Abstract
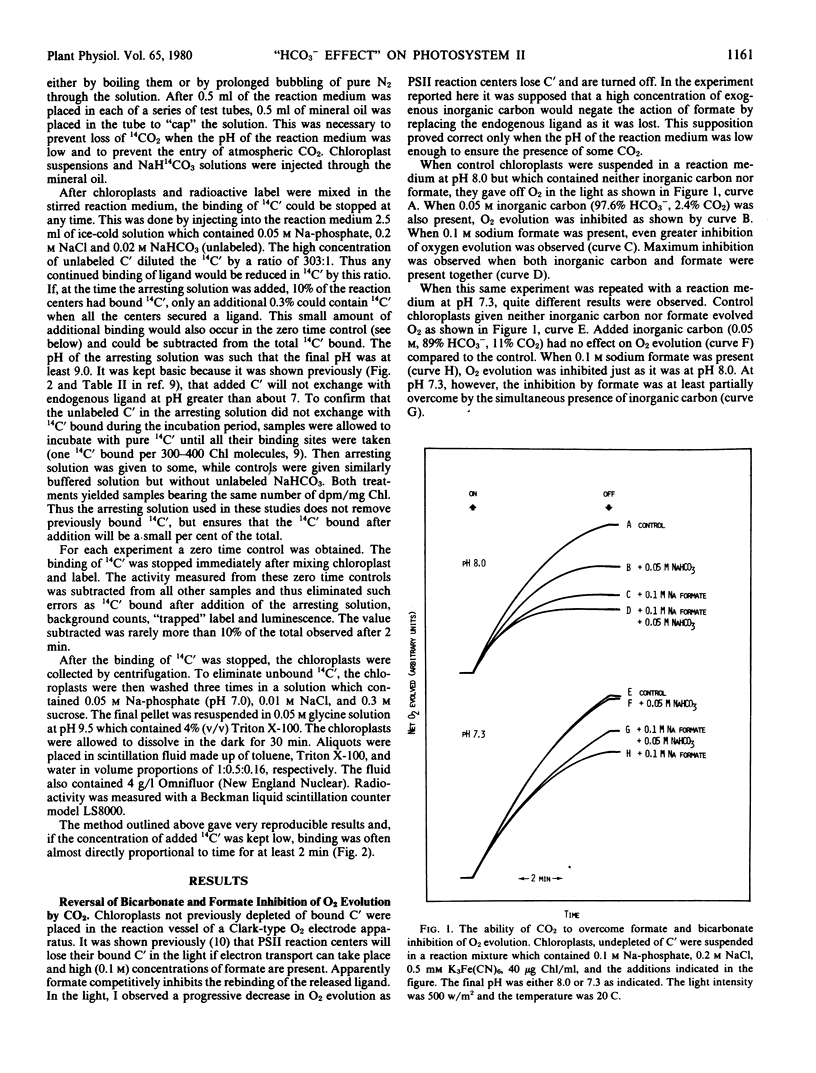
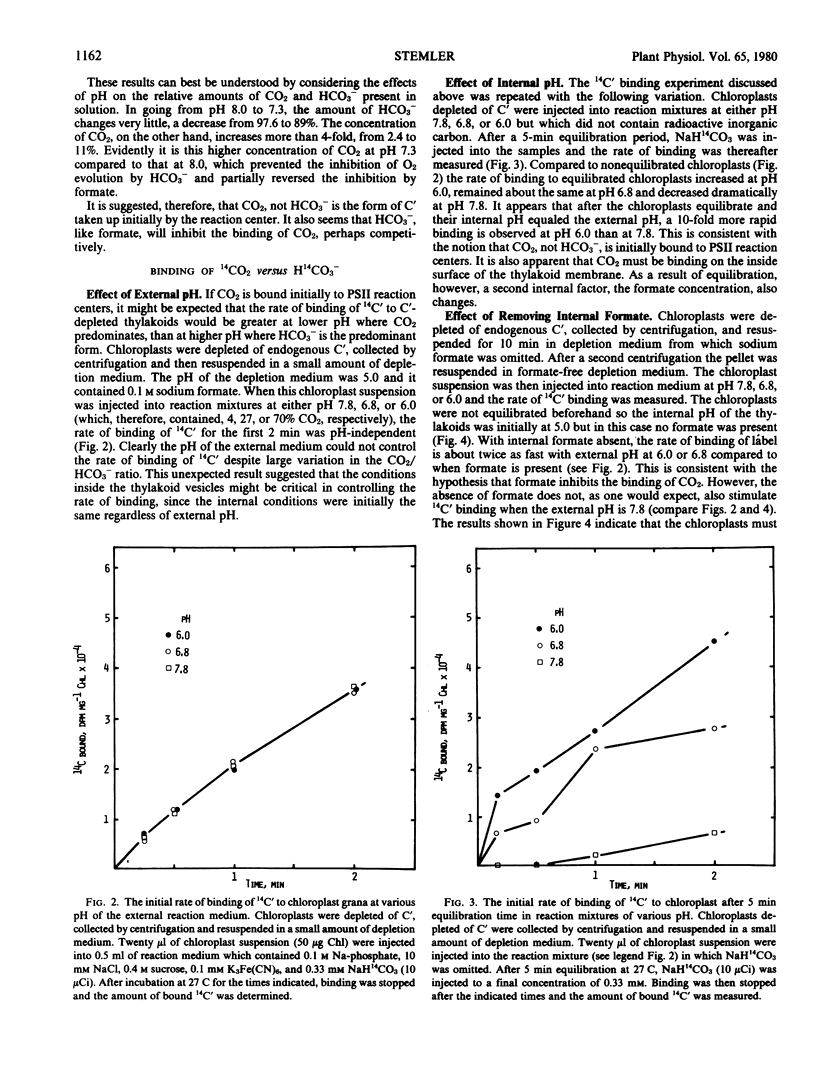
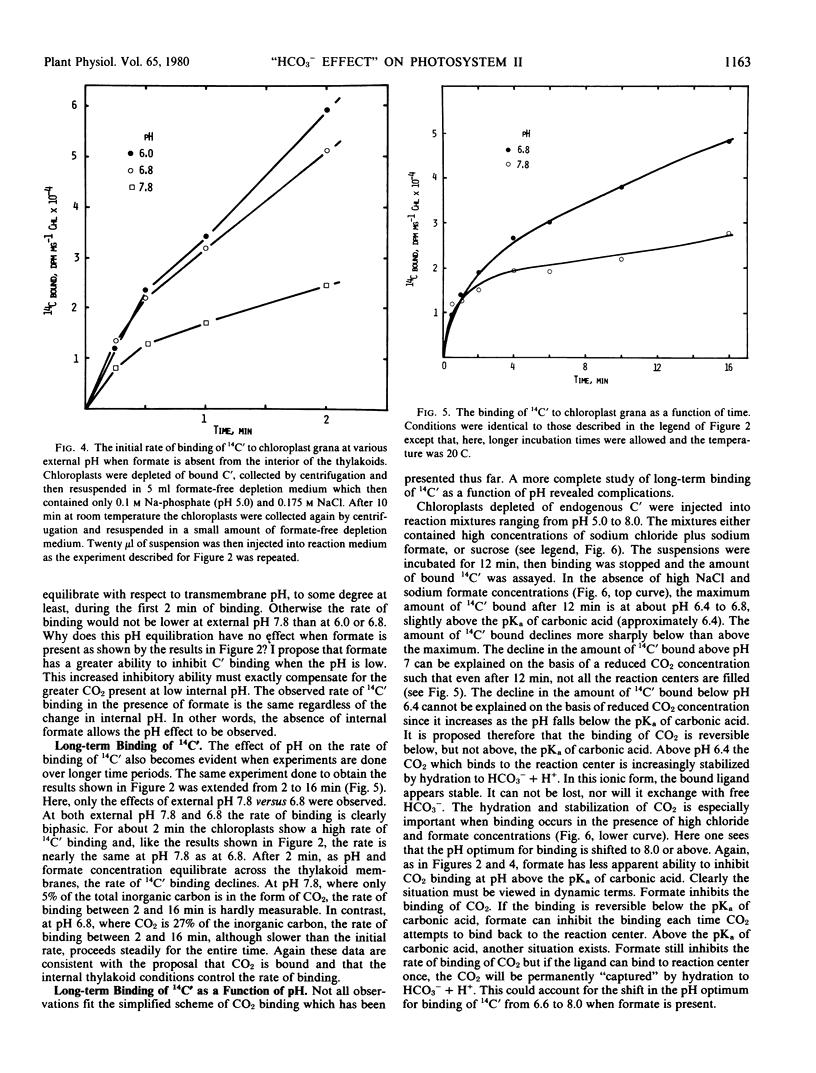
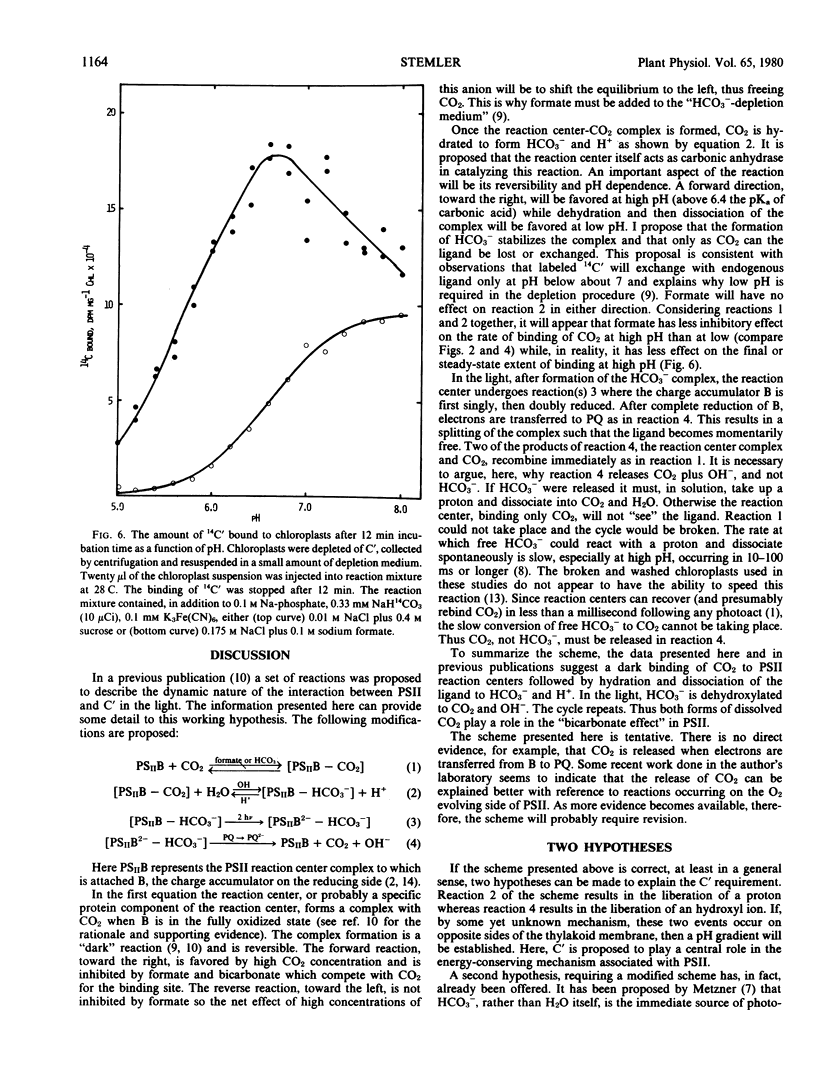
High concentrations of both bicarbonate and formate inhibit photosynthetic O2 evolution at pH 8.0. At this pH, only 2.4% of the total dissolved carbon dioxide exists as CO2. At pH 7.3, where 11% of the total dissolved carbon dioxide exists as CO2, HCO3− no longer inhibits. While formate still inhibits O2 evolution at pH 7.3, its effect can be partially overcome if CO2 is also present. The rate of binding of added 14C-labeled inorganic carbon is nearly 10-fold more rapid when the internal pH of thylakoid membranes is at 6.0 than when it is at 7.8. These observations suggest that CO2, not HCO3−, is initially bound to the photosystem II reaction center and that the location of the binding site is on the inside thylakoid surface. However, additional data presented here suggest that, after binding, CO2 is hydrated to HCO3− + H+ in a pH-dependent reaction. Two possible explanations of the “bicarbonate effect” are presented.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouges-Bocquet B. Electron transfer between the two photosystems in spinach chloroplasts. Biochim Biophys Acta. 1973 Aug 31;314(2):250–256. doi: 10.1016/0005-2728(73)90140-0. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Limiting steps in photosystem II and water decomposition in Chlorella and spinach chloroplasts. Biochim Biophys Acta. 1973 Apr 5;292(3):772–785. doi: 10.1016/0005-2728(73)90024-8. [DOI] [PubMed] [Google Scholar]
- Good N. E. Carbon Dioxide & the Hill Reaction. Plant Physiol. 1963 May;38(3):298–304. doi: 10.1104/pp.38.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee, van Rensen J. J. Bicarbonate effects on the electron flow in isolated broken chloroplasts. Biochim Biophys Acta. 1978 Oct 23;505(2):183–213. doi: 10.1016/0304-4173(78)90012-5. [DOI] [PubMed] [Google Scholar]
- Jursinic P., Warden J., Govindjee A major site of bicarbonate effect in system II reaction. Evidence from ESR signal IIvf, fast fluorescence yield changes and delayed light emission. Biochim Biophys Acta. 1976 Aug 13;440(2):322–330. doi: 10.1016/0005-2728(76)90066-9. [DOI] [PubMed] [Google Scholar]
- Khanna R., Govindjee, Wydrzynski T. Site of bicarbonate effect in Hill reaction. Evidence from the use of artificial electron acceptors and donors. Biochim Biophys Acta. 1977 Oct 12;462(1):208–214. doi: 10.1016/0005-2728(77)90203-1. [DOI] [PubMed] [Google Scholar]
- Stemler A. A dynamic interaction between the bicarbonate ligand and photosystem II reaction center complexes in chloroplasts. Biochim Biophys Acta. 1979 Jan 11;545(1):36–45. doi: 10.1016/0005-2728(79)90111-7. [DOI] [PubMed] [Google Scholar]
- Stemler A., Babcock G. T., Govindjee The effect of bicarbonate on photosynthetic oxygen evolution in flashing light in chloroplast fragments. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4679–4683. doi: 10.1073/pnas.71.12.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemler A., Govindjee Bicarbonate ion as a critical factor in photosynthetic oxygen evolution. Plant Physiol. 1973 Aug;52(2):119–123. doi: 10.1104/pp.52.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemler A. The binding of bicarbonate ions to washed chloroplast grana. Biochim Biophys Acta. 1977 Jun 9;460(3):511–522. doi: 10.1016/0005-2728(77)90089-5. [DOI] [PubMed] [Google Scholar]
- WARBURG O., KRIPPAHL G. [The necessity of carbon dioxide for quinone and ferricyanide reactions in green grain]. Z Naturforsch B. 1960 Jun;15B:367–369. [PubMed] [Google Scholar]


