Abstract
We here discuss the role of brown adipose tissue on energy homeostasis and assess its potential as a target for body weight management. Because of their high number of mitochondria and the presence of uncoupling protein 1, brown fat adipocytes can be termed as energy inefficient for adenosine-5′-triphosphate (ATP) production but energy efficient for heat production. Thus, the energy inefficiency of ATP production, despite high energy substrate oxidation, allows brown adipose tissue to generate heat for body temperature regulation. Whether such thermogenic property also plays a role in body weight regulation is still debated. The recent (re)discovery of brown adipose tissue in human adults and a better understanding of brown adipose tissue development have encouraged the quest for new alternatives to treat obesity since obese individuals seem to have less brown adipose tissue mass/activity than do their lean counterparts. In this review, we discuss the physiological relevance of brown adipose tissue on thermogenesis and its potential usefulness on body weight control in humans.
Keywords: adaptive thermogenesis, uncoupling protein, mitochondria, proton leak, obesity
INTRODUCTION
The number of overweight/obese individuals is exceeding the number of lean people in many developed and developing countries, challenging the concept of “body weight normality” in these populations (60). Growing interest exists in finding strategies to reduce excess body weight; however, at present very few—if any—of those strategies have met the essential criteria of being safe and efficacious (86).
Decreasing energy intake seems a difficult goal in societies for which plenty of high-energy foods are available. To tip energy balance toward weight loss, not only should food intake be decreased, but also increased energy expenditure may help. Even if increasing physical activity seems the most effective and safe way to enhance energy expenditure, not many people are willing to follow the minimal recommendations to maintain an active lifestyle. As a consequence, the search for a safe and efficacious way to increase metabolic rate has become important.
Until a couple of years ago, a potential role of brown adipose tissue (BAT) in the regulation of body temperature and energy balance in adult humans was dismissed. However, very recently, BAT has been consistently identified in adult humans undergoing positron emission tomography (PET; see sidebar Positron Emission Tomography as a Tool to Measure Brown Adipose Tissue) and computed tomography (CT) technologies (18). This (re)discovery of BAT and its known role in adaptive thermogenesis regulation has opened an interest in activating BAT and enhancing energy expenditure in order to control body weight and prevent metabolic disorders (15, 45, 55, 57, 66, 67, 85, 86). In this review, we discuss the evidence and the putative roles of BAT in the regulation of body weight in humans.
ANATOMICAL, HISTOLOGICAL, AND MOLECULAR CHARACTERISTICS OF BROWN FAT CELLS
From an anatomical point of view, brown fat cells are localized in two types of depots: discrete and diffuse. In humans, BAT of discrete location is found in cervical-supraclavicular (the most common location), perirenal/adrenal, and paravertebral regions around the major vessels (the aorta and its main branches: carotids, subclavian, intercostal, and renal arteries) and is probably present to generate and distribute heat to maintain core temperature (42) (Figure 1). In distinction, diffuse BAT is found in coexistence with white adipose and skeletal muscle tissues.
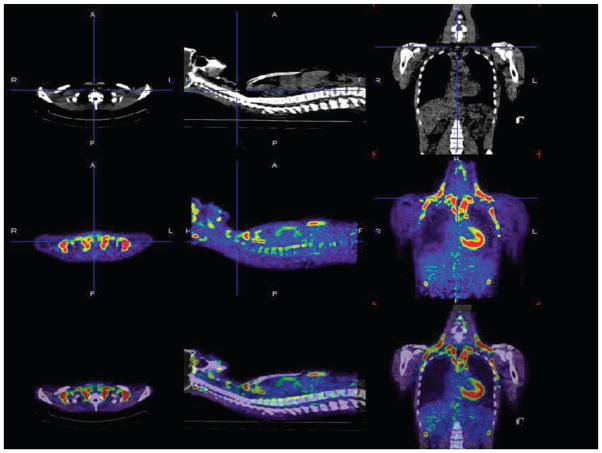
Figure 1.
Anatomical location of discrete brown adipose tissue measured by positron emission tomography (PET). Computed tomography (CT; upper panel ) and PET (with 18F-fluorodeoxyglucose; middle panel ) images from the neck and thoracic region in one lean individual. The bottom panel shows the superimposition of the CT and PET scans. In the three panels (from left to right) are (a) a transverse slice at the level of the clavicles, (b) a sagittal slice at the level of the spine, and (c) a coronal slice of the thorax. Activated brown adipose tissue areas (red and green) are in the cervical-supraclavicular (most common), perirenal/adrenal, and paravertebral regions. Illustration prepared by Dr. Wouter van Marken Lichtenbelt (Maastricht University, The Netherlands).
Brown fat cells are characterized by a polygonal shape with multilocular lipid droplets and an increased number of large and spherical mitochondria, which give their brown coloration (13) (Figure 2). In addition, BAT is highly irrigated with blood vessels and innervated with noradrenergic fibers. Uncoupling protein 1 (UCP1), a highly specialized protein, is expressed in brown fat cells and can therefore be considered as a marker of BAT. Additional genes showing increased expression in these cells are peroxisome proliferator–activated receptor γ coactivator 1α (PGC1α), deiodinase iodothyronine type 2 (DIO2), cytochrome c, PR domain containing 16 (PRDM16), and β3 adrenergic receptor (33, 91) (Figure 3). All of these genes are closely related to the main role of brown fat cells, i.e., heat production.
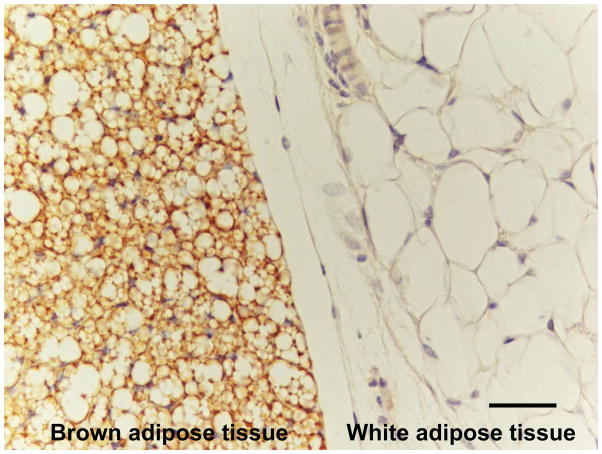
Figure 2.
Histology of brown and white fat cells. Light microscopic image of mouse anterior subcutaneous fat depot in an area where white and brown adipose tissues are close to each other. Uncoupling protein 1 (UCP1)-immunoreactive fat cells corresponding to brown adipose tissue (left) and UCP1-negative white fat cells corresponding to white adipose tissue (right) are visible. Immunohistochemistry by ABC method. Sheep antirat UCP1 primary antibody diluted 1:500. Antibody kindly provided by Daniel Ricquier (Paris). Bar represents 40 microns. Illustration prepared by Dr. Saverio Cinti (University of Ancona, Italy).
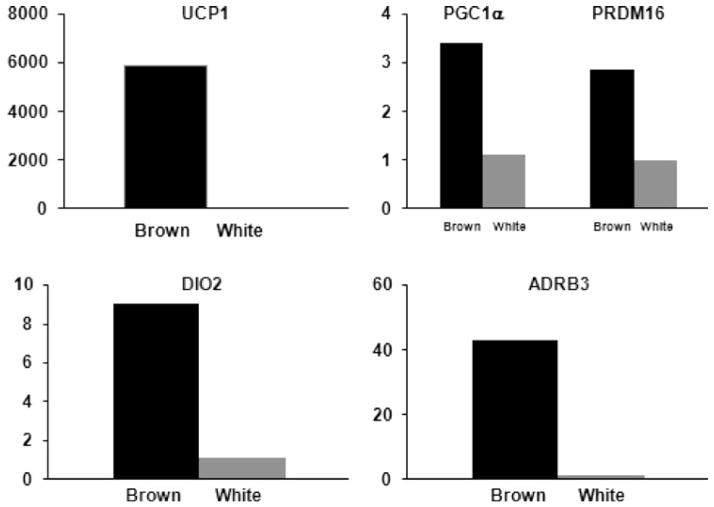
Figure 3.
Gene expression profile in brown and white fat cells. Gene expression unit corresponds to brown adipose tissue as a multiple of white adipose tissue. UCP1, uncoupling protein 1; PGC1α, peroxisome-proliferator–activated receptor γ coactivator 1α; PRDM16, PR domain containing 16; DIO2, deiodinase iodothyronine type 2; ADRB3, β3 adrenergic receptor. Data modified from Virtanen et al. (91).
EMBRYONIC ORIGIN OF BROWN FAT CELLS
BAT, dermis, and some skeletal muscle cells are derived from the central dermomyotome, specifically from a group of cells positive for engrailed-1, which is a homeobox transcription factor (5). Indeed, brown but not white fat precursor cells express genes that are characteristics of muscle precursors such as Myf5, an early myogenic transcription factor (33, 82, 93). There is another population of brown fat cells called brite cells, which, in response to cold exposure or chronic catecholamine stimulation, appear in white fat depots. These cells are not derived from a Myf5-expressing cell lineage (74), thus reinforcing the notion that brown fat cells in discrete locations are from a different lineage than brown fat cells in diffuse locations (82, 100). These newly formed brown adipocytes could derive from existing stem cells in the tissue, from migrating stem cells, or from the direct transformation of differentiated white adipocytes (transdifferentiation) (13, 25, 31). In this regard, Petrovic et al. (62) found that precursors from mouse white adipose tissue treated with rosiglitazone—a PPARγ agonist—promote PGC1α expression, mitochondrial biogenesis, and a noradrenaline-dependent UCP1 gene expression in a subset of cells. However, these cells do not express PRDM16 or myocyte-associated genes (Myf5), typical molecular signatures of brown fat cells found in discrete locations.
REGULATION OF BROWN ADIPOGENESIS
Brown adipogenesis is a highly regulated process, with several growth factors and transcription factors playing significant roles (Figure 4). Bone morphogenetic proteins (BMPs) are main regulators of brown adipogenesis. Indeed, exposure of brown preadipocytes to BMP7 induces a full program of brown fat differentiation, including induction of PRDM16, PGC1α, PPARγ, C/EBPs, and UCP1 genes (87).
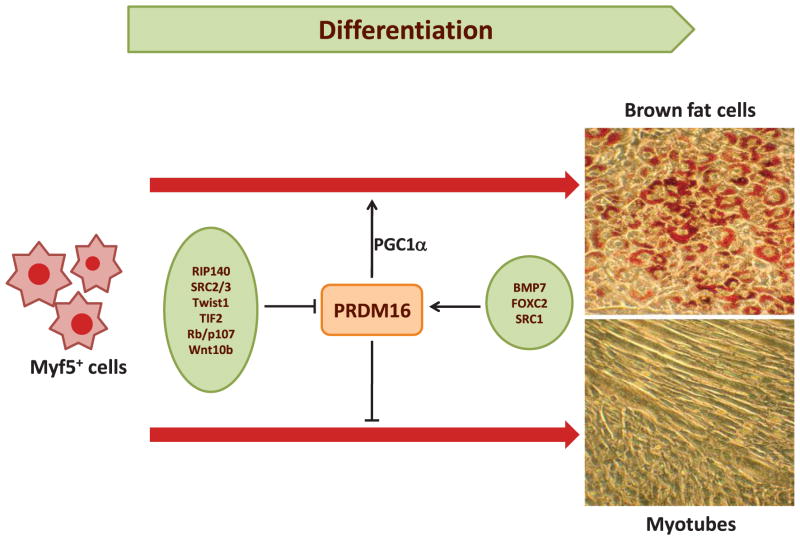
Figure 4.
Regulation of brown adipogenesis. Myf5+ cells are early brown fat or muscle cell precursors. Activation of PRDM16 in combination with other proteins such as PGC1α suppresses myocyte differentiation and promotes brown fat cell phenotype. Proteins on the left and right have a negative and positive action on PRDM16 activity, respectively.
POSITRON EMISSION TOMOGRAPHY AS A TOOL TO MEASURE BROWN ADIPOSE TISSUE.
Positron emission tomography is a functional imaging technique using specific ligands labeled with positron-emitting isotopes for the monitoring of in vivo molecular processes. The use of a wide range of radioactive elements (such as 13N, 11C, 15O, and 18F) provides a strong basis for molecular imaging using PET. However, the ligands used by PET originate from pharmacological agents that demonstrate specific biochemical interactions. The most widely used PET radioisotope to date is the 18F-labeled glucose analogue known as 2-fluoro-2-deoxy-D-glucose (FDG). FDG allows the monitoring of molecular glucose metabolism since it is taken up and phosphorylated in cells in proportion to the rate of glycolysis (92).
The limitation of FDG PET for BAT activity is that glucose accounts for only a small fraction of the total energy supply to BAT. Differences in BAT glucose uptake and plasma FFA concentration may modify the fuel mix oxidized. As an example, a condition in which GLUT-1-dependent glucose uptake is decreased and plasma FFA concentration is increased will underscore the presence of BAT because less FDG activity will be detected. Therefore, FDG activity represents only a semi-quantitative measure of BAT activity. Whether the presence of excess white adipose tissue impairs the detection of brown adipocytes in obese individuals remains to be established.
At the transcriptional level, several proteins enhance or inhibit brown fat development. PPARγ (8, 58, 62, 70, 84) and the C/EBP family (36) are classical transcription factors promoting both white and brown adipogenesis, which in combination with proteins such as PGC1α and PRDM16 have a critical role on determining brown adipogenesis. Table 1 shows different genetically engineered mouse models having an impact on BAT development.
Table 1.
Animal models targeting brown adipose tissue–related proteins
| Reference | Model (KO/TG) | Changes in brown adipose tissue | Whole-body metabolic phenotype |
|---|---|---|---|
| (12) | TG FoxC2 in fat cells | Higher interscapular BAT and increased expression of C/EBPα, PPARγ, SREBP1, and metabolic rate in TG WAT | Lower HFD-induced weight gain and fat accumulation and improved glucose control |
| (63) | SRC1 KO | Higher lipid infiltration and lower expression of UCP1 and PGC1α | Higher HFD-induced weight gain, reduced metabolic rate, and body temperature at 4°C |
| (63) | TIF2 KO | Lower lipid infiltration and higher expression of UCP1 and PGC1α | Lower HFD-induced weight gain and fat accumulation and improved glucose control. Higher metabolic rate and body temperature at 4°C |
| (14) | SRC3 KO | Lower lipid infiltration and increased mitochondrial number | Lower HFD-induced weight gain and fat accumulation and improved glucose control. Higher metabolic rate, body temperature, and muscle mitochondrial content |
| (73) | p107 KO | White fat cells with multilocular lipid droplets and high UCP1 and PGC1α levels | Lower WAT mass and increased metabolic rate |
| (87) | BMP7 KO | Lower BAT mass at birth | Mice are not viable after birth |
| (87) | BMP7 TG | Increase in brown but not white fat mass | Higher metabolic rate and body temperature and lower weight gain |
| (44) | RIP140 KO | Higher expression of UCP1 and CPT1b | Lower HFD-induced weight gain and liver fat accumulation. Higher metabolic rate |
| (35) | UCP1-Wnt10b TG | Lack of functional BAT with lower expression of PGC1α and UCP1 | Blunted increase in body temperature after β-agonist stimulation |
| (61) | Adipose tissue TG twist-1 | Lower expression of PGC1α and UCP1. Lower metabolic rate and mitochondrial density. Higher lipid infiltration | Higher HFD-induced weight gain and lower body temperature at night |
| (61) | Twist-1+/− | Lower lipid infiltration. Higher mitochondrial number and metabolic rate | Lower HFD-induced weight gain and higher body temperature at night |
Abbreviations: BAT, brown adipose tissue; HFD, high-fat diet; KO, knockout mice; TG, transgenic mice; WAT, white adipose tissue.
A master gene involved in BAT development is PGC1α, which plays a critical role in enhancing mitochondrial biogenesis and oxidative metabolic pathways (99). Ectopic expression of PGC1α both in human and mouse white fat cells induces a number of mitochondrial and thermogenic genes including UCP1 (65, 83), whereas genetic ablation of PGC1α in mice reduces cold-induced thermogenesis (CIT) capacity (46). PGC1α activity is regulated through several molecules including RIP140, which binds to PGC1α and antagonizes its transcriptional function of several target gene promoters (27). Genetic ablation of RIP140 causes the emergence of brown fat-like cells in white adipose tissue in mice (44).
The SRC family of proteins also has a regulatory role in PGC1α activity (50). For instance, SRC1 reinforces the coactivation of PGC1α on PPARγ transcriptional activity (63), whereas SRC2 and SRC3 inhibit PGC1α activity. Specifically, SRC2 inhibits the PPARγ-PGC1α interaction (63), while SRC3 promotes PGC1α acetylation (14, 49). Additional inhibitory action on PGC1α activity is accounted for by twist proteins. Twist1 knockout mice showed increased brown fat–related gene expression (94), whereas overexpression represses those genes in a PGC1α-dependent fashion (61).
A recently discovered, critical transcription factor playing a role in brown fat cell differentiation is PRDM16 (Figure 4). This is a potent coactivator of the transcriptional activity of PGC1α and β as well as PPARα and γ (75). PRDM16 directly binds to PGC-1α, PGC-1β, PPARα, PPARγ, p53, and several members of the C/EBP family, resulting in enhanced coactivation of their transcriptional activities (34, 74, 75). The PRDM16-C/EBPβ complex is particularly relevant and appears to control the initiating events of the conversion from myoblastic precursors to brown fat cells. Depletion of C/EBPβ significantly blunts the ability of PRDM16 to induce brown fat differentiation in mice (33). PRDM16 is also a suppressor of specific genes present in white fat (resistin and angiotensinogen) and muscle (myoD, myogenin, and myosin heavy chain) cells. Thus, when PRDM16 is expressed in mouse white fat preadipocytes or white adipocytes, brown fat differentiation is induced, including activation of thermogenic and mitochondrial genes (34, 74, 75). In contrast, depletion of PRDM16 from cultured brown fat cells causes a loss of the brown fat characteristics in parallel with the development of skeletal muscle differentiation features (75). Consistently, BAT from PRDM16−/− mice at embryonic day 17 exhibits decreased expression of thermogenic genes and increased expression of muscle-specific genes (74).
PHYSIOLOGICAL ROLE OF BROWN ADIPOSE TISSUE
In newborns, the sudden change from an intrauterine environment at 37°C to the external environment at lower temperature in combination with a high surface area-to-volume ratio (~twofold higher than in adults) represent an important challenge for thermoregulation (76). As an adaptive mechanism, the appearance of BAT around 150 million years ago allowed mammals to maintain body temperature significantly higher than ambient temperature (18). As mentioned above, the increased capacity to release heat is due to the UCP1. This protein, located in the inner mitochondrial membrane, allows protons to move down their electrochemical gradient, bypassing adenosine-5′-triphosphate (ATP) synthase and therefore ATP production (52). As a result, brown adipocytes oxidize their own fat stores and circulating substrates at a fast rate, thus releasing heat (Figure 5).
Figure 5.
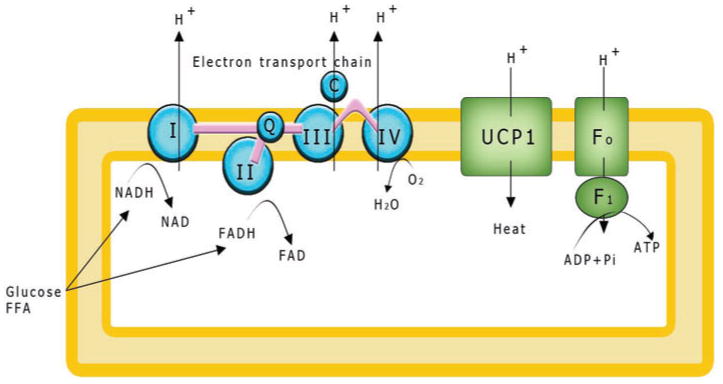
The role of uncoupling protein 1 (UCP1) on mitochondrial heat production. Free fatty acids and glucose are oxidized to generate nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH)2, which donate electrons to the electron transport chain. At the end of the transport chain, these electrons are accepted by molecular oxygen (O2). A proton electrochemical potential gradient is created when protons a repumped out. Adenosine-5′-triphosphate (ATP) is generated when protons reenter the mitochondrial matrix through the F0/F1-ATPase. Protons may also reenter through an UCP1, with energy being released in the form of heat.
The BAT thermogenic process is mostly induced by noradrenaline. In response to cold [ambient temperature ≤23°C for humans, ≤28°C for rats, and ≤30°C for mice (76)], noradrenaline is secreted by the BAT sympathetic nerve terminals, leading to increased UCP1 expression with concomitant thermogenesis (37). In fact, BAT can be activated and expanded by treatment with β-adrenergic agonists in animals (69) or by cathecolamine-secreting tumors in humans (41). In contrast, when BAT is not adrenergically activated, it loses most of its histological and molecular properties (e.g., multilocular lipid droplets and high UCP1 expression), becoming like a white adipocyte. Interestingly, both cold exposure and cathecolamine stimulation cause the emergence of UCP1-expressing brown fat cells in rat white adipose tissue depots (24). The role of these cells in thermoregulation remains elusive.
Thyroid hormones, particularly triiodothyronine (T3), also have well known thermogenic properties (51, 76). T3 availability in brown fat cells is regulated by DIO2, which is highly expressed in BAT (91). For example, brown fat cells from DIO2−/− mice have impaired cathecolamine-stimulated UCP1 expression accompanied by impaired cold tolerance (17). In addition, T3 action on BAT activity may not be solely restricted to a direct effect at the tissue level. Recently, Lopez et al. (48) showed in rats that direct injection of T3 into the brain results in activation of sympathetic activity and increased expression of BAT-related markers in parallel with weight loss.
Besides the role of BAT on core temperature regulation, this tissue has also been proposed to play a role in the regulation of body weight (71). For instance, rodents fed a “cafeteria diet” have an expansion and activation of BAT (71). This thermogenic effect in response to hypercaloric diets was proposed to be a defense mechanism against excess energy supply by dissipating part of the energy excess and thus reducing body weight gain and its comorbidities (12, 38, 39, 88, 100). In contrast, genetic ablation of BAT or β-adrenergic receptors causes a propensity toward obesity and metabolic diseases (7, 53), mostly because of hyperphagia. Similarly, deletion of UCP1 in mice causes increased weight gain when mice are housed at thermoneutrality (20) but not at lower temperature (47).
The hypothesized role of BAT on body weight regulation, however, has been strongly refuted. Kozak (40) doubts that BAT evolved to burn off excess calories in mammals—including premodern humans—that rarely had a plethora of food available. In support of this view, increased oxygen consumption in rats fed cafeteria diets was not accounted for by increased BAT oxygen consumption (54). In fact, in response to such a diet, oxygen consumption increased at a comparable rate in UCP1+/+ and UCP1−/− mice, indicating that diet-induced thermogenesis (DIT) may be independent of UCP1 expression (4).
In humans, despite easier measures of oxygen consumption, the evidence of the role of BAT in the regulation of body weight is no less controversial. As early as 1902, Neumann realized that the increase in his body weight was not proportional to the excess energy intake (59). This observation led him to propose that some of the excess energy intake was dissipated as heat or “luxuskonsumption” (59). More than half a century later, Miller et al. (56) revived Neumann’s concept by conducting human overfeeding studies in which they described that only part of the body weight gain was related to increased energy intake, thus implying that some of the excess was dissipated as increased energy expenditure. Such an idea was supported by the findings from the Vermont studies of prisoners in whom almost 50% more energy intake was necessary to maintain their new body weights after long-term overfeeding (77). It is, however, only in the late nineties that Stock (79) confirmed these findings in a careful review of the literature showing that weight gain after overfeeding was quite variable across individuals, up to four times per unit of energy excess. Besides the level dietary compliance, such differences in weight gain can be explained by our inability to assess weight-maintenance energy requirements and therefore the actual energy excess. Differences in digestion and absorption may also modify the amount of bioavailable energy, affecting the actual positive energy balance. The composition of weight gain (fat mass and lean mass) also needs to be considered because the energy cost of protein deposition is higher than that of adipose tissue. Stock also identified that part of the variability in weight gain was related to the dietary composition, particularly to dietary protein content (79). Finally, differences in mitochondrial energy efficiency may also represent an underlying cause of the variability in weight gain. All these confounding factors decrease our chances to isolate any potential role of BAT mass/activity on adaptive thermogenesis and body weight regulation until more reliable methods to assess energy homeostasis become available.
Despite the above caveats in both animal and human studies, there is some evidence that DIT may also be mediated by BAT, mainly in response to adrenergic stimulation, but to a lower extent than CIT (reviewed in 11). This is also indirectly suggested by recent data showing a significant relationship between the magnitude of the increase in energy expenditure in response to cold (CIT) and to overfeeding (DIT) (98).
PRESENCE OF BROWN ADIPOSE TISSUE IN HUMANS
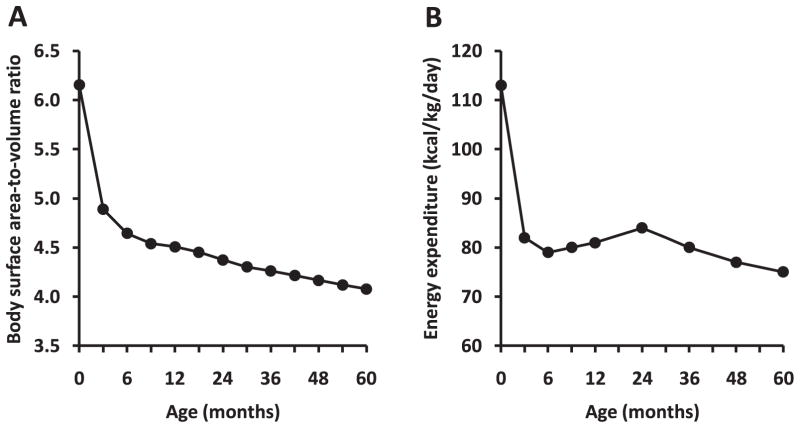
Newborns are particularly dependent on BAT to keep their body temperature within a homeothermic range. In humans, the need for active brown adipocytes decreases after a few months of life, as the surface area-to-volume ratio is reduced in parallel with a reduction in metabolic rate on a body-size basis (Figure 6). For instance, at age 5 months, the surface area-to-volume ratio has decreased by one-third, while in adulthood this ratio goes down to one-half to two-thirds in comparison with newborns. The decreased need to rely on BAT thermogenesis in adults may explain why it has been so difficult to identify BAT in adult humans for a long time. However, the detection by PET of “hot spots” in the neck, roots of the upper limbs, and the intercostal spaces near the spine from adult humans reopened a scientific interest for the search of BAT (2, 28, 81, 95, 101).
Figure 6.
Change in body surface area-to-volume ratio and metabolic rate during the first five years of life. Body surface area estimated by Dubois’s equation [weight (kg)0.425 × height (cm)0.725/1000]. Body volume was estimated from body weight assuming a body density equal to one. Body weight, height, and energy expenditure measurements were taken from average values reported in children having normal growth (1).
Three independent publications reported in a comprehensive manner the presence of BAT in adult humans (16, 89, 91). Virtanen et al. (91) studied five subjects during cold exposure (2 h at 17–19°C; one of the subject’s feet was placed intermittently in ice water 5–9°C for five minutes) and warm conditions using 2-fluoro-2-deoxy-D-glucose (FDG) in combination with PET/CT technologies. In response to cold, a 15-fold increase in FDG uptake in the supraclavicular area was observed. From three of the volunteers, white adipose tissue and BAT biopsies were taken to measure the expression of informative genes for cellular origin. Increased expression of UCP1, DIO2, PGC1α, PRDM16, β3 adrenergic receptor, and cytochrome c were observed in BAT versus white adipose tissue from surrounding areas (Figure 3).
Van Marken Lichtenbelt et al. (89) used a similar approach in a larger group of subjects (10 lean and 14 overweight or obese volunteers) and found increased FDG uptake potentially attributable to BAT activity in the neck, supraclavicular region, chest, and abdomen. It is noteworthy that the activity of BAT was approximately fourfold higher in the lean group than in the overweight/obese group. However, it will be important to investigate whether the presence of excess white adipose tissue may impair the detection of brown adipocytes in obese individuals before definitively concluding that obese people have lower BAT. Furthermore, the same investigators reported a positive relationship between resting metabolic rate and BAT activity at thermo-neutrality or during cold exposure, while an inverse association between BAT amount/activity and body mass index was found. Similarly, an inverse association between cold-stimulated FDG uptake and body mass index was reported by Saito et al. (72) in healthy volunteers ages 23–65 years. The latter study also pointed out a seasonal variation, with increased cold-activated FDG uptake during winter versus summer, which was later confirmed by others (6). BAT activity was surprisingly more prevalent in women than in men (7% versus 3%) in retrospective readings of FDG PET/CT in approximately 2,000 subjects (16). This sexual dimorphism was recently reported by others under more controlled conditions (42).
Using a histological approach to detect brown fat adipocytes, Zingaretti et al. (103) collected some adipose tissue in the necks of patients undergoing surgery for thyroid diseases. In one-third of the patients, clear evidence of BAT was found. Again, BAT amount was inversely related to age and obesity. At the molecular level, the proportion of UCP1-positive fat cells among all adipocytes in these individuals ranged from 3% to 31% (average 13%). Together, the data show that BAT is indeed present in variable amounts in human adults.
INCREASING BROWN ADIPOSE TISSUE MASS OR ACTIVITY AS A PUTATIVE ALTERNATIVE TO CONTROL BODY WEIGHT
The finding that body mass index and BAT activity are inversely related has encouraged the assessment of the role of BAT on whole-body energy homeostasis, particularly in response to overfeeding. Whether obesity is partially driven by impaired BAT activity as suggested by mouse models (Table 1) or whether decreased BAT activity in obese individuals is due to lower surface area-to-volume ratio and enhanced thermal insulation is unknown (97).
Regarding the effect of BAT on energy expenditure, Virtanen et al. (91) estimated that the size of the supraclavicular BAT depot (both sides included) was 63 g, and the rate of glucose disposal was 2.2 mg/100 g/min, which is equivalent to 1.4 mg/min for the entire depot or approximately 2 g for a day (~8 kcal). Considering that only 10% of the fuel oxidation is derived from glucose in rat brown adipose (53b) and assuming a similar contribution in humans, one could anticipate a total 80 kcal/d for fully activated BAT. Even if such a small increase in thermogenesis could offset a slightly positive energy balance, it has been clearly established that 80 kcal/d is not sufficient to cause obesity unless the positive energy balance is maintained over time (10, 30, 80, 96). An increased and active mass of BAT could, however, help to maintain body weight, if not contribute to weight loss. This can be particularly relevant in individuals losing weight because they often experience a “metabolic adaptation,” i.e., a fall in energy expenditure larger than what can be accounted for by the loss of fat-free mass and fat mass (23, 43, 68).
The notion that body weight could be modified by manipulating energy efficiency is not new. In the 1930s, 2,4-dinitrophenol (a mitochondrial uncoupler) became a popular strategy to lose weight by increasing metabolic rate (up 20% to 30%). However, ingestion of slightly higher doses than prescribed caused serious side effects, sometimes including death (29). The role of energy efficiency was reconsidered when the existence of UCP1 was first described in human BAT (9) and again later, when uncoupling protein 2, a more ubiquitous protein, was proposed to play a role on energy homeostasis (22). However, a lack of differences in metabolic rate between obese and lean individuals decreased the enthusiasm for a potential use of UCPs for boosting energy expenditure (64).
At present, the better understanding of BAT development and regulation has opened a new research avenue that is geared toward finding new targets to increase BAT mass and activity. For example, PPARγ agonists increase the expression of brown fat–selective proteins (e.g., PGC1α, UCP1) both in fat cell lines and in white adipose tissue from mice (62, 90). Consistently, PPARγ+/− mice have a reduced metabolic rate, and their adipose tissue shows an impaired capacity to generate ATP (3). One can therefore hypothesize that PPARγ agonists may increase metabolic rate by stimulating BAT. However, people with type 2 diabetes treated with pioglitazone (a PPARγ agonist; 45 mg/d for 24 weeks) showed no changes in metabolic rate and gained weight (78).
Other strategies to enhance BAT activity may include increasing the activity of BMP7 since mesenchymal progenitor cells treated with BMP7 develop a brown adipocyte phenotype. When such cells were implanted into nude mice, adipose tissue showed a dramatic increase in the amount of brown fat cells (87). Similarly, PRDM16 was shown to induce brown adipocyte differentiation in a fibroblast cell line (75), primary mouse (74), and human myoblasts (34). These findings were accompanied by increased glucose uptake measured by PET scanning and enhanced in vitro respiration (33). Further studies should assess the potential of PRDM16 to increase BAT activity at the whole-body level. Another potential target to increase metabolic rate is to suppress the activity of cell death–inducing DNA fragmentation factor-α-like effector A (CIDEA). This protein increases mitochondrial coupling by suppressing UCP1 expression, which is consistent with the observation that CIDEA−/− mice are resistant to diet-induced obesity (102). In addition, a recent report in humans found an inverse association between adipose tissue CIDEA expression and metabolic rate (26). Sirtuins (NAD-dependent deacetylases) have also been proposed to activate BAT activity via PGC1α (21). After mice were treated with synthetic activators of sirtuin 1, increased PGC1α and enhanced oxidative capacity were observed in skeletal muscle, liver, and BAT, all associated with higher metabolic rate and reduced weight gain (19, 32).
Finally, it should be considered that increased thermogenesis might still be insufficient to decrease body mass because energy homeostasis is tightly regulated, and compensatory increases in energy intake are likely to happen. Such up-regulation of energy intake would only lead to a new higher energy flux, probably at higher body mass.
CONCLUSIONS
Brown adipose tissue plays a critical role for heat production in mammals, especially in newborns. However, its role in the regulation of body weight remains more controversial, if not unknown. The recent discovery of BAT in adult humans has caused a resurgence of interest in this potential therapeutic target because reports have suggested it may represent a novel strategy in the treatment of obesity. Before conclusions are reached, it will be necessary to investigate:
How much variance of the metabolic rate is accounted for by BAT mass/activity in adult humans?
What are the behavioral or pharmacological means to increase the amount and activity of human BAT?
Is increased BAT mass and activity related to enhanced CIT and/or increased DIT?
Does increased energy expenditure (independent of changes in physical activity) represent a way to control body weight?
Only when such questions are answered will it be possible to determine whether strategies to increase the mass and/or activity of BAT can be used to improve the control of body weight.
SUMMARY POINTS.
Increasing energy expenditure independent of changes in physical activity may play a role in body weight regulation.
The recent confirmation of the presence of brown adipose tissue in adult humans and a better understanding of brown adipose tissue development and activity may increase our potential to enhance energy expenditure.
The existence of brown fat cells in white adipose tissue and the reported transdifferentiation from white into brown adipocytes may open a new avenue to boost metabolic rate.
The evidence of lower BAT mass/activity in obese and elderly versus lean and young individuals may offer a potential to increase BAT mass/activity and prevent obesity- and aging-related metabolic disorders.
FUTURE ISSUES.
Future research is needed on:
Improvement in our technical capabilities to determine brown adipose tissue activity.
Diet-induced thermogenesis and its relationship to brown adipose tissue activity.
Identification of cellular/molecular mechanisms explaining differential BAT mass/activity according to sex, age, and fatness.
Acknowledgments
E.R. has support for brown adipose tissue work from an NORC Center Grant #1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK. J.E.G., is supported by The Chilean Commission of Science and Technology (Fondecyt #11090007). We thank Dr. Saverio Cinti (University of Ancona, Italy) and Dr. Wouter van Marken Lichtenbelt (Maastricht University, The Netherlands) for sharing their work on brown adipose tissue. We are grateful to Prof. Les Kozak for his comments on the manuscript.
Glossary
- BAT
brown adipose tissue
- PET
positron emission tomography
- CT
computed tomography
- Core temperature
the defended temperature of the body and its vital organs
- UCP1
uncoupling protein 1
- PGC1α
peroxisome proliferator–activated receptor γ coactivator 1α
- DIO2
deiodinase iodothyronine type 2
- PRDM16
PR domain containing 16
- Brite cells
brown fat–like white fat cells
- Transdifferentiation
process by which white fat cells can turn into brown fat cells in response to hormonal signals
- Cold-induced thermogenesis (CIT)
the increase in energy expenditure above basal fasting level during cold exposure
- Diet-induced thermogenesis (DIT)
the increase in energy expenditure above basal fasting level divided by the energy content of the food ingested
- Energy efficiency
moles of mitochondrial ATP generated relative to moles of oxygen consumed
- Adaptive thermogenesis
changes in energy expenditure not attributable to the changes in the size of the body and its tissue composition in response to alterations in energy balance. Those alterations may be caused by excess caloric intake, caloric restriction, increase or decrease in physical activity levels, cold or heat exposure, drugs, and other environmental agents
- FDG
2-fluoro-2-deoxy-D-glucose
Footnotes
Errata
An online log of corrections to Annual Review of Nutrition articles may be found at http://nutr.annualreviews.org/errata.shtml
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Human energy requirements. Scientific background papers from the Joint FAO/WHO/UNU Expert Consultation. Oct 17–24, 2001 Rome, Italy. Public Health Nutr. 2005;8:929–1228. doi: 10.1079/phn2005778. [DOI] [PubMed] [Google Scholar]
- 2.Alkhawaldeh K, Alavi A. Quantitative assessment of FDG uptake in brown fat using standardized uptake value and dual-time-point scanning. Clin Nucl Med. 2008;33:663–67. doi: 10.1097/RLU.0b013e318184b3de. [DOI] [PubMed] [Google Scholar]
- 3.Anghel SI, Bedu E, Vivier CD, Descombes P, Desvergne B, Wahli W. Adipose tissue integrity as a prerequisite for systemic energy balance: a critical role for peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:29946–57. doi: 10.1074/jbc.M702490200. [DOI] [PubMed] [Google Scholar]
- 4.Anunciado-Koza R, Ukropec J, Koza RA, Kozak LP. Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. J Biol Chem. 2008;283:27688–97. doi: 10.1074/jbc.M804268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–76. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 6.Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–87. doi: 10.2337/db09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–45. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 8.Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–95. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 9.Bouillaud F, Combes-George M, Ricquier D. Mitochondria of adult human brown adipose tissue contain a 32 000-Mr uncoupling protein. Biosci Rep. 1983;3:775–80. doi: 10.1007/BF01120989. [DOI] [PubMed] [Google Scholar]
- 10.Butte NF, Ellis KJ. Comment on “Obesity and the environment: where do we go from here? Science. 2003;301:598. doi: 10.1126/science.1085985. author reply 598. [DOI] [PubMed] [Google Scholar]
- 11.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 12.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–73. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 13.Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab. 2009;297:E977–86. doi: 10.1152/ajpendo.00183.2009. [DOI] [PubMed] [Google Scholar]
- 14.Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, et al. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1α. Proc Natl Acad Sci USA. 2008;105:17187–92. doi: 10.1073/pnas.0808207105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crisan M, Casteilla L, Lehr L, Carmona M, Paoloni-Giacobino A, et al. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells. 2008;26:2425–33. doi: 10.1634/stemcells.2008-0325. [DOI] [PubMed] [Google Scholar]
- 16.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, et al. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest. 2001;108:1379–85. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enerback S. Human brown adipose tissue. Cell Metab. 2010;11:248–52. doi: 10.1016/j.cmet.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–58. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–9. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–91. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–72. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 23.Galgani JE, Greenway FL, Caglayan S, Wong ML, Licinio J, Ravussin E. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J Clin Endocrinol Metab. 2010;95:851–55. doi: 10.1210/jc.2009-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–75. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- 25.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–16. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 26.Gummesson A, Jernas M, Svensson PA, Larsson I, Glad CA, et al. Relations of adipose tissue CIDEA gene expression to basal metabolic rate, energy restriction, and obesity: population-based and dietary intervention studies. J Clin Endocrinol Metab. 2007;92:4759–65. doi: 10.1210/jc.2007-1136. [DOI] [PubMed] [Google Scholar]
- 27.Hallberg M, Morganstein DL, Kiskinis E, Shah K, Kralli A, et al. A functional interaction between RIP140 and PGC-1alpha regulates the expression of the lipid droplet protein CIDEA. Mol Cell Biol. 2008;28:6785–95. doi: 10.1128/MCB.00504-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–98. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 29.Harper ME, Green K, Brand MD. The efficiency of cellular energy transduction and its implications for obesity. Annu Rev Nutr. 2008;28:13–33. doi: 10.1146/annurev.nutr.28.061807.155357. [DOI] [PubMed] [Google Scholar]
- 30.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–61. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 31.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–81. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 32.Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp Neurol. 2010;225:74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–58. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008;22:1397–409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang S, Bajnok L, Longo KA, Petersen RK, Hansen JB, et al. Effects of Wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1alpha. Mol Cell Biol. 2005;25:1272–82. doi: 10.1128/MCB.25.4.1272-1282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karamanlidis G, Karamitri A, Docherty K, Hazlerigg DG, Lomax MA. C/EBPbeta reprograms white 3T3-L1 preadipocytes to a brown adipocyte pattern of gene expression. J Biol Chem. 2007;282:24660–69. doi: 10.1074/jbc.M703101200. [DOI] [PubMed] [Google Scholar]
- 37.Klingenspor M. Cold-induced recruitment of brown adipose tissue thermogenesis. Exp Physiol. 2003;88:141–48. doi: 10.1113/eph8802508. [DOI] [PubMed] [Google Scholar]
- 38.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–23. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopecky J, Hodny Z, Rossmeisl M, Syrovy I, Kozak LP. Reduction of dietary obesity in aP2-Ucp transgenic mice: physiology and adipose tissue distribution. Am J Physiol. 1996;270:E768–75. doi: 10.1152/ajpendo.1996.270.5.E768. [DOI] [PubMed] [Google Scholar]
- 40.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–67. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue in patients with phaeochromocytoma. Int J Obes. 1986;10:219–27. [PubMed] [Google Scholar]
- 42.Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–6. doi: 10.1152/ajpendo.00298.2010. [DOI] [PubMed] [Google Scholar]
- 43.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–28. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 44.Leonardsson G, Steel JH, Christian M, Pocock V, Milligan S, et al. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci USA. 2004;101:8437–42. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lidell ME, Enerback S. Brown adipose tissue-a new role in humans? Nat Rev Endocrinol. 2010;6:319–25. doi: 10.1038/nrendo.2010.64. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–35. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–8. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Louet JF, Coste A, Amazit L, Tannour-Louet M, Wu RC, et al. Oncogenic steroid receptor coactivator-3 is a key regulator of the white adipogenic program. Proc Natl Acad Sci USA. 2006;103:17868–73. doi: 10.1073/pnas.0608711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Louet JF, O’Malley BW. Coregulators in adipogenesis: What could we learn from the SRC (p160) coactivator family? Cell Cycle. 2007;6:2448–52. doi: 10.4161/cc.6.20.4777. [DOI] [PubMed] [Google Scholar]
- 51.Lovejoy JC, Smith SR, Bray GA, DeLany JP, Rood JC, et al. A paradigm of experimentally induced mild hyperthyroidism: effects on nitrogen balance, body composition, and energy expenditure in healthy young men. J Clin Endocrinol Metab. 1997;82:765–70. doi: 10.1210/jcem.82.3.3827. [DOI] [PubMed] [Google Scholar]
- 52.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–60. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 53.Lowell BB, Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–42. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 53b.Ma SW, Foster DO. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol. 1986;64:609–14. doi: 10.1139/y86-101. [DOI] [PubMed] [Google Scholar]
- 54.Ma SW, Foster DO, Nadeau BE, Triandafillou J. Absence of increased oxygen consumption in brown adipose tissue of rats exhibiting “cafeteria” diet-induced thermogenesis. Can J Physiol Pharmacol. 1988;66:1347–54. doi: 10.1139/y88-221. [DOI] [PubMed] [Google Scholar]
- 55.Mattson MP. Perspective: Does brown fat protect against diseases of aging? Ageing Res Rev. 2010;9:69–76. doi: 10.1016/j.arr.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller DS, Mumford P, Stock MJ. Gluttony. 2 Thermogenesis in overeating man. Am J Clin Nutr. 1967;20:1223–29. doi: 10.1093/ajcn/20.11.1223. [DOI] [PubMed] [Google Scholar]
- 57.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–72. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Nedergaard J, Petrovic N, Lindgren EM, Jacobsson A, Cannon B. PPARgamma in the control of brown adipocyte differentiation. Biochim Biophys Acta. 2005;1740:293–304. doi: 10.1016/j.bbadis.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Neumann R. Experimentelle Beitrage zur lehre von dem taglichen Nahrungsbedarf des Menschen unter besonderer Berucksichtigung der notwendigen Eiweissmenge. Arch Hyg. 1902;45:1–87. [Google Scholar]
- 60.Nishida C, Uauy R, Kumanyika S, Shetty P. The Joint WHO/FAO Expert Consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 2004;7:245–50. doi: 10.1079/phn2003592. [DOI] [PubMed] [Google Scholar]
- 61.Pan D, Fujimoto M, Lopes A, Wang YX. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Picard F, Gehin M, Annicotte J, Rocchi S, Champy MF, et al. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–41. doi: 10.1016/s0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 64.Prentice A, Jebb S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr Rev. 2004;62:S98–104. doi: 10.1111/j.1753-4887.2004.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 65.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 66.Purnak T, Ozaslan E, Efe C, Sevimler H. A missing link in the puzzle: brown adipose tissue. Hepatology. 2010;51:1470–71. doi: 10.1002/hep.23559. author reply 1471–72. [DOI] [PubMed] [Google Scholar]
- 67.Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes Rev. 2009;10:265–68. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, et al. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robidoux J, Martin TL, Collins S. Beta-adrenergic receptors and regulation of energy expenditure: a family affair. Annu Rev Pharmacol Toxicol. 2004;44:297–323. doi: 10.1146/annurev.pharmtox.44.101802.121659. [DOI] [PubMed] [Google Scholar]
- 70.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–17. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 71.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 72.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, et al. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005;2:283–95. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Seale P, Bjork B, Yang W, Kajimura S, Chin S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–67. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–64. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 77.Sims EA, Danforth E, Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–96. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- 78.Smith SR, De Jonge L, Volaufova J, Li Y, Xie H, Bray GA. Effect of pioglitazone on body composition and energy expenditure: a randomized controlled trial. Metabolism. 2005;54:24–32. doi: 10.1016/j.metabol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 79.Stock MJ. Gluttony and thermogenesis revisited. Int J Obes Relat Metab Disord. 1999;23:1105–17. doi: 10.1038/sj.ijo.0801108. [DOI] [PubMed] [Google Scholar]
- 80.Swinburn BA, Jolley D, Kremer PJ, Salbe AD, Ravussin E. Estimating the effects of energy imbalance on changes in body weight in children. Am J Clin Nutr. 2006;83:859–63. doi: 10.1093/ajcn/83.4.859. [DOI] [PubMed] [Google Scholar]
- 81.Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med. 2004;45:1189–93. [PubMed] [Google Scholar]
- 82.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–6. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, et al. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem. 2003;278:33370–76. doi: 10.1074/jbc.M305235200. [DOI] [PubMed] [Google Scholar]
- 84.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–56. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 85.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–82. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–4. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–32. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 89.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 90.Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, et al. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–28. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 92.Visvikis D, Cheze-Le Rest C, Jarritt P. PET technology: current trends and future developments. Br J Radiol. 2004;77:906–10. doi: 10.1259/bjr/32045866. [DOI] [PubMed] [Google Scholar]
- 93.Walden TB, Timmons JA, Keller P, Nedergaard J, Cannon B. Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J Cell Physiol. 2009;218:444–49. doi: 10.1002/jcp.21621. [DOI] [PubMed] [Google Scholar]
- 94.Wallberg-Henriksson H, Zierath JR. A new twist on brown fat metabolism. Cell. 2009;137:22–24. doi: 10.1016/j.cell.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 95.Weber WA. Brown adipose tissue and nuclear medicine imaging. J Nucl Med. 2004;45:1101–3. [PubMed] [Google Scholar]
- 96.Weinsier RL, Bracco D, Schutz Y. Predicted effects of small decreases in energy expenditure on weight gain in adult women. Int J Obes Relat Metab Disord. 1993;17:693–700. [PubMed] [Google Scholar]
- 97.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Cold-induced adaptive thermogenesis in lean and obese. Obesity (Silver Spring) 2010;18:1092–99. doi: 10.1038/oby.2010.74. [DOI] [PubMed] [Google Scholar]
- 98.Wijers SL, Saris WH, van Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab. 2007;92:4299–305. doi: 10.1210/jc.2007-1065. [DOI] [PubMed] [Google Scholar]
- 99.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 100.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med. 2003;44:1789–96. [PubMed] [Google Scholar]
- 102.Zhou Z, Yon Toh S, Chen Z, Guo K, Ng CP, et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 103.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–20. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]