Abstract
Plasma membrane preparations from soybean root and hypocotyl contained the following free sterols: cholesterol, campesterol, stigmasterol, and sitosterol. The cholesterol level was relatively low in root plasma membrane (less than 0.5%) but was 1.4 to 2.4% in hypocotyl membrane. The relative levels of the three other sterols fluctuated with cellular development and tissue source. Campesterol level decreased with the development of both root and hypocotyl membrane. With development, stigmasterol increased greatly in root membrane but remained constant in hypocotyl membrane, and sitosterol, the major free sterol component of all membrane preparations, decreased in root membrane but increased slightly in hypocotyl membrane.
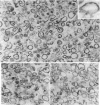
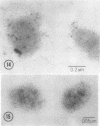
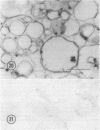
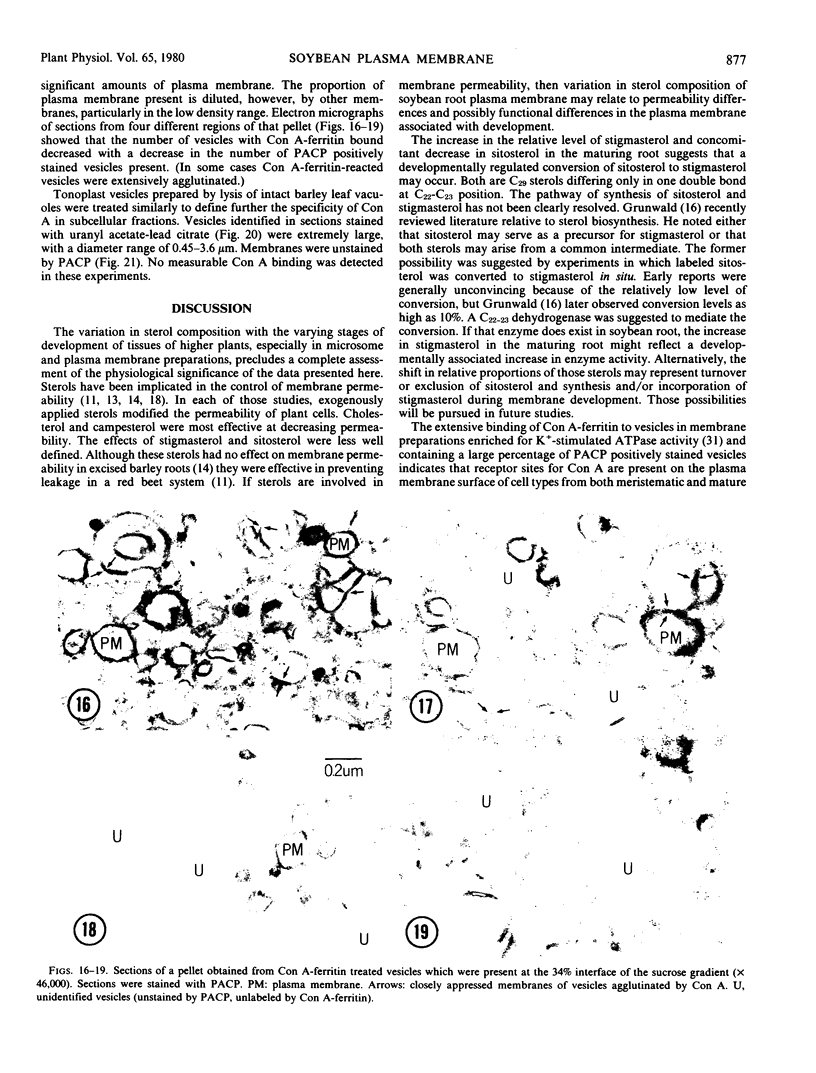
Electron microscope studies indicated that all root plasma membrane preparations were equivalent in terms of relative purity. Hypocotyl membrane preparations contained significantly greater levels of contaminating membrane components.
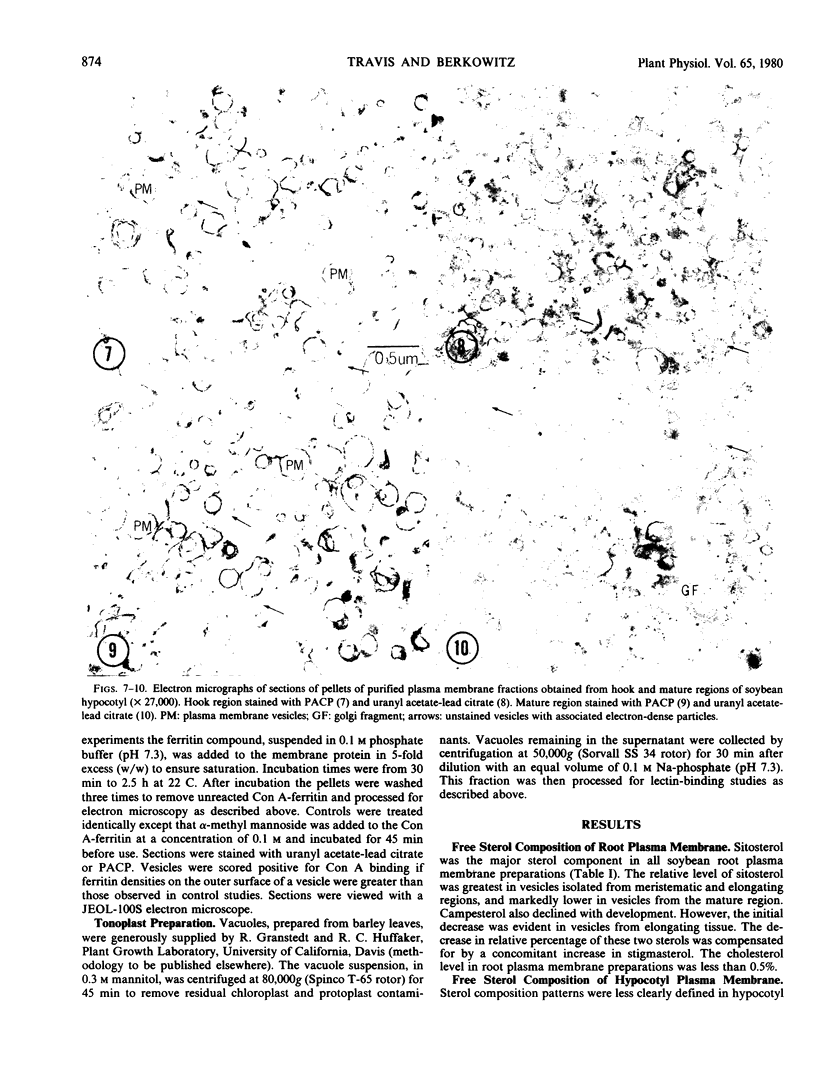
Root plasma membrane fractions were between 70 and 80% pure as determined by staining with the phosphotungstic acid-chromic acid procedure (PACP). Staining was most definitive for vesicles present in complete cross-section. Electron micrographs showed that vesicles treated with concanavalin A (Con A)-ferritin were extensively labeled at the outer surface indicating the presence of mannosyl and/or glucosyl residues at the vesicle surface. Densities of ferritin were highest on vesicles present in oblique section. PACP and Con A-ferritin were thus complementary with respect to topological specificity.
The percentage of Con A-ferritin-labeled and/or PACP-stained vesicles in plasma membrane root preparations was greater than 80%. Con A did not bind in purified tonoplast preparations, and binding was reduced in regions of low PACP reactivity in a root membrane fraction containing a lowered proportion of plasma membrane. Con A specificity for the plasma membrane in subcellular membrane preparations is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Ray P. M. Labeling of the Plasma Membrane of Pea Cells by a Surface-localized Glucan Synthetase. Plant Physiol. 1978 May;61(5):723–730. doi: 10.1104/pp.61.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz R. L., Travis R. L. An electron microscope comparison of plasma membrane vesicles from meristematic and mature soybean root tissue. Plant Physiol. 1979 Jun;63(6):1191–1197. doi: 10.1104/pp.63.6.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R. D., Benveniste P. Isolation and identification of sterols from subcellular fractions of bean leaves (Phaseolus vulgaris). Biochim Biophys Acta. 1972 Sep 1;282(1):85–92. doi: 10.1016/0005-2736(72)90313-6. [DOI] [PubMed] [Google Scholar]
- Bush P. B., Grunwald C., Davis D. L. Changes in Sterol Composition during Greening of Etiolated Barley Shoots. Plant Physiol. 1971 Jun;47(6):745–749. doi: 10.1104/pp.47.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. L., Poneleit C. G. Sterol Accumulation and Composition in Developing Zea mays L. Kernels. Plant Physiol. 1974 Nov;54(5):794–796. doi: 10.1104/pp.54.5.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P., Beevers H. Lipid composition of organelles from germinating castor bean endosperm. Plant Physiol. 1977 Feb;59(2):259–263. doi: 10.1104/pp.59.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Kayman S. C., Kuhlenschmidt M. S. Studies on the binding of concanavalin A to rat liver mitochondria. J Biol Chem. 1973 May 10;248(9):3137–3145. [PubMed] [Google Scholar]
- Grunwald C. Effect of sterols on the permeability of alcohol-treated red beet tissue. Plant Physiol. 1968 Apr;43(4):484–488. doi: 10.1104/pp.43.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Effects of free sterols, steryl ester, and steryl glycoside on membrane permeability. Plant Physiol. 1971 Nov;48(5):653–655. doi: 10.1104/pp.48.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Sterol distribution in intracellular organelles isolated from tobacco leaves. Plant Physiol. 1970 Jun;45(6):663–666. doi: 10.1104/pp.45.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Sterol molecular modifications influencing membrane permeability. Plant Physiol. 1974 Oct;54(4):624–628. doi: 10.1104/pp.54.4.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix D. L., Higinbotham N. Effects of Filipin and Cholesterol on K Movement in Etiolated Stem Cells of Pisum sativum L. Plant Physiol. 1973 Aug;52(2):93–97. doi: 10.1104/pp.52.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix D. L., Kennedy R. M. Adenosine triphosphatase from soybean callus and root cells. Plant Physiol. 1977 Feb;59(2):264–267. doi: 10.1104/pp.59.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan T. W., Franke W. W., Kartenbeck J. Concanavalin A binding by isolated plasma membranes and endomembranes from liver and mammary gland. FEBS Lett. 1974 Aug 30;44(3):274–278. doi: 10.1016/0014-5793(74)81156-7. [DOI] [PubMed] [Google Scholar]
- Kemp R. J., Mercer E. I. Studies on the sterols and sterol esters of the intracellular organelles of maize shoots. Biochem J. 1968 Nov;110(1):119–125. doi: 10.1042/bj1100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi G., Leonard R. T., Thomson W. W. Purification of plasma membranes from roots of barley: specificity of the phosphotungstic Acid-chromic Acid stain. Plant Physiol. 1978 Jun;61(6):993–999. doi: 10.1104/pp.61.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Singer S. J. Ferritin-conjugated plant agglutinins as specific saccharide stains for electron microscopy: application to saccharides bound to cell membranes. Proc Natl Acad Sci U S A. 1971 May;68(5):942–945. doi: 10.1073/pnas.68.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Booz M. L. Partial Characterization of a Potassium-stimulated Adenosine Triphosphatase from the Plasma Membrane of Meristematic and Mature Soybean Root Tissue. Plant Physiol. 1979 Mar;63(3):573–577. doi: 10.1104/pp.63.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I., Wartiovaara J. Lectin receptor sites on rat liver cell nuclear membranes. J Cell Sci. 1976 Nov;22(2):335–344. doi: 10.1242/jcs.22.2.335. [DOI] [PubMed] [Google Scholar]