Abstract
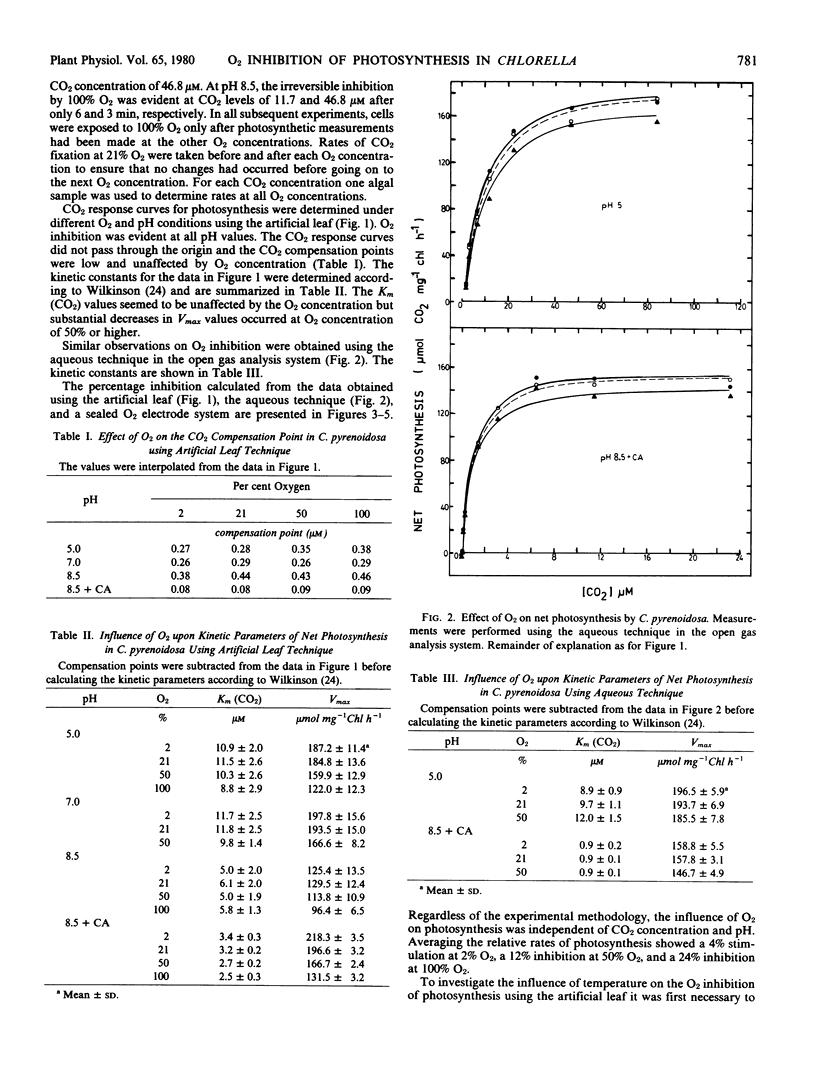
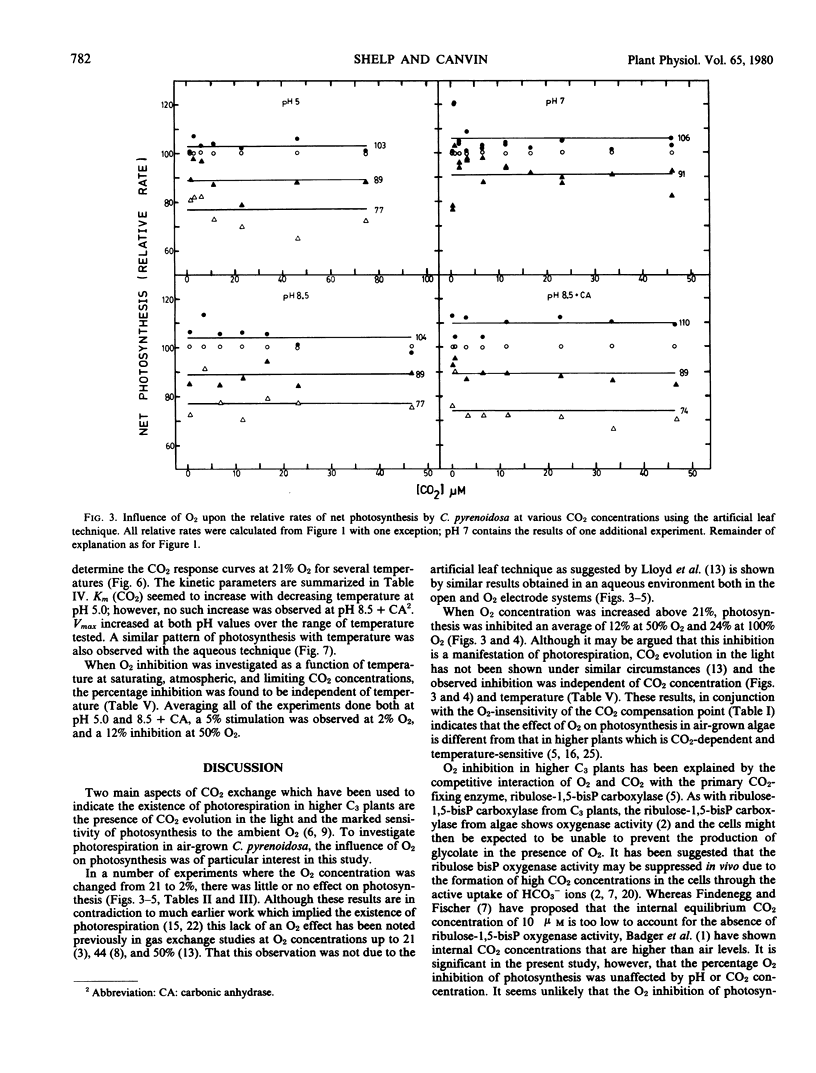
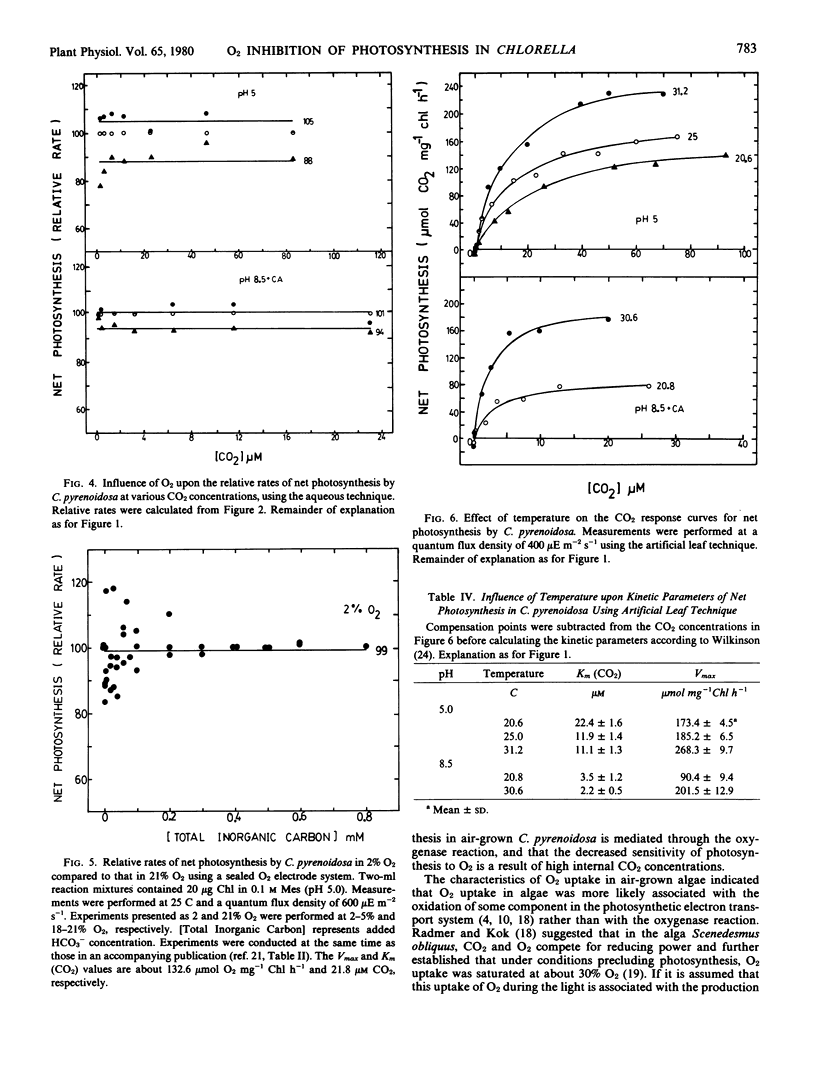
The inhibition of photosynthesis by O2 in air-grown Chlorella pyrenoidosa was investigated using three experimental techniques (artificial leaf, aqueous method, and O2 electrode) to measure carbon assimilation. CO2 response curves were determined under different O2, pH, and temperature conditions. Regardless of the experimental technique and condition, O2 inhibition was not evident until a concentration of 50% was reached; Vmax values were reduced whereas Km (CO2) values were unaffected by the increasing O2 concentration. The response of photosynthesis to O2 was independent of CO2 and HCO3− concentrations as well as temperature. Relative rates of photosynthesis showed a 4 to 5% stimulation in 2% O2, a 12% inhibition in 50% O2, and a 24% inhibition in 100% O2. The inhibition by 50% O2 was still reversible after 20 minutes exposure whereas 100% O2 caused irreversible inhibition after only 4 minutes.
The O2 inhibition is discussed in terms of the oxygenase reaction and a Mehler reaction supporting pseudocyclic electron flow. The results are inconsistent with the proposals that photorespiration exists in these algae and that a CO2-concentrating mechanism suppresses the O2 inhibition of photosynthesis.
Full text
PDF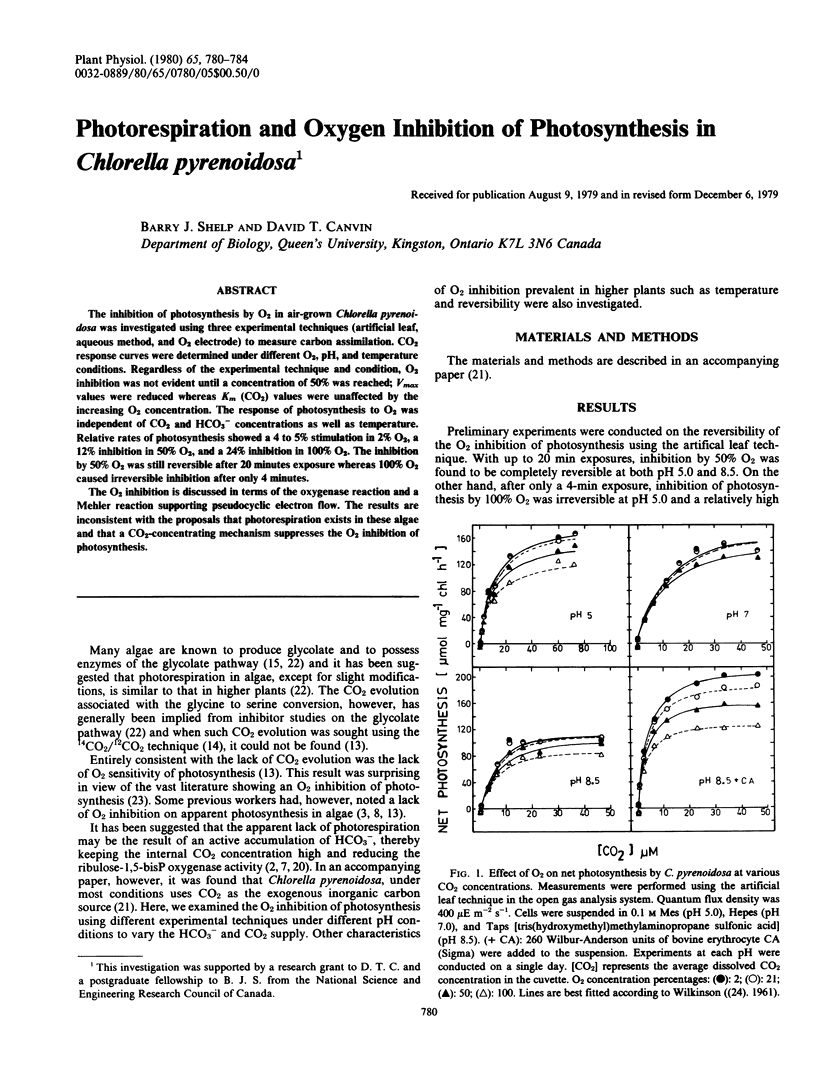
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunt J. S., Heeb M. A. Consumption of O-2 in the light by Chlorella pyrenoidosa and Chlamydomonas reinhardtii. Biochim Biophys Acta. 1971 Mar 2;226(2):354–359. doi: 10.1016/0005-2728(71)90102-2. [DOI] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd N. D., Canvin D. T., Culver D. A. Photosynthesis and photorespiration in algae. Plant Physiol. 1977 May;59(5):936–940. doi: 10.1104/pp.59.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren W. L., Hunt L. D. Comparative biochemistry of ribulose bisphosphate carboxylase in higher plants. Basic Life Sci. 1978;11:127–128. doi: 10.1007/978-1-4684-8106-8_9. [DOI] [PubMed] [Google Scholar]
- Patterson C. O., Myers J. Photosynthetic Production of Hydrogen Peroxide by Anacystis nidulans. Plant Physiol. 1973 Jan;51(1):104–109. doi: 10.1104/pp.51.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R., Kok B., Ollinger O. Kinetics and Apparent K(m) of Oxygen Cycle under Conditions of Limiting Carbon Dioxide Fixation. Plant Physiol. 1978 Jun;61(6):915–917. doi: 10.1104/pp.61.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelp B. J., Canvin D. T. Utilization of Exogenous Inorganic Carbon Species in Photosynthesis by Chlorella pyrenoidosa. Plant Physiol. 1980 May;65(5):774–779. doi: 10.1104/pp.65.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER J. S., BRITTAIN E. G. Oxygen as a factor in photosynthesis. Biol Rev Camb Philos Soc. 1962 Feb;37:130–170. doi: 10.1111/j.1469-185x.1962.tb01607.x. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


