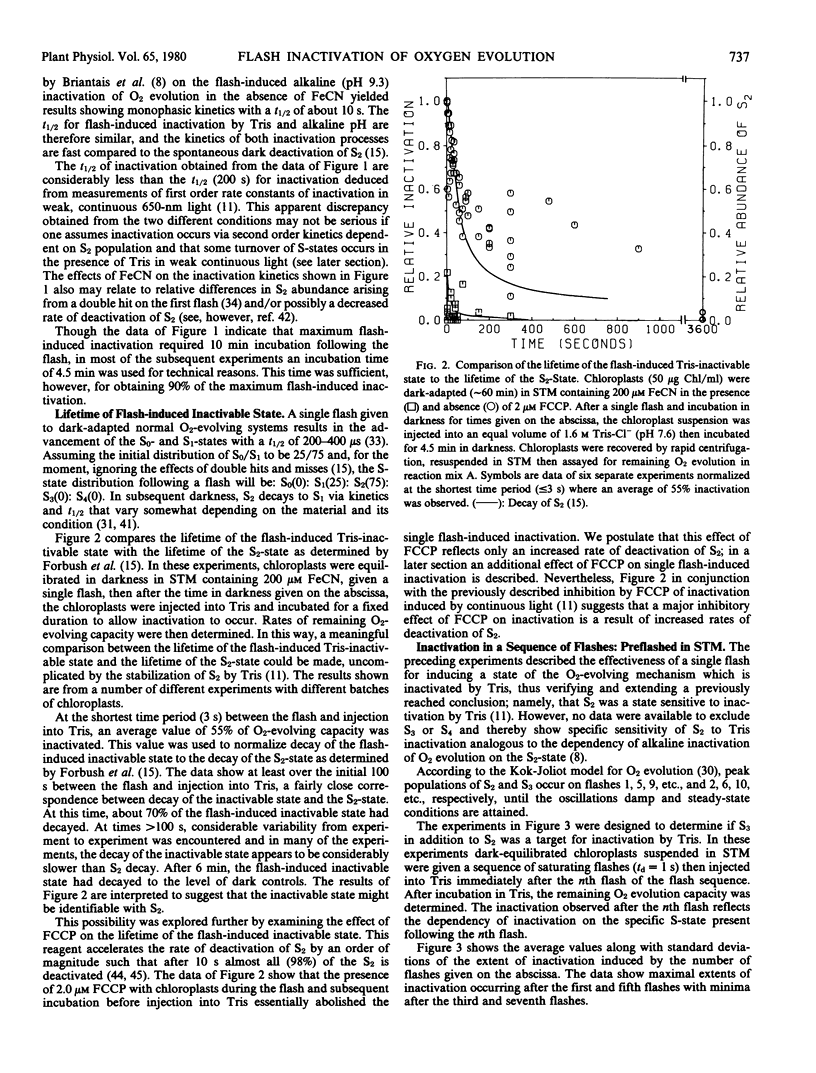
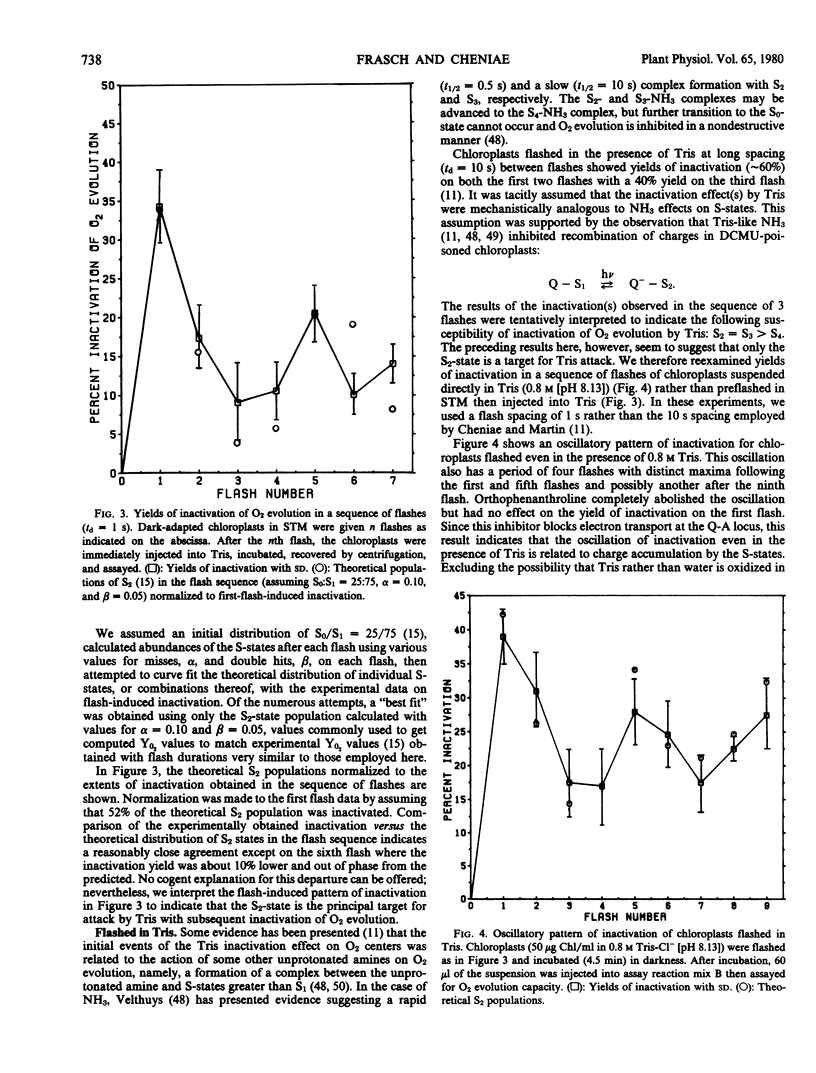
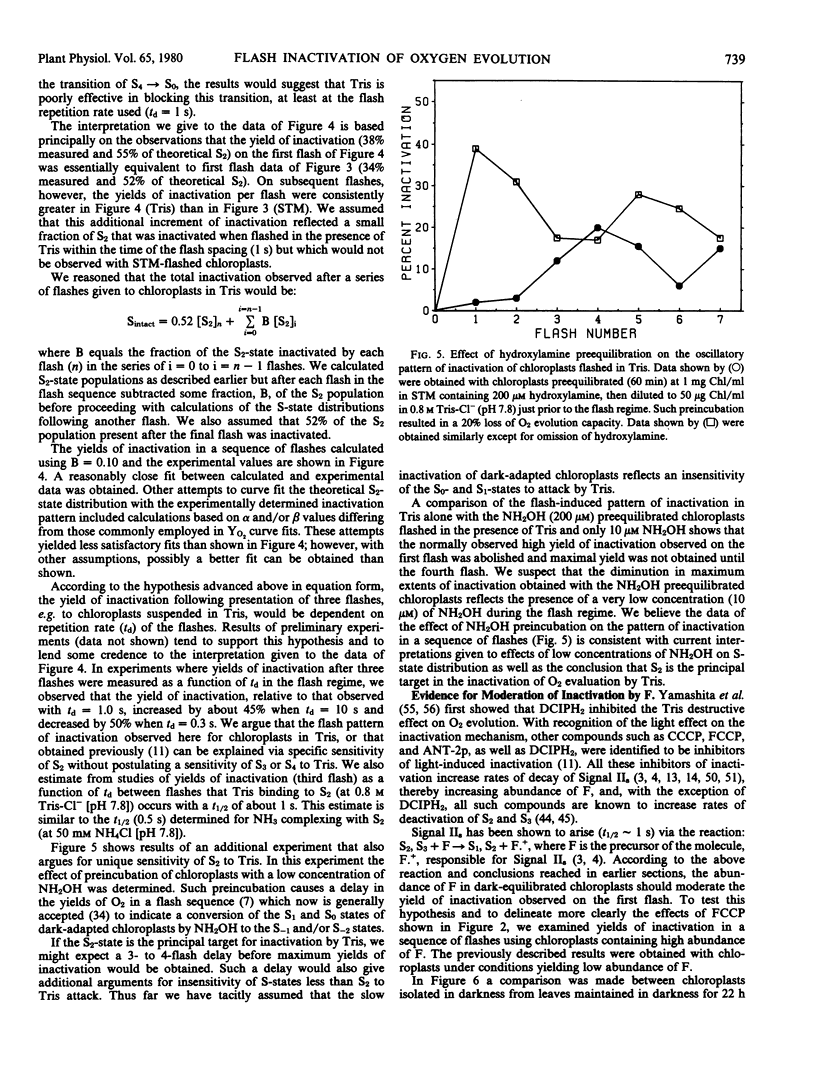
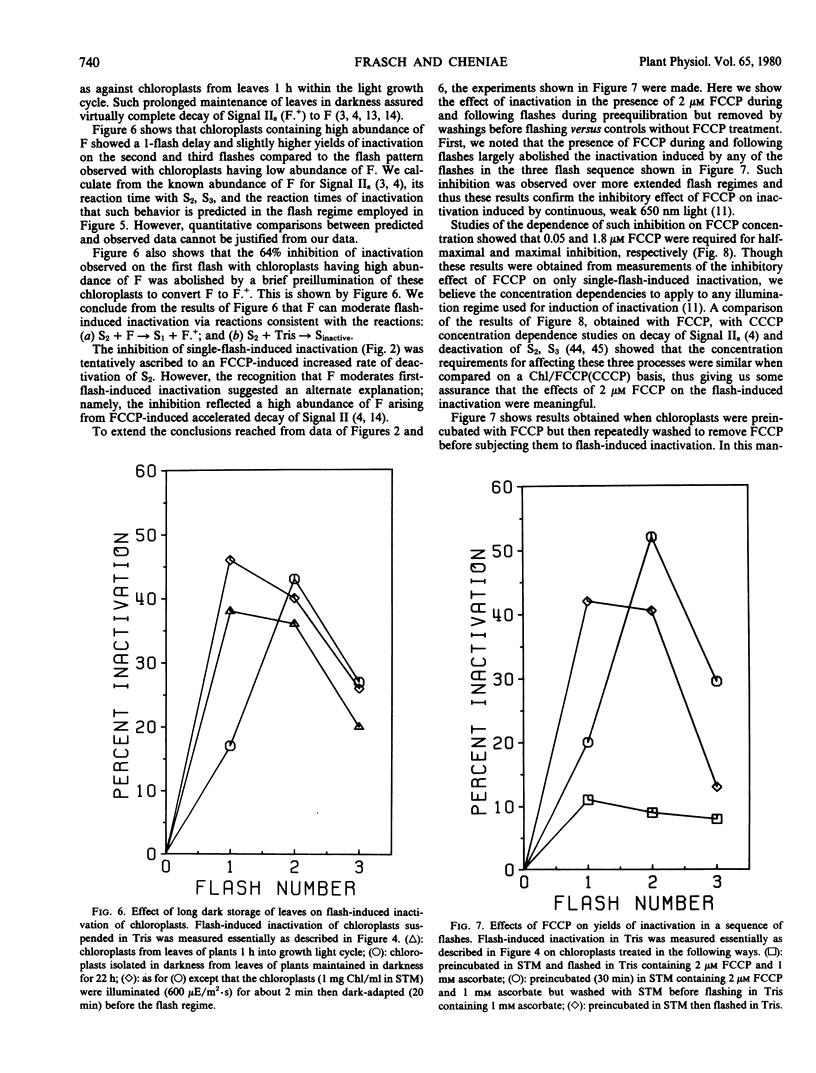
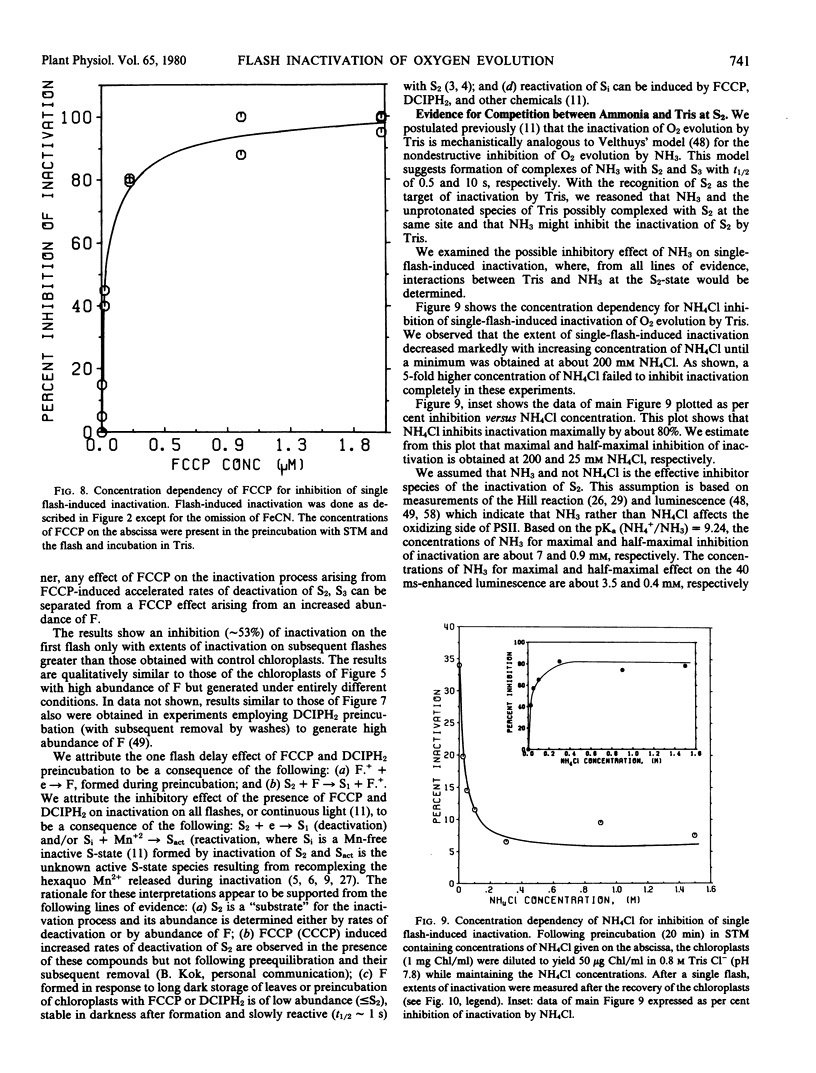
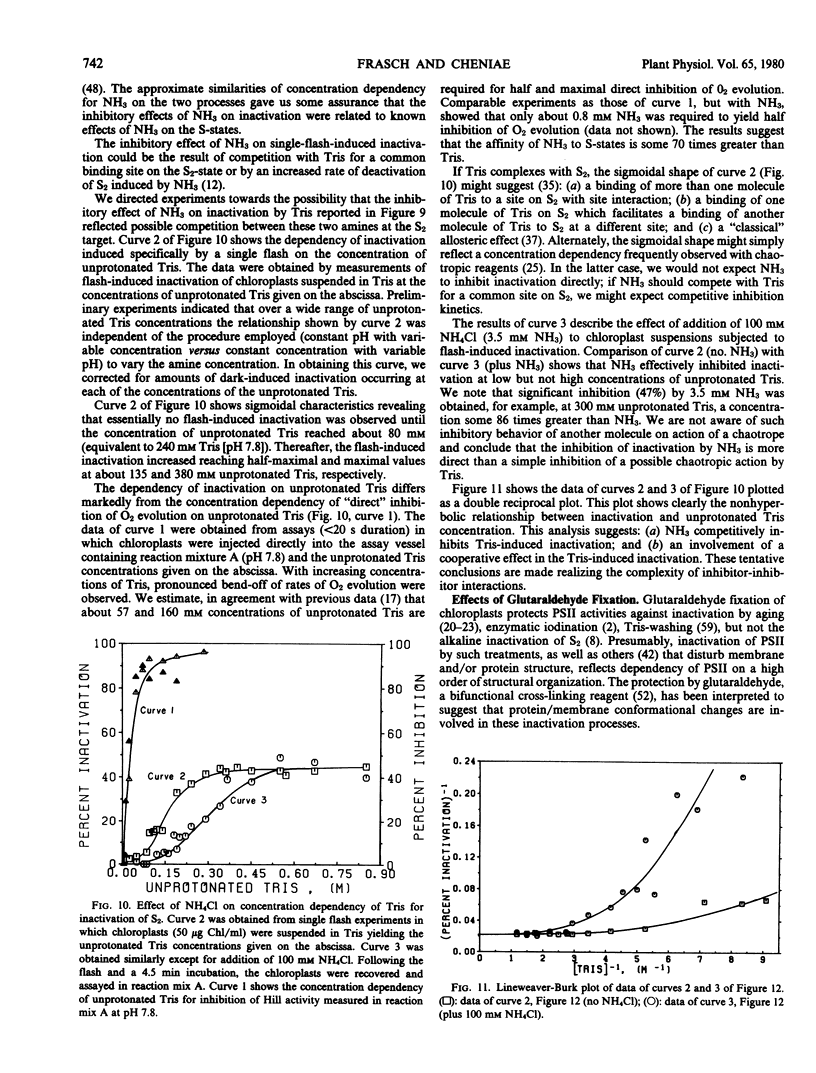
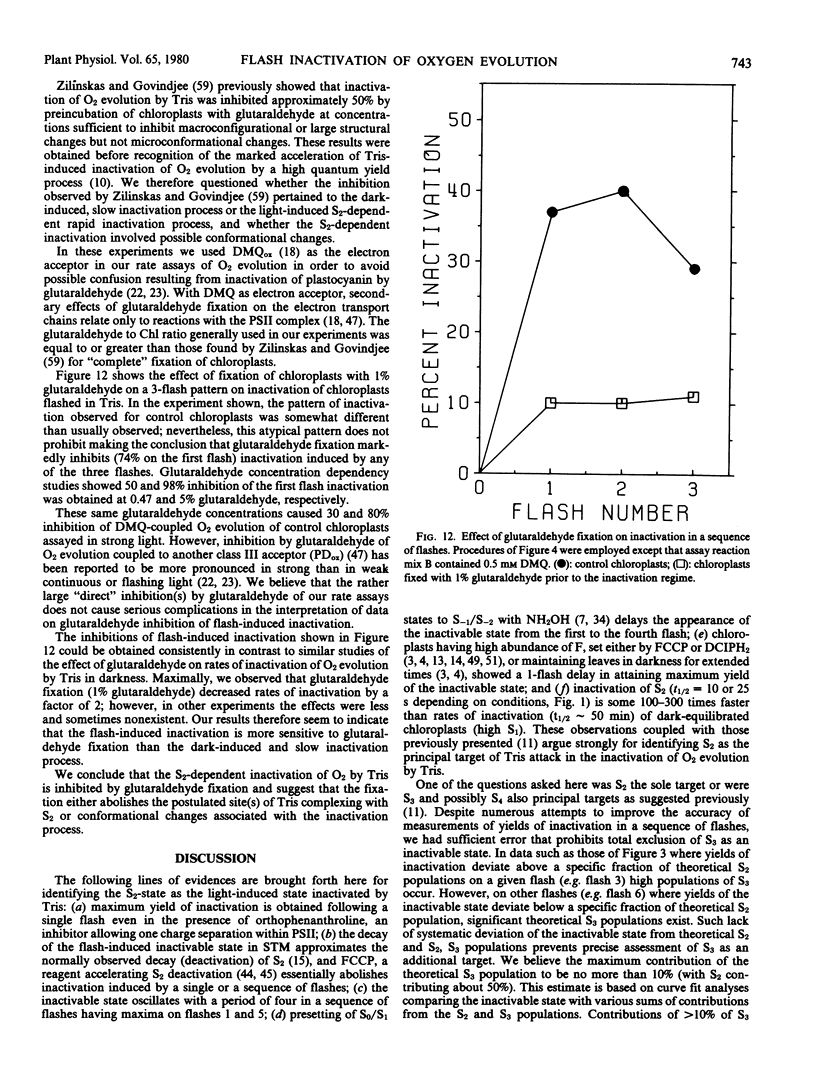
Brief saturating light flashes were used to probe the mechanism of inactivation of O2 evolution by Tris in chloroplasts. Maximum inactivation with a single flash and an oscillation with period of four on subsequent flashes was observed. Analyses of the oscillations suggested that only the charge-collecting O2-evolving catalyst of photosystem II (S2-state) was a target of inactivation by Tris. This conclusion was supported by the following observations: (a) hydroxylamine preequilibration caused a three-flash delay in the inactivation pattern; (b) the lifetimes of the Tris-inactivable and S2-states were similar; and (c) reagents accelerating S2 deactivation decreased the lifetime of the inactivable state. Inactivation proved to be moderated by F, the precursor of Signal IIs, as shown by a one flash delay with chloroplasts having high abundance of F. Evidence was obtained for cooperativity effects in inactivation and NH3 was shown to be a competitive inhibitor of the Tris-induced inactivation. S2-dependent inactivation was inhibited by glutaraldehyde fixation of chloroplasts, possibly suggesting that inactivation proceeds via conformational changes of the S2-state.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arntzen C. J., Vernotte C., Briantais J. M., Armond P. Lactoperoxidase-catalyzed iodination of chloroplast membranes. II. Evidence for surface localization of photosystem II reaction centers. Biochim Biophys Acta. 1974 Oct 18;368(1):39–53. doi: 10.1016/0005-2728(74)90095-4. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Sauer K. Electron paramagnetic resonance signal II in spinach chloroplasts. I. Kinetic analysis for untreated chloroplasts. Biochim Biophys Acta. 1973 Dec 14;325(3):483–503. doi: 10.1016/0005-2728(73)90209-0. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Sauer K. Electron paramagnetic resonance signal II in spinach chloroplasts. II. Alternative spectral forms and inhibitor effects on kinetics of signal II in flashing light. Biochim Biophys Acta. 1973 Dec 14;325(3):504–519. doi: 10.1016/0005-2728(73)90210-7. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E., Babcock G. T., Sauer K. Kinetic study of oxygen evolution parameters in Triswashed, reactivated chloroplasts. Biochim Biophys Acta. 1975 Apr 14;387(1):165–175. doi: 10.1016/0005-2728(75)90061-4. [DOI] [PubMed] [Google Scholar]
- Blankenship R. E., Sauer K. Manganese in photosynthetic oxygen evolution. I. Electron paramagnetic resonance study of the environment of manganese in Tris-washed chloroplasts. Biochim Biophys Acta. 1974 Aug 23;357(2):252–266. doi: 10.1016/0005-2728(74)90065-6. [DOI] [PubMed] [Google Scholar]
- Bouges B. Action de faibles concentrations d'hydroxylamine sur l'émission d'oxygène des algues Chlorella et des chloroplastes d'épinards. Biochim Biophys Acta. 1971 Apr 6;234(1):103–112. doi: 10.1016/0005-2728(71)90135-6. [DOI] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Lavergne J., Arntzen C. J. Identification of S2 as the sensitive state to alkaline photoinactivation of photosystem II in chloroplasts. Biochim Biophys Acta. 1977 Jul 7;461(1):61–74. doi: 10.1016/0005-2728(77)90069-x. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: I. Factors Affecting the Decay of O(2) Evolution. Plant Physiol. 1971 Apr;47(4):568–575. doi: 10.1104/pp.47.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Sites of function of manganese within photosystem II. Roles in O2 evolution and system II. Biochim Biophys Acta. 1970 Mar 3;197(2):219–239. doi: 10.1016/0005-2728(70)90033-2. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Studies on the mechanism of Tris-induced inactivation of oxygen evolution. Biochim Biophys Acta. 1978 May 10;502(2):321–344. doi: 10.1016/0005-2728(78)90053-1. [DOI] [PubMed] [Google Scholar]
- Giaquinta R. T., Ort D. R., Dilley R. A. The possible relationship between a membrane conformational change and photosystem II dependent hydrogen ion accumulation and adenosine 5'-triphosphate synthesis. Biochemistry. 1975 Oct 7;14(20):4392–4396. doi: 10.1021/bi00691a008. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Ort D. R. Studies on the energy coupling sites of photophosphorylation. 3. The different effects of methylamine and ADP plus phosphate on electron transport through coupling sites I and II in isolated chloroplasts. Biochim Biophys Acta. 1973 Oct 19;325(1):157–166. doi: 10.1016/0005-2728(73)90161-8. [DOI] [PubMed] [Google Scholar]
- Hallier U. W., Park R. B. Photosynthetic Light Reactions in Chemically Fixed Anacystis nidulans, Chlorella pyrenoidosa, and Porphyridium cruentum. Plant Physiol. 1969 Apr;44(4):535–539. doi: 10.1104/pp.44.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallier U. W., Park R. B. Photosynthetic light reactions in chemically fixed spinach thylakoids. Plant Physiol. 1969 Apr;44(4):544–546. doi: 10.1104/pp.44.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt H., Kok B. Plastocyanin as the possible site of photosynthetic electron transport inhibition by glutaraldehyde. Plant Physiol. 1977 Aug;60(2):225–229. doi: 10.1104/pp.60.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt H., Kok B. Stabilization by glutaraldehyde of high-rate electron transport in isolated chloroplasts. Biochim Biophys Acta. 1976 Oct 13;449(1):125–135. doi: 10.1016/0005-2728(76)90012-8. [DOI] [PubMed] [Google Scholar]
- Harth E., Reimer S., Trebst A. Control of photosynthetic oxygen evolution by the internal pH of the chloroplast thylakoid. Inhibition of photosynthetic oxygen evolution by uncouplers at high pH and restoration of electron flow by an artificial electron donor for photosystem II. FEBS Lett. 1974 Jun 1;42(2):165–168. doi: 10.1016/0014-5793(74)80777-5. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Destabilization of membranes with chaotropic ions. Methods Enzymol. 1974;31:770–790. doi: 10.1016/0076-6879(74)31080-4. [DOI] [PubMed] [Google Scholar]
- Homann P. H. Effects of manganese on the fluorescence of chloroplasts. Biochem Biophys Res Commun. 1968 Oct 24;33(2):229–234. doi: 10.1016/0006-291x(68)90773-0. [DOI] [PubMed] [Google Scholar]
- Horton P., Croze E. The relationship between the activity of chloroplast photosystem II and the midpoint oxidation-reduction potential of cytochrome b-559. Biochim Biophys Acta. 1977 Oct 12;462(1):86–101. doi: 10.1016/0005-2728(77)90191-8. [DOI] [PubMed] [Google Scholar]
- Izawa S., Heath R. L., Hind G. The role of chloride ion in photosynthesis. 3. The effect of artificial electron donors upon electron transport. Biochim Biophys Acta. 1969 Jun 24;180(2):388–398. doi: 10.1016/0005-2728(69)90123-6. [DOI] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. Ascorbate-supported NADP photoreduction by heated Euglena chloroplasts. Arch Biochem Biophys. 1967 Oct;122(1):144–152. doi: 10.1016/0003-9861(67)90133-6. [DOI] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Verschoor G. A. Primary reactions of photosystem II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):98–106. doi: 10.1016/0005-2728(76)90116-x. [DOI] [PubMed] [Google Scholar]
- Radmer R., Kok B. A kinetic analysis of the oxidizing and reducing sides of the O2-evolving system of photosynthesis. Biochim Biophys Acta. 1973 Jul 26;314(1):28–41. doi: 10.1016/0005-2728(73)90061-3. [DOI] [PubMed] [Google Scholar]
- Renger G., Bouges-Bocquet B., Delosme R. Studies on the ADRY agent-induced mechanism of the discharge of the holes trapped in the photosynthetic watersplitting enzyme system Y. Biochim Biophys Acta. 1973 Apr 5;292(3):796–807. doi: 10.1016/0005-2728(73)90026-1. [DOI] [PubMed] [Google Scholar]
- Saha S., Izawa S., Good N. E. Photophosphorylation as a function of light intensity. Biochim Biophys Acta. 1970 Nov 3;223(1):158–164. doi: 10.1016/0005-2728(70)90140-4. [DOI] [PubMed] [Google Scholar]
- Saha S., Ouitrakul R., Izawa S., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971 May 25;246(10):3204–3209. [PubMed] [Google Scholar]
- Velthuys B. R., Amesz J. Temperature and preillumination dependence of delayed fluorescence of spinach chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):162–168. doi: 10.1016/0005-2728(75)90214-5. [DOI] [PubMed] [Google Scholar]
- Velthuys B. R. Binding of the inhibitor NH3 to the oxygen-evolving apparatus of spinach chloroplasts. Biochim Biophys Acta. 1975 Sep 8;396(3):392–401. doi: 10.1016/0005-2728(75)90145-0. [DOI] [PubMed] [Google Scholar]
- Velthuys B. R., Visser J. W. The reactivation of EPR signal II in chloroplasts treated with reduced dichlorophenol-indophenol: evidence against a dark equilibrium between two oxidation states of the oxygen evolving system. FEBS Lett. 1975 Jul 15;55(1):109–112. doi: 10.1016/0014-5793(75)80971-9. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Inhibition of chloroplasts by UV-irradiation and heat-treatment. Plant Physiol. 1968 Dec;43(12):2037–2040. doi: 10.1104/pp.43.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankel K. Rapid delayed luminescence from chloroplasts: kinetic analysis of components; the relationship to the O 2 evolving system. Biochim Biophys Acta. 1971 Sep 7;245(2):373–385. doi: 10.1016/0005-2728(71)90156-3. [DOI] [PubMed] [Google Scholar]


