Abstract
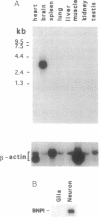
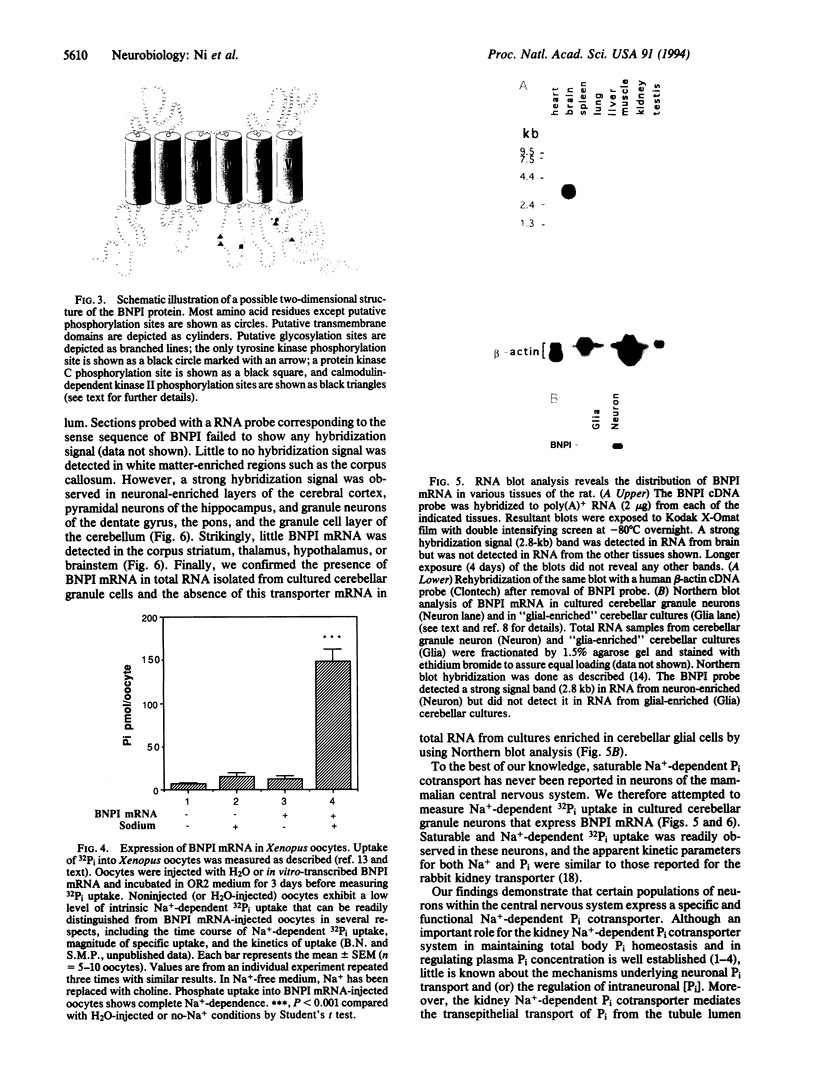
We have isolated a brain-specific cDNA that encodes a Na(+)-dependent inorganic phosphate (Pi) cotransporter (BNPI). The nucleotide sequence of BNPI predicts a protein of 560 amino acids with 6-8 putative transmembrane-spanning segments that is approximately 32% identical to the rabbit kidney Na(+)-dependent Pi cotransporter. Expression of BNPI mRNA in Xenopus oocytes results in Na(+)-dependent Pi transport similar to that reported for the recombinantly expressed or native kidney Na(+)-dependent cotransporter. RNA blot analysis reveals that BNPI mRNA is expressed predominantly (if not exclusively) in brain, and in situ hybridization histochemistry reveals BNPI transcripts in neurons of the cerebral cortex, hippocampus, and cerebellum. Furthermore, we have confirmed the presence of saturable Na(+)-dependent Pi cotransport in cultured cerebellar granule cells. Together, these data demonstrate the presence of a specific neuronal Na(+)-dependent transport system for Pi in brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anner B., Ferrero J., Jirounek P., Jones G. J., Salamin A., Straub R. W. Sodium-dependent influx of orthophosphate in mammalian non-myelinated nerve. J Physiol. 1976 Sep;260(3):667–686. doi: 10.1113/jphysiol.1976.sp011538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anner B., Ferrero J., Jirounek P., Straub R. W. Uptake of orthophosphate by rabbit vagus nerve fibres. J Physiol. 1975 Jun;247(3):759–771. doi: 10.1113/jphysiol.1975.sp010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Morrissey R. L., Zolock D. T., Rasmussen H. The intestinal response to vitamin D. Rev Physiol Biochem Pharmacol. 1981;89:63–142. doi: 10.1007/BFb0035265. [DOI] [PubMed] [Google Scholar]
- Chuang D. M., Gao X. M., Paul S. M. N-methyl-D-aspartate exposure blocks glutamate toxicity in cultured cerebellar granule cells. Mol Pharmacol. 1992 Aug;42(2):210–216. [PubMed] [Google Scholar]
- Deguchi Y., Yamato I., Anraku Y. Nucleotide sequence of gltS, the Na+/glutamate symport carrier gene of Escherichia coli B. J Biol Chem. 1990 Dec 15;265(35):21704–21708. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G. C., Pratt R. D., Pedersen P. L. Energy-linked anion transport. Cloning, sequencing, and characterization of a full length cDNA encoding the rat liver mitochondrial proton/phosphate symporter. J Biol Chem. 1989 Sep 15;264(26):15628–15633. [PubMed] [Google Scholar]
- Fulceri R., Bellomo G., Gamberucci A., Romani A., Benedetti A. Physiological concentrations of inorganic phosphate affect MgATP-dependent Ca2+ storage and inositol trisphosphate-induced Ca2+ efflux in microsomal vesicles from non-hepatic cells. Biochem J. 1993 Jan 1;289(Pt 1):299–306. doi: 10.1042/bj2890299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Burgess R. R. Sigma factors from E. coli, B. subtilis, phage SP01, and phage T4 are homologous proteins. Nucleic Acids Res. 1986 Aug 26;14(16):6745–6763. doi: 10.1093/nar/14.16.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himori N., Imai M., Akaike N., Matsukura T., Watanabe H. Replenishment of brain adenosine triphosphate content by morphinan-type N-methyl-D-asparatate receptor antagonists, dextrorphan and dextromethorphan through the activation of adenylate kinase. Arzneimittelforschung. 1992 May;42(5):595–598. [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Marini A. M., Schwartz J. P., Kopin I. J. The neurotoxicity of 1-methyl-4-phenylpyridinium in cultured cerebellar granule cells. J Neurosci. 1989 Oct;9(10):3665–3672. doi: 10.1523/JNEUROSCI.09-10-03665.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Werner A., Reshkin S., Wuarin F., Biber J. Cellular mechanisms in proximal tubular reabsorption of inorganic phosphate. Am J Physiol. 1991 May;260(5 Pt 1):C885–C899. doi: 10.1152/ajpcell.1991.260.5.C885. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Greengard P. Protein phosphorylation in the brain. Nature. 1983 Oct 13;305(5935):583–588. doi: 10.1038/305583a0. [DOI] [PubMed] [Google Scholar]
- Ni B., Landry C. F., Brown I. R. Developmental expression of neuronal calmodulin mRNA species in the rat brain analyzed by in situ hybridization. J Neurosci Res. 1992 Dec;33(4):559–567. doi: 10.1002/jnr.490330408. [DOI] [PubMed] [Google Scholar]
- Ni B., Rush S., Gurd J. W., Brown I. R. Molecular cloning of calmodulin mRNA species which are preferentially expressed in neurons in the rat brain. Brain Res Mol Brain Res. 1992 Mar;13(1-2):7–17. doi: 10.1016/0169-328x(92)90039-e. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993 May;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- STRICKLER J. C., THOMPSON D. D., KLOSE R. M., GIEBISCH G. MICROPUNCTURE STUDY OF INORGANIC PHOSPHATE EXCRETION IN THE RAT. J Clin Invest. 1964 Aug;43:1596–1607. doi: 10.1172/JCI105035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J., Du Z., Thomas K., Wilson R., Hillier L., Staden R., Halloran N., Green P., Thierry-Mieg J., Qiu L. The C. elegans genome sequencing project: a beginning. Nature. 1992 Mar 5;356(6364):37–41. doi: 10.1038/356037a0. [DOI] [PubMed] [Google Scholar]
- Werner A., Biber J., Forgo J., Palacin M., Murer H. Expression of renal transport systems for inorganic phosphate and sulfate in Xenopus laevis oocytes. J Biol Chem. 1990 Jul 25;265(21):12331–12336. [PubMed] [Google Scholar]
- Werner A., Moore M. L., Mantei N., Biber J., Semenza G., Murer H. Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9608–9612. doi: 10.1073/pnas.88.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]