Abstract
Objective
Antiretroviral therapy (ART) has been implicated in bone loss in HIV. The role of inflammation and vitamin D is unclear and better investigated in ART-naïve individuals.
Design and Methods
This is a 48-week, prospective, cohort study to compare baseline and change in hip and spine bone mineral density (BMD) measured by dual energy Xray absorptiometry (DXA) in HIV-infected, ART-naïve adults and healthy controls matched by age, sex, and race. We also studied associations between bone loss and inflammation markers and plasma 25-hydroxyvitamin D (25(OH)D) using logistic regression.
Results
47 HIV-infected adults and 41 controls were included. Baseline 25(OH)D, BMD at total hip, trochanter, and spine, and prevalence of osteopenia and osteoporosis were similar between groups. In the HIV-infected group, total hip and trochanter, but not spine, BMD decreased over 48 weeks (hip −0.005 (−0.026-0.008)g/cm2, p=0.02 within-group; trochanter −0.013 (−0.03-0.003), p<0.01). BMD did not change at any site within controls. The HIV-infected group was more likely to have bone loss at the trochanter (p=0.03). This risk persisted after adjustment for age, sex, race, BMI, smoking, and hepatitis C (OR 4 (95% CI 1.2-15.8)). In the HIV-infected group, higher IL-6 concentrations (p=0.04) and Caucasian race (p<0.01) were independently associated with progression to osteopenia or osteoporosis, but not 25(OH)D levels.
Conclusion
BMD at the total hip and trochanter sites decreased in the HIV-infected, ART-naïve adults, but not controls, over this 48-week study. Higher serum IL-6 concentrations were associated with progression to osteopenia or osteoporosis status in the HIV-infected group.
Keywords: Bone Mineral Density, Bone loss, Antiretroviral-Naïve, Inflammation, Vitamin D
Introduction
People are living with HIV infection longer than ever before due to advances in antiretroviral therapy (ART) over the past two decades.[1] Bone health has become an increasingly important aspect of the long term care of HIV-infected individuals because of the higher prevalence of osteoporosis[2] and demonstrated higher risk of fractures including fragility fractures compared with the general population.[3] Understanding the pathogenesis of bone loss in HIV is imperative for developing targeted approaches to fracture prevention in this high risk group.
Multiple factors likely contribute to the pathogenesis of low bone mineral density (BMD) and bone loss in HIV. Some traditional osteoporosis risk factors may disproportionately affect HIV-infected individuals including low body weight, hypogonadism, and smoking, and these have been shown to be important causes of low BMD in HIV.[4, 5] Direct effects of ART, specifically tenofovir and protease inhibitors, have been implicated as well.[6-9] However, regardless of the regimen selected, ART initiation has been shown to result in a 2-6% loss of BMD after 48-96 weeks[10, 11] with subsequent stabilization.[12] Further, the degree of bone loss after ART initiation has been linked with CD4+ T cell count suggesting a role for degree of pre-ART immunodeficiency.[13] Most recently, the effect of HIV itself and consequent chronic inflammation has been suggested as a contributor.[14] Low BMD is prevalent in ART-naïve, HIV-infected individuals.[8, 11, 15] At the time of enrollment into A5224s, a substudy of ACTG A5202, 39% of HIV-infected, ART-naïve participants were osteopenic at the hip or spine.[11] It is notable that 85% of these participants were men and the median age was 38 years, which if HIV-uninfected, should have been considered at low risk for bone disease. This suggests that HIV infection and/or heightened inflammation may be impacting BMD in HIV, independently of the effect of ART. Higher markers of inflammation have been linked with risk of fracture in HIV-uninfected adults as well.[16]
To date, studies evaluating changes in BMD over time in HIV have included participants initiating ART[10, 13] or ART-experienced.[4, 5, 17-22] The aim of this study was to evaluate the change in BMD without the confounding effect of ART with a well-matched HIV-uninfected comparator group and report the association with markers of systemic inflammation. In addition, given the very high prevalence of vitamin D deficiency in HIV,[23] we aimed to assess the effect of vitamin D status on skeletal health. The hypothesis of this study was that over 48 weeks HIV-infected, ART-naïve adults would have greater loss of BMD at the hip and spine measured by dual energy Xray absorptiometry (DXA) compared to HIV-uninfected controls matched by age, sex, and race. Secondary hypotheses were that bone loss in the HIV-infected group would be associated with higher markers of systemic inflammation and lower vitamin D levels.
Methods
Study design
A 48-week, prospective, matched, cohort study was performed on HIV-infected, ART-naïve adults and HIV-uninfected controls matched by age, sex, and race to determine the effect of HIV, systemic inflammation, and vitamin D concentration, assessed by plasma 25-hydroxyvitamin D, on BMD over time. HIV-infected individuals ≥18 years old who were naïve to ART and unlikely to require ART for at least 48 weeks based on ART treatment guidelines at the time (antiretroviral therapy initiation recommended at CD4 cell count ≤350 cells/mm3)[24] were eligible for inclusion in the HIV-infected group. Individuals ≥18 years old without known HIV infection or any medical condition requiring the use of prescription medications were eligible for the control group. Exclusion criteria for both groups included: diabetes mellitus defined as fasting blood glucose level >126 mg/dL, any active infectious or inflammatory condition, pregnancy or breastfeeding. HIV-infected individuals were recruited from the John T. Carey Special Immunology Unit at University Hospitals Case Medical Center (UHCMC) in Cleveland, Ohio. Control participants were UHCMC employees, friends and family members of the HIV- infected participants and employees, and community members responding to an invitation to participate received through the mail. Controls were matched by age within three years, sex, and race to a previously enrolled HIV-infected participant. All participants signed a written informed consent prior to enrollment into this study. Both the study consent and protocol were approved by the UHCMC Institutional Review Board prior to the enrollment of any participants.
The primary outcomes in this study were changes in hip and spine BMD measured by DXA over 48 weeks. Secondary outcomes of interest included: changes in plasma 25-hydroxyvitamin D and markers of inflammation over 48 weeks; proportion of participants with bone loss over 48 weeks; and proportion of participants with progression from normal bone to osteopenia or osteopenia to osteoporosis over 48 weeks. The 48 week time point was selected for follow-up in this study for two reasons. First, the aim of this study was to evaluate ART-naïve HIV-infected individuals who remained ART-naïve through study follow-up to assess the effect of HIV on BMD progression without the confounding effect of ART. At the time this study began, average time to ART initiation was approximately 2 years in our clinic. Therefore, choosing 48-week follow-up would permit 2 DXAs prior to ART initiation on most enrolled participants. Second, in ART initiation studies, 48 weeks has been sufficient to detect a significant change in BMD.[10]
Bone mineral density measurement
DXA of the spine and left hip was performed at study entry and week 48. All DXA scans were performed at a single site (UHCMC) on all study participants using a Hologic QDR-4500A (Hologic Inc, Bedford, MA, USA). The scans were assessed with a dedicated densitometer and a technologist who was blinded to the HIV status of the study participants. Osteopenia was defined as a T-score ≤−1 and >−2.5 at any of the sites measured. Osteoporosis was defined as a T-score ≤−2.5 at any of the sites measured.
Inflammatory biomarkers and vitamin D
Participants had blood drawn after a 12 hour fast at entry and week 48. Plasma was stored at −80°C and never thawed until analysis. Stored samples were batched and tested for inflammatory markers including high sensitivity C-reactive protein (hsCRP), interleukin-6 (IL-6), soluble tumor necrosis factor-α receptors (sTNFR-l and sTNFR-II), adhesion molecules/endothelial activation markers including soluble vascular cell adhesion molecule-1 (sVCAM-1) and soluble intercellular adhesion molecule-1 (sICAM-1) and 25-hydroxyvitamin D (25(OH)D). Interleukin-6, sTNFR-I and –II, sVCAM-1 and sICAM-1 were determined by quantitative sandwich ELISAs (R&D Systems, Minneapolis, MN, USA). Inter-assay variability ranged from 2.02%-15.36%, 3.66%-5.77%, 2.13%-3.79%, 4.76%-8.77% and 3.43%-7.37%, respectively. High sensitivity CRP was determined by particle enhanced immunonephelometric assays on a BNII nephelometer (Siemens, Indianapolis, IN, USA). Inter assay variability ranged from 3.01%-6.46%. All inflammatory marker assays were performed at the Laboratory for Clinical Biochemistry Research University of Vermont.
Plasma 25(OH)D was determined by chemiluminescent technique with the iSYS fully automated system (IDS, LTD, Fountain Hills, AZ, USA). All samples were analyzed in the same co-investigator's laboratory (VT) at Emory University by experienced personnel. This laboratory participates in the National Institutes of Health Vitamin D Quality Control Project as well as the Vitamin D External Quality Assessment Scheme (DEQAS, site 606). Intra- and inter-assay variability is <8% and <10%, respectively, and this assay correlates well with established methods to determine 25(OH)D (R2=0.89 vs radioimmunoassay). In this study vitamin D insufficiency was defined as plasma 25(OH)D <30 ng/ml and ≥20 ng/ml and vitamin D deficiency plasma 25(OH)D <20 ng/ml.[25]
CD4+ T-cell counts and HIV-1 RNA levels were measured as part of clinical care concurrently with the other measures.
Data analysis
Demographics are presented overall and by group at baseline. Median and interquartile range (IQR) are reported for continuous variables and frequency and percent for categorical variables. All baseline demographics as well as endpoints were compared between groups using unpaired t-tests or Wilcoxon Rank Sum tests as distributionally appropriate for continuous variables and by Chi-Square tests, Fisher's Exact tests, or Pearson Exact Chi-Square tests as appropriate for categorical variables. Within-group changes were tested using paired t-tests or Wilcoxon Signed Rank tests as distributionally appropriate. ANCOVA was used to adjust baseline BMD at each site for age, sex, race, body mass index (BMI), and smoking status. Adjusted means were compared between groups using unpaired t-tests. Univariable followed by multivariable linear regression was used to determine associations between baseline BMD at each site and variables of interest including HIV status, demographics, inflammatory markers, and 25(OH)D levels. Stepwise selection was used to generate final models considering all variables with p≤0.25 in univariable analyses.
Bone loss was defined as a negative change in BMD over 48 weeks. Proportion of participants with bone loss at each site separately, and at any site (composite outcome), as well as proportion of participants with progression from normal bone to osteopenia or osteopenia to osteoporosis at any site (composite outcome), were compared between groups using Chi-Square tests or Fisher's Exact tests as appropriate. With bone loss at each site, bone loss at any site, and progression from normal bone to osteopenia or osteopenia to osteoporosis, as the dependent variables, logistic regression was used to adjust for age, sex, race, BMI, smoking, and Hepatitis C status. Next, an exploratory analysis was performed using logistic regression to determine if baseline inflammation or 25(OH)D level was in the causal pathway of bone loss at the trochanter site. A model was created with bone loss at the trochanter site as the dependent variable and HIV status as the only independent variable. To this model each inflammatory marker and 25(OH)D level at baseline were added in turn to ascertain the effect of this added variable on the HIV status odds ratio. A change in the HIV status odds ratio of >10% was considered suggestive that this factor was in the causal pathway of why HIV was associated with bone loss at this site. Lastly, in the HIV-infected group, logistic regression was used to explore predictors of progression from normal bone mineral density to osteopenia or osteopenia to osteoporosis. For this model, stepwise selection was used considering the following variables of interest: IL-6, hsCRP, sTNFR-I, sTNFR-II, sVCAM-1, sICAM-1, 25(OH)D, age, sex, race, BMI, smoking, baseline CD4+ T cell count, HIV-1 RNA level, and known duration of HIV infection.
All statistical tests were two-sided and considered significant with p<0.05. Analyses were performed using SAS v. 9.2 (The SAS Institute, Carey, North Carolina, USA).
Results
From July 13, 2010 to August 22, 2012, 88 participants were enrolled including 47 HIV-infected individuals and 41 controls. Of the 88 participants enrolled, 11 (12.5%) did not complete the study (7/47 (8.5%) in the HIV-infected group and 4/41 (9%) in the control group). Two participants moved, one was incarcerated and the rest were lost to follow-up. As such, for the longitudinal analysis of BMD, 40 HIV-infected participants and 37 controls were included.
Table 1 shows baseline demographic factors by group. The groups were similar with regard to all baseline factors except in the HIV-infected group there were more current smokers (72% vs 15%; p<0.0001) and participants with Hepatitis C (19% vs 2%; p=0.017). Overall, 69% were men and 68% were African American. Median (interquartile range, IQR) age was 38 (25-49) years and BMI was 26.5 (23.3-29.6) kg/m2. The median (IQR) baseline and nadir CD4+ T cell counts were 625 (533-844) and 520 (452-618) cells/mm3, respectively, HIV-1 RNA was 4638 (783-20600) copies/ml, and known duration of HIV infection was 4 (1.1-12.4) years. All participants were ART-naïve at study entry and remained so through this 48 week study. For women, menopausal status was not ascertained.
Table 1.
Baseline Demographics by Group
| ART-Naïve HIV+ (n=47) | HIV- (n=41) | P | |
|---|---|---|---|
| Age, years | 40 (25-50) | 37 (25-49) | 0.85 |
| Men | 33 (70) | 28 (68) | 0.85 |
| Race | |||
| Caucasian | 13 (28) | 14 (34) | 0.73 |
| African American | 33 (70) | 27 (66) | |
| Hispanic | 1 (2) | 0 (0) | |
| BMI, kg/m2 | 26 (22.9-28.7) | 27.4 (24.6-30.8) | 0.33 |
| Current Smoker | 34 (72) | 6 (15) | <0.0001 |
| Current Heavy Alcohol Use* | 1 (2) | 0 (0) | >0.99 |
| Hepatitis C | 9 (19) | 1 (2) | 0.02 |
| Family History of Hip Fracture | 0 (0) | 1 (2) | 0.47 |
Continuous variables are reported as median (interquartile range) and categorical variables as frequency (percent)
Current heavy alcohol use was defined as greater than 2 drinks of alcohol per day on average. BMI, body mass index
Baseline, week 48, and absolute change in inflammatory markers, 25(OH)D levels, and BMD at each site are shown in Tables 2a and 2b by group. At baseline, IL-6, sTNFR-II, sVCAM-1, and sICAM-1 were higher in the HIV-infected group (p<0.01 for all). High sensitivity CRP, sTNFR-I, and 25(OH)D levels were similar between groups. Overall, only 3/88 (3.4%) participants had a plasma 25(OH)D concentration ≥ 30 ng/ml at baseline and the proportion of participants with vitamin D insufficiency (27.7% vs 17.1%; p=0.24 for HIV-infected vs controls) and deficiency (70.2% vs 78.1%; p=0.4) were similar between groups. Baseline BMD at the total hip, femoral neck, trochanter, and spine were similar between groups. Adjusting mean BMD at each site for variables known to effect BMD did not change the between group results although there was a trend towards lower BMD at the femoral neck in the HIV-infected group (adjusted mean 1.074 vs. 1.145 g/cm2 for HIV-infected vs controls; p=0.054). Table 3 shows variables independently associated with baseline BMD at each site. Older age, low BMI, female sex, and being a race other than Caucasian were associated with low BMD at the total hip and femoral neck; older age, low BMI, and female sex were associated with low BMD at the trochanter; and low BMI was associated with low BMD at the spine. HIV status, inflammatory makers, and 25(OH)D levels were not independently associated with baseline BMD at any site. The proportion of participants with osteopenia (33.3% vs 32.5% for HIV-infected vs controls) or osteoporosis (4.4% vs 0%) at baseline were similar between groups as well (p=0.58).
Table 2a.
Baseline, Week 48 and Absolute Change Over 48 Weeks in Inflammatory Markers and 25-Hydroxy-Vitamin D by Group
| Entry | ART-Naïve HIV+ (n=47) | HIV- (n=41) | P |
|---|---|---|---|
| IL-6, pg/ml | 3.026 (1.99-5.59) | 2.21 (1.52-3.254) | <0.01 |
| hsCRP, μg/ml | 1.5 (0.56-4.37) | 0.92 (0.25-2.1) | 0.11 |
| sTNFR-I, pg/ml | 1720.31 (1301.59-3154.05) | 1846.87 (1263.86-2409.57) | 0.56 |
| sTNFR-II, pg/ml | 2433.83 (1421.74-3333.03) | 1965.14 (1275.05-2194.04) | <0.01 |
| sVCAM-1, ng/ml | 782.33 (552.92-1045.95) | 543.544 (393.091-608.86) | <0.0001 |
| sICAM-1, ng/ml | 299.61 (196.372-344.93) | 187.05 (128.65-237.842) | <0.0001 |
| 25(OH)D, ng/ml | 13.245 (9.707-20.855) | 15.1 (10.335-19.528) | 0.63 |
| Week 48 | ART-Naïve HIV+ (n=40)a | HIV-(n=37)b | |
| IL-6, pg/ml | 2.59 (1.79-3.94) | 1.89 (1.1-2.78) | 0.02 |
| hsCRP, μg/ml | 1.34 (0.63-2.72) | 1.05 (0.38-2.87) | 0.53 |
| sTNFR-I, pg/ml | 1317.03 (1142.98-1553.38) | 1332.21 (1102.01-1636.04) | 0.97 |
| sTNFR-II, pg/ml | 3257.38 (2728.18-3588.5) | 2332.42 (1890.03-2750.32) | <0.0001 |
| sVCAM-1, ng/ml | 878.5 (672.71-1160.1) | 574.11 (462.56-710.28) | <0.0001 |
| sICAM-1, ng/ml | 305.19 (211.25-355.42) | 207.39 (141.26-262.85) | <0.01 |
| 25(OH)D, ng/ml | 17.5 (13-23.5) | 15.9 (11.1-23.1) | 0.65 |
| Change Over 48 Weeks | ART-Naïve HIV+ (n=40)a | HIV-(n=37)b | |
| IL-6, pg/ml | −0.38 (−1.709-0.78) | −0.32 (−0.981-0.153)c | 0.73 |
| hsCRP, μg/ml | −0.16 (−1.61-0.38) | 0 (−0.57-0.19) | 0.38 |
| sTNFR-I, pg/ml | −167.755 (−1596.48- −56.28)c | −288.28 (−1071.78- −33.77) c | 0.91 |
| sTNFR-II, pg/ml | 24.43 (−228.39-1454.57) | 518.22 (−73.96-1131.65) c | 0.66 |
| sVCAM-1, ng/ml | 88.453 (6.663-222.338)c | 79.941 (36.75-192.53)c | 0.91 |
| sICAM-1, ng/ml | 9.545 (−13.565-36.91) | 12.415 (−4.98-29.776)c | 0.63 |
| 25(OH)D, ng/ml | 1.826 (−2.045-6.184) | 1.122 (−3.364-4.319) | 0.26 |
All values are reported as median (interquartile range)
n=38 for inflammatory markers and 25(OH)D
n=34 for inflammatory markers and n=33 for 25(OH)D
p<0.05 within-group
IL-6, interleukin-6; hsCRP, high sensitivity C-reactive protein; sTNFR-I, soluble tumor necrosis factor receptor-I; sTNFR-II, soluble tumor necrosis factor receptor-II; sVCAM-1, soluble vascular cell adhesion molecule-1; sICAM-1, soluble intracellular adhesion molecule-1; 25(OH)D, 25-hydroxy-vitamin D
Table 2b.
Baseline, Week 48 and Absolute Change Over 48 Weeks in Bone Mineral Density at Each Site by Group
| Entry | ART-Naïve HIV+ (n=47) | HIV- (n=41) | P |
|---|---|---|---|
| Total Hip BMD, g/cm2 | 1.081 (0.917-1.182) | 1.04 (0.942-1.168) | 0.85 |
| Femoral Neck BMD, g/cm2 | 1.063 (0.972-1.264) | 1.115 (0.979-1.216) | 0.39 |
| Trochanter BMD, g/cm2 | 0.867 (0.761-0.973) | 0.903 (0.771-0.998) | 0.82 |
| Spine BMD, g/cm2 | 1.259 (1.152-1.361) | 1.312 (1.206-1.376) | 0.37 |
| Week 48 | ART-Naïve HIV+ (n=40) | HIV- (n=37) | |
| Total Hip BMD, g/cm2 | 1.0315 (0.906-1.16) | 1.037 (0.916-1.141) | 0.68 |
| Femoral Neck BMD, g/cm2 | 1.047 (0.935-1.209) | 1.09 (0.96-1.209) | 0.47 |
| Trochanter BMD, g/cm2 | 0.848 (0.747-0.953) | 0.907 (0.758-0.97) | 0.65 |
| Spine BMD, g/cm2 | 1.235 (1.159-1.351) | 1.301 (1.211-1.374) | 0.29 |
| Change Over 48 Weeks | ART-Naïve HIV+ (n=40) | HIV- (n=37) | |
| Total Hip BMD, g/cm2 | −0.005 (−0.026-0.008)a | 0 (−0.024-0.012) | 0.23 |
| Femoral Neck BMD, g/cm2 | −0.003 (−0.03-0.013) | −0.006 (−0.024-0.011) | 0.87 |
| Trochanter BMD, g/cm2 | −0.013 (−0.03-0.003)a | 0.001 (−0.023-0.014) | 0.09 |
| Spine BMD, g/cm2 | −0.003 (−0.024-0.027) | 0 (−0.015-0.02) | 0.89 |
All values are reported as median (interquartile range)
p<0.05 within-group
BMD, bone mineral density
Table 3.
Factors Independently Associated with Baseline BMD in Multivariable Linear Regression
| Parameter Estimate (Standard Error) | P | |
|---|---|---|
| Total Hip BMD | ||
| Age, years | −0.004 (0.001) | <0.01 |
| BMI, kg/m2 | 0.012 (0.002) | <0.0001 |
| Sex | 0.077 (0.035) | 0.03 |
| Race | −0.07 (0.033) | 0.04 |
| Femoral Neck BMD | ||
| Age, years | −0.006 (0.001) | <0.0001 |
| BMI, kg/m2 | 0.01 (0.002) | <0.0001 |
| Sex | 0.058 (0.035) | 0.1 |
| Race | −0.081 (0.033) | 0.02 |
| Trochanter BMD | ||
| Age | −0.002 (0.001) | 0.05 |
| BMI, kg/m2 | 0.011 (0.002) | <0.0001 |
| Sex | 0.106 (0.034) | <0.01 |
| Spine BMD | ||
| BMI, kg/m2 | 0.00713 (0.00225) | <0.01 |
Variables considered for inclusion: HIV status, age, BMI, sex, race, smoking status, alcohol use, hepatitis C status, IL-6, hsCRP, sTNFR-I, sTNFR-II, sVCAM-1, sICAM-1, 25(OH)D
BMD, bone mineral density; BMI, body mass index
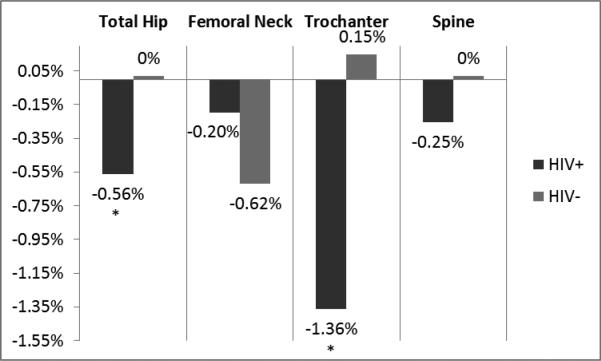
Figure 1 shows percent change in BMD at each site by group. In the HIV-infected group, BMD at the total hip and trochanter decreased over 48 weeks (median absolute change in BMD (IQR) at total hip −0.005 (−0.026-0.008) g/cm2, p=0.023 within-group; trochanter −0.013 (−0.03-0.003), p=0.002). In contrast, BMD did not change significantly at any site within the control group. Changes in BMD did not reach statistical significance between groups, however. In comparing the proportion of participants with bone loss at each site, the HIV-infected group was 2.8 times more likely than controls to have bone loss at the trochanter site (73% vs 49% for HIV-infected vs controls; OR 2.8 (95% CI 1.1-7.2); p=0.034). This risk persisted after adjustment for age, sex, race, BMI, smoking, and hepatitis C (OR 4.3 (95% CI 1.2-15.8); p=0.026). In an exploratory analysis to assess if heightened inflammation or vitamin D status was in the causal pathway between HIV and bone loss at the trochanter site, adding IL-6, sTNFR-II, sVCAM-1, or sICAM-1 attenuated the OR for HIV status by greater than 10%. Further, with each of these inflammatory markers in the model, HIV status was no longer independently predictive of bone loss at the trochanter site (Table 4). Taken together, this suggests that heightened inflammation is in the causal pathway and is a possible explanation for why HIV status was associated with this outcome.
Figure 1.
Percent Change in Bone Mineral Density at Each Site by Group
Table 4.
The Effect of Inflammatory Markers and Vitamin D on the Odds Ratio for HIV Status in a Logistic Regression Model of Bone Loss at the Trochanter Site
| Odds Ratio (95% CI)a | Adjusted Odds Ratio (95% CI)b | p | |
|---|---|---|---|
| HIV status | 2.8 (1.1-7.2) | 0.03 | |
| IL-6 | 2.2 (0.8-5.9)c | 0.12 | |
| hsCRP | 2.8 (1.1-7.2) | 0.03 | |
| sTNFR-I | 2.7 (1.1-7.1) | 0.04 | |
| sTNFR-II | 2.1 (0.8-5.8) c | 0.14 | |
| sVCAM-1 | 1.4 (0.5-4.2) c | 0.51 | |
| sICAM-1 | 2.1 (0.8-5.8) c | 0.14 | |
| 25(OH)D | 3 (1.1-7.9) | 0.03 |
Unadjusted odds ratio for HIV status
Odds ratio for HIV status with each inflammatory marker or 25(OH)D added to the model separately
Odds ratio for HIV status changed by greater than 10% with this variable in the model
IL-6, interleukin-6; hsCRP, high sensitivity C-reactive protein; sTNFR-I, soluble tumor necrosis factor receptor-I; sTNFR-II, soluble tumor necrosis factor receptor-II; sVCAM-1, soluble vascular cell adhesion molecule-1; sICAM-1, soluble intracellular adhesion molecule-1; 25(OH)D, 25-hydroxy-vitamin D
20.5% of the HIV-infected participants progressed from normal bone to osteopenia or from osteopenia to osteoporosis vs 5.6 % of controls (p=0.089). In the HIV-infected group, higher baseline IL-6 (OR 1.1 (95% CI 1-1.2); p=0.036) and Caucasian race (OR 17.4 (95% CI 2.1-142); p=0.008) were independently associated with this outcome in multivariable modeling. Other inflammatory markers, 25(OH)D level and HIV-related factors including baseline CD4+ T cell count were not independently associated with this outcome.
Discussion
In this study, we have comprehensively assessed change in BMD and the association with inflammation and vitamin D status in HIV-infected adults naïve to ART with an age, sex, and race matched healthy control group for comparison. Similar to previous reports,[8, 11, 15] the prevalence of low BMD in the ART-naive HIV-infected group at baseline was high (36%); however, it was similar to what was seen in matched controls (32%). Low baseline BMD in this study was associated with traditional osteoporosis risk factors including older age, low BMI, and female sex. Over 48 weeks, BMD decreased significantly at both the total hip and trochanter sites in the HIV-infected group, but again the changes in BMD were not statistically different than matched healthy controls. However, after adjustment for traditional osteoporosis risk factors, the ART-naive HIV-infected adults were more likely to have bone loss at the trochanter site than controls and this risk appeared to be associated with heightened inflammation. Also, progression from normal bone to osteopenia or from osteopenia to osteoporosis was independently associated with higher baseline IL-6 levels in the HIV-infected group. Last, although vitamin D deficiency was common in both groups in this study, vitamin D status was not associated with change in BMD.
It has been suggested that the process of bone resorption and bone formation (or bone remodeling) which is normally a tightly regulated balance is uncoupled in HIV.[26] HIV viral proteins have been shown to directly stimulate osteoclast activity[27] and suppress osteoblast activity.[28] Further, tumor necrosis factor α and IL-6, inflammatory cytokines known to be elevated in both ART-treated and untreated-HIV-infected individuals compared to uninfected controls,[29, 30] both promote osteoclast formation.[31-33] In this study, given the suggestion that systemic inflammation contributes to the risk of bone loss at the trochanter site, perhaps with longer follow-up greater differences would be seen with regard to change in BMD over time. A major strength of this study was the large number of ART-naïve HIV-infected individuals who remained ART-naïve through 48 weeks of follow-up allowing the opportunity to study the effect of HIV on bone without the confounding effect of ART. However, with current ART treatment guidelines recommending ART initiation at any CD4+ T cell count, longer duration of follow-up was not feasible. Indeed, over half (24/40) of the study participants had initiated ART within one year after completion of this 48-week study.
Low vitamin D has been linked to low BMD[34, 35] and replacement of vitamin D has been shown to improve BMD[36] outside HIV; however, we did not find a link in our study. In HIV, our study is congruent with the study by Sherwood JE, et al which showed that vitamin D deficiency (25(OH)D < 30 ng/dl) was not associated with low BMD in military beneficiaries with HIV[37] and with a study by El-Maouche D, et al which showed that vitamin D deficiency (25(OH)D < 15 ng/dl) was not associated with low BMD in HCV/HIV co-infected adults.[38] Potential explanations include the fact that African American race, which has been shown to effect the relationship between 25(OH)D and BMD,[39, 40] was the most predominant race in this study. Also, it was recently recognized that in the general population, measurements of free 25(OH)D, and not just total 25(OH)D, are better correlated with BMD.[41] Last, most of the participants in this study, HIV-infected and controls, were vitamin D deficient, so we were unable to compare change in BMD with a vitamin D sufficient group. Ongoing vitamin D supplementation trials will be able to establish a causal link in the relationship between vitamin D status and BMD in HIV.
Despite the uniqueness of our study and our population, we should point out some limitations. The study was powered to detect large differences between groups in change in BMD over 48 weeks which were not seen. Assuming a standard deviation of changes from baseline to week 48 within an arm of 0.03, a sample size of 77 participants had 80% power (two-sided t test with an alpha of 0.05) to detect an absolute difference in mean change between arms of 0.0194 g/cm2. In this study, the actual difference in BMD between groups at the trochanter site was 0.0095 g/cm2. Further, although studies evaluating changes in BMD after ART initiation have shown significant differences after 48 weeks, 48 week duration remains relatively short for the evaluation of changes in BMD. Further, because we have only evaluated participants at two time points, it is not clear that the effect of HIV on change in BMD at the trochanter site is constant over time, ie a linear relationship, or that the risk of bone loss at the trochanter site seen in this study will be the similar over longer periods of follow-up. Next, we did not adjust for multiple comparisons given the exploratory nature of the inflammation analyses. Last, the menopausal status of the women in this study was not captured; however, the control group was matched by both age and gender.
In conclusion, HIV-infected, ART-naïve adults, but not controls, had BMD loss at the total hip and trochanter sites over this 48-week, matched, prospective, cohort study; although changes in BMD did not reach statistical significance between groups. HIV-infected individuals were more likely to have bone loss at the trochanter site and this may be related to heightened inflammation. Further, higher baseline IL-6 was associated with progression to osteopenia or osteoporosis in the HIV-infected group. 25(OH)D concentration at baseline was not associated with baseline or change in BMD in this study. Therapeutics targeting inflammation may benefit bone health in HIV-infected adults naïve to ART and further study in this area is needed.
Acknowledgements
The authors would like to thank the patients that participated in this research. The results of this study were presented at the Conference on Retroviruses and Opportunistic Infections in Seattle, Washington, March 5-8, 2012 and at ID Week 2013 in San Francisco, California on October 4, 2013.
This study was funded by an independent research grant to GM from Bristol-Myers Squibb. The content is solely the responsibility of the authors and does not necessarily represent the official views of BMS. BMS has no access to the study data and did not contribute to any decision related to presentation and publication of the study data. This study was also supported by the National Institutes of Health (grant number K23 HL116209-02 to CH).
Role of each author: Corrilynn Hileman assisted in recruitment and study design relating to the statistical analysis, performed the statistical analysis and drafted the manuscript. Danielle Labbato and Norma Storer assisted in recruitment, study visits and contributed to the manuscript. Vin Tangpricha performed the vitamin D assays for this study and contributed to the manuscript. Grace McComsey conceived the study concept and design, oversaw all study procedures and contributed to the manuscript.
Footnotes
Disclaimer: The results of this study were presented at the Conference on Retroviruses and Opportunistic Infections in Seattle, Washington, March 5-8, 2012 and at ID Week 2013 in San Francisco, California on October 4, 2013.
Conflicts of Interest and Source of Funding:
Grace A McComsey has served as a scientific advisor or speaker for Bristol Myers Squibb, GlaxoSmithKline, Janssen, Merck and Gilead Sciences, has received research grants from Bristol Myers Squibb, GlaxoSmithKline and Gilead Sciences and is currently serving as the Data Safety and Monitoring Board Chair for a Pfizer-sponsored study. For the remaining authors no conflicts were declared.
References
- 1.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 3.Shiau S, Broun EC, Arpadi SM, Yin MT. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS. 2013;27:1949–1957. doi: 10.1097/QAD.0b013e328361d241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson DL, Spiegelman D, Knox TK, Wilson IB. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr. 2008;49:298–308. doi: 10.1097/QAI.0b013e3181893e8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TT, Ross AC, Storer N, Labbato D, McComsey GA. Bone turnover, osteoprotegerin/RANKL and inflammation with antiretroviral initiation: tenofovir versus non-tenofovir regimens. Antivir Ther. 2011;16:1063–1072. doi: 10.3851/IMP1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haskelberg H, Hoy JF, Amin J, Ebeling PR, Emery S, Carr A, et al. Changes in bone turnover and bone loss in HIV-infected patients changing treatment to tenofovir-emtricitabine or abacavir-lamivudine. PLoS ONE. 2012;7:e38377. doi: 10.1371/journal.pone.0038377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23:817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 9.McComsey GA, Tebas P, Shane E, Yin MT, Overton ET, Huang JS, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 11.McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolland MJ, Wang TK, Grey A, Gamble GD, Reid IR. Stable bone density in HAART-treated individuals with HIV: a meta-analysis. J Clin Endocrinol Metab. 2011;96:2721–2731. doi: 10.1210/jc.2011-0591. [DOI] [PubMed] [Google Scholar]
- 13.Grant PM, Kitch D, McComsey GA, Dube MP, Haubrich R, Huang J, et al. Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis. 2013;57:1483–1488. doi: 10.1093/cid/cit538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ofotokun I, McIntosh E, Weitzmann MN. HIV: inflammation and bone. Curr HIV/AIDS Rep. 2012;9:16–25. doi: 10.1007/s11904-011-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown TT, Chen Y, Currier JS, Ribaudo HJ, Rothenberg J, Dube MP, et al. Body composition, soluble markers of inflammation, and bone mineral density in antiretroviral therapy-naive HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2013;63:323–330. doi: 10.1097/QAI.0b013e318295eb1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cauley JA, Danielson ME, Boudreau RM, Forrest KY, Zmuda JM, Pahor M, et al. Inflammatory markers and incident fracture risk in older men and women: the Health Aging and Body Composition Study. J Bone Miner Res. 2007;22:1088–1095. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- 17.Yin MT, Lu D, Cremers S, Tien PC, Cohen MH, Shi Q, et al. Short-term bone loss in HIV-infected premenopausal women. J Acquir Immune Defic Syndr. 2010;53:202–208. doi: 10.1097/QAI.0b013e3181bf6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma A, Flom PL, Weedon J, Klein RS. Prospective study of bone mineral density changes in aging men with or at risk for HIV infection. AIDS. 2010;24:2337–2345. doi: 10.1097/QAD.0b013e32833d7da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin MT, Zhang CA, McMahon DJ, Ferris DC, Irani D, Colon I, et al. Higher rates of bone loss in postmenopausal HIV-infected women: a longitudinal study. J Clin Endocrinol Metab. 2012;97:554–562. doi: 10.1210/jc.2011-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91:2938–2945. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonjoch A, Figueras M, Estany C, Perez-Alvarez N, Rosales J, del Rio L, et al. High prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort study. AIDS. 2010;24:2827–2833. doi: 10.1097/QAD.0b013e328340a28d. [DOI] [PubMed] [Google Scholar]
- 22.Assoumou L, Katlama C, Viard JP, Bentata M, Simon A, Roux C, et al. Changes in bone mineral density over a 2-year period in HIV-1-infected men under combined antiretroviral therapy with osteopenia. AIDS. 2013;27:2425–2430. doi: 10.1097/QAD.0b013e32836378c3. [DOI] [PubMed] [Google Scholar]
- 23.Dao CN, Patel P, Overton ET, Rhame F, Pals SL, Johnson C, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 24.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Jan 29, 2008. [December 2, 2013]. pp. 1–128. Available at http://aidsinfo.nih.gov/guidelines/archive/adult-and-adolescent-guidelines. [Google Scholar]
- 25.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 26.Walker Harris V, Brown TT. Bone loss in the HIV-infected patient: evidence, clinical implications, and treatment strategies. J Infect Dis. 2012;205(Suppl 3):S391–398. doi: 10.1093/infdis/jis199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278:48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 28.Cotter EJ, Malizia AP, Chew N, Powderly WG, Doran PP. HIV proteins regulate bone marker secretion and transcription factor activity in cultured human osteoblasts with consequent potential implications for osteoblast function and development. AIDS Res Hum Retroviruses. 2007;23:1521–1530. doi: 10.1089/aid.2007.0112. [DOI] [PubMed] [Google Scholar]
- 29.Hileman CO, Carman TL, Longenecker CT, Labbato DE, Storer NJ, White CA, et al. Rate and predictors of carotid artery intima media thickness progression in antiretroviral-naive HIV-infected and uninfected adults: a 48-week matched prospective cohort study. Antivir Ther. 2013 doi: 10.3851/IMP2651. [DOI] [PubMed] [Google Scholar]
- 30.Ross AC, Rizk N, O'Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 32.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Zhang J, Yan C, Shen X. Relationships between serum 25-hydroxyvitamin D and quantitative ultrasound bone mineral density in 0-6 year old children. Bone. 2013;53:306–310. doi: 10.1016/j.bone.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Ooms ME, Lips P, Roos JC, van der Vijgh WJ, Popp-Snijders C, Bezemer PD, et al. Vitamin D status and sex hormone binding globulin: determinants of bone turnover and bone mineral density in elderly women. J Bone Miner Res. 1995;10:1177–1184. doi: 10.1002/jbmr.5650100806. [DOI] [PubMed] [Google Scholar]
- 36.Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P. Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab. 1995;80:1052–1058. doi: 10.1210/jcem.80.4.7714065. [DOI] [PubMed] [Google Scholar]
- 37.Sherwood JE, Mesner OC, Weintrob AC, Hadigan CM, Wilkins KJ, Crum-Cianflone NF, et al. Vitamin D deficiency and its association with low bone mineral density, HIV-related factors, hospitalization, and death in a predominantly black HIV-infected cohort. Clin Infect Dis. 2012;55:1727–1736. doi: 10.1093/cid/cis785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Maouche D, Mehta SH, Sutcliffe CG, Higgins Y, Torbenson MS, Moore RD, et al. Vitamin D deficiency and its relation to bone mineral density and liver fibrosis in HIV-HCV coinfection. Antivir Ther. 2013;18:237–242. doi: 10.3851/IMP2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman BI, Register TC. Effect of race and genetics on vitamin D metabolism, bone and vascular health. Nat Rev Nephrol. 2012;8:459–466. doi: 10.1038/nrneph.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93:40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almas B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Invest. 2014 doi: 10.3109/00365513.2013.869701. [DOI] [PubMed] [Google Scholar]