Abstract
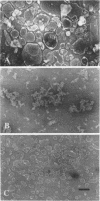
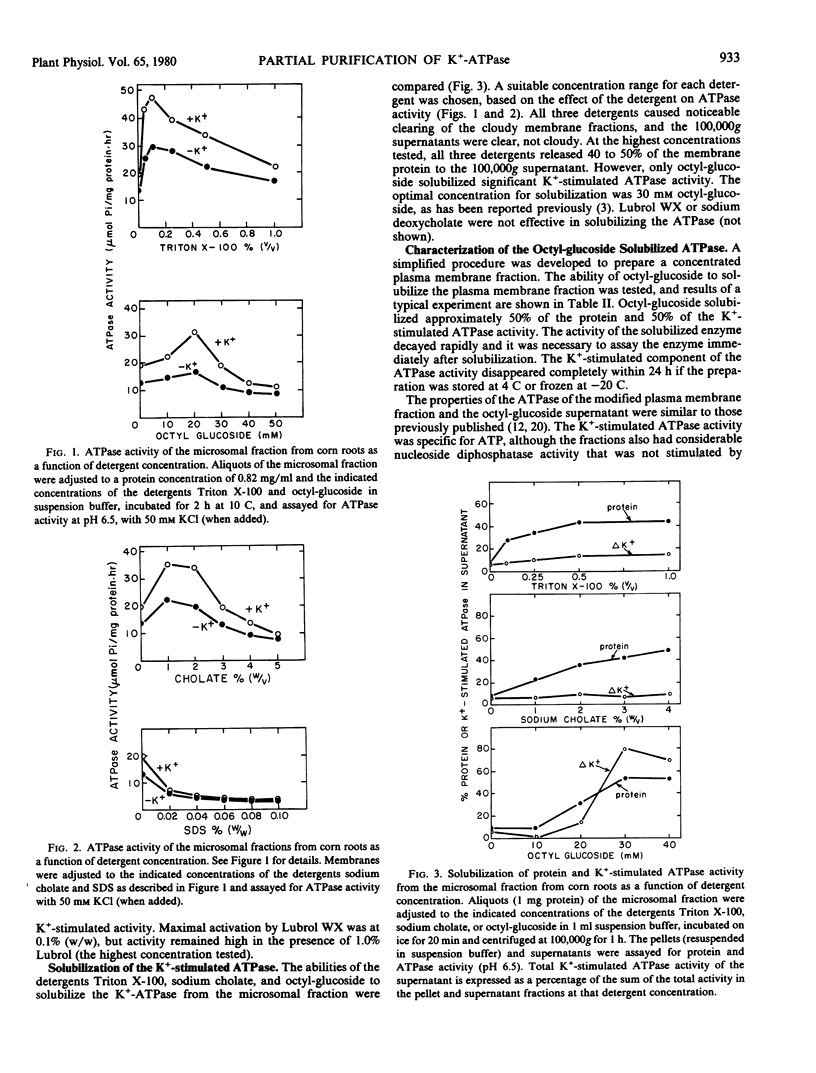
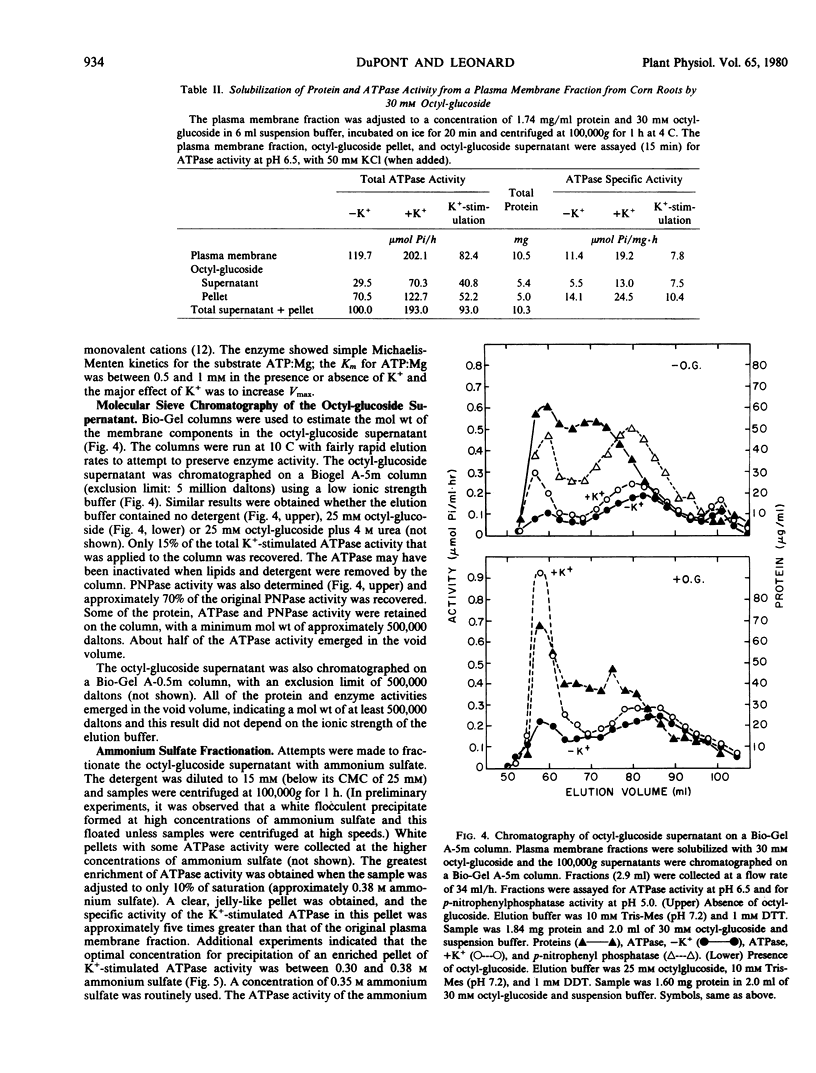
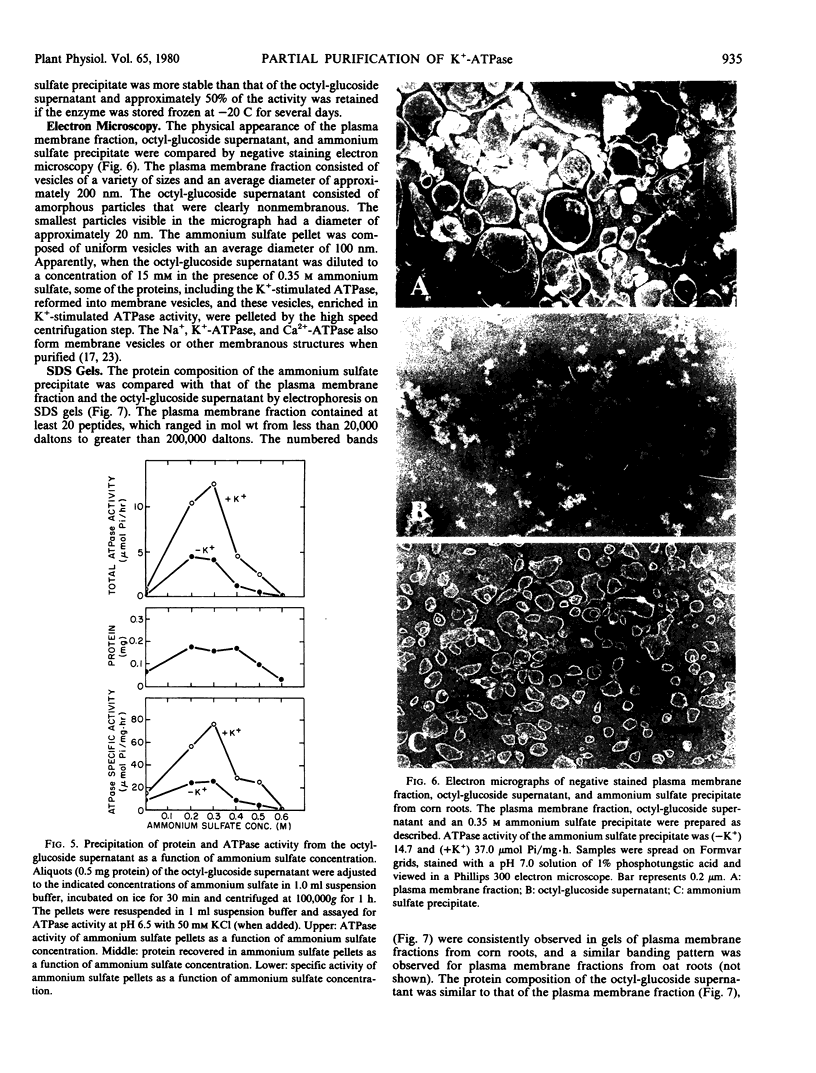
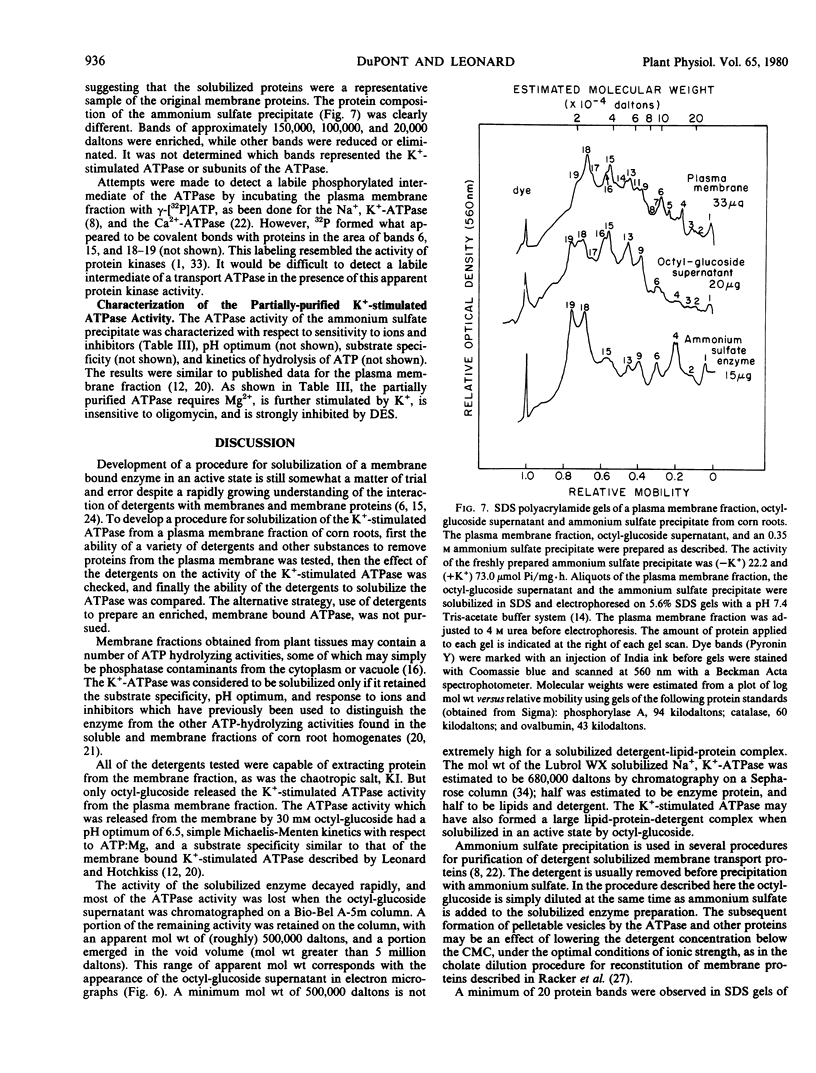
The K+-stimulated ATPase was partially purified from a plasma membrane fraction from corn roots (WF9 × Mo 17) by solubilization with 30 millimolar octyl-β-d-glucopyranoside followed by precipitation with dilute ammonium sulfate. The specific activity of the enzyme was increased about five times by this procedure. The molecular weight of the detergent-extracted ATPase complex was estimated to be at least 500,000 daltons by chromatography on a Bio-Gel A-5m column. Negative staining electron microscopy indicated that the detergent-extracted material consisted of amorphous particles, while the ammonium sulfate precipitate was composed of uniform vesicles with an average diameter of 100 nanometers. The protein composition of the ammonium sulfate precipitate was significantly different from that of the plasma membrane fraction when compared by sodium dodecyl sulfate gel electrophoresis. The characteristics of the partially purified ATPase resembled those of the plasma membrane associated enzyme. The ATPase required Mg2+, was further stimulated by K+, was almost completely inhibited by 0.1 millimolar diethylstilbestrol, and was not affected by 5.0 micrograms per milliliter oligomycin. Although the detergents sodium cholate, deoxycholate, Triton X-100 and Lubrol WX also solubilized some membrane protein, none solubilized the K+-stimulated ATPase activity. Low concentrations of each detergent, including octyl-β-d-glucopyranoside, activated the ATPase and higher concentrations inactivated the enzyme. These results suggest that the plasma membrane ATPase is a large, integral membrane protein or protein complex that requires lipids to maintain its activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Fairbanks G. Phosphorylation of endogenous substrates by erythrocyte membrane protein kinases. I. A monovalent cation-stimulated reaction. Biochemistry. 1974 Dec 31;13(27):5507–5514. doi: 10.1021/bi00724a009. [DOI] [PubMed] [Google Scholar]
- Balke N. E., Hodges T. K. Comparison of Reductions in Adenosine Triphosphate Content, Plasma Membrane-associated Adenosine Triphosphatase Activity, and Potassium Absorption in Oat Roots by Diethylstilbestrol. Plant Physiol. 1979 Jan;63(1):53–56. doi: 10.1104/pp.63.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Thompson T. E. Solubilization of bacterial membrane proteins using alkyl glucosides and dioctanoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Mar 25;382(3):276–285. doi: 10.1016/0005-2736(75)90270-9. [DOI] [PubMed] [Google Scholar]
- Benson M. J., Tipton C. L. Purification and Characterization of a Cation-stimulated Adenosine Triphosphatase from Corn Roots. Plant Physiol. 1978 Aug;62(2):165–172. doi: 10.1104/pp.62.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross J. W., Briggs W. R. Auxin receptors of maize coleoptile membranes do not have ATPase activity. Plant Physiol. 1978 Apr;61(4):581–584. doi: 10.1104/pp.61.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- Delhez J., Dufour J. P., Thines D., Goffeau A. Comparison of the properties of plasma membrane-bound and mitochondria-bound ATPases in the yeast Schizosaccharmoyces pombe. Eur J Biochem. 1977 Sep 15;79(1):319–328. doi: 10.1111/j.1432-1033.1977.tb11812.x. [DOI] [PubMed] [Google Scholar]
- Dufour J. P., Goffeau A. Solubilization by lysolecithin and purification of the plasma membrane ATPase of the yeast Schizosaccharomyces pombe. J Biol Chem. 1978 Oct 10;253(19):7026–7032. [PubMed] [Google Scholar]
- Dulley J. R. Determination of inorganic phosphate in the presence of detergents or protein. Anal Biochem. 1975 Jul;67(1):91–96. doi: 10.1016/0003-2697(75)90275-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hokin L. E., Dahl J. L., Deupree J. D., Dioxon J. F., Hackney J. F., Perdue J. F. Studies on the characterization of the sodium-potassium transport adenosine triphosphatase. X. Purification of the enzyme from the rectal gland of Squalus acanthias. J Biol Chem. 1973 Apr 10;248(7):2593–2605. [PubMed] [Google Scholar]
- Korenbrot J. I. Ion transport in membranes: incorporation of biological ion-translocating proteins in model membrane systems. Annu Rev Physiol. 1977;39:19–49. doi: 10.1146/annurev.ph.39.030177.000315. [DOI] [PubMed] [Google Scholar]
- Leonard R. T., Hotchkiss C. W. Cation-stimulated Adenosine Triphosphatase Activity and Cation Transport in Corn Roots. Plant Physiol. 1976 Sep;58(3):331–335. doi: 10.1104/pp.58.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H., Holland P. C. Calcium transport in sarcoplasmic reticulum. Annu Rev Biophys Bioeng. 1975;4(00):377–404. doi: 10.1146/annurev.bb.04.060175.002113. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H., Seeman P., Iles G. H., Yip C. C. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1971 Apr 25;246(8):2702–2710. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Racker E., Knowles A. F., Eytan E. Resolution and reconstitution of ion-transport systems. Ann N Y Acad Sci. 1975 Dec 30;264:17–33. doi: 10.1111/j.1749-6632.1975.tb31473.x. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. Properties of Neurospora crassa plasma membrane ATPase. Arch Biochem Biophys. 1977 Apr 30;180(2):384–393. doi: 10.1016/0003-9861(77)90052-2. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. The neurospora plasma membrane ATPase is an electrogenic pump. Proc Natl Acad Sci U S A. 1976 May;73(5):1485–1488. doi: 10.1073/pnas.73.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Hodges T. K. Characterization of passive ion transport in plasma membrane vesicles of oat roots. Plant Physiol. 1976 Sep;58(3):304–308. doi: 10.1104/pp.58.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi S., Kahlenberg A., Medzihradsky F., Hokin L. E. Studies on the characterization of the sodium-potassium transport adenosinetriphosphatase. IV. Properties of a lubrol-solubilized beef brain microsomal enzyme. Arch Biochem Biophys. 1969 Mar;130(1):156–163. doi: 10.1016/0003-9861(69)90021-6. [DOI] [PubMed] [Google Scholar]
- Wilson D. B. Cellular transport mechanisms. Annu Rev Biochem. 1978;47:933–965. doi: 10.1146/annurev.bi.47.070178.004441. [DOI] [PubMed] [Google Scholar]
- Zobel R. W., Del Tredici P., Torrey J. G. Method for growing plants aeroponically. Plant Physiol. 1976 Mar;57(3):344–346. doi: 10.1104/pp.57.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]