Abstract
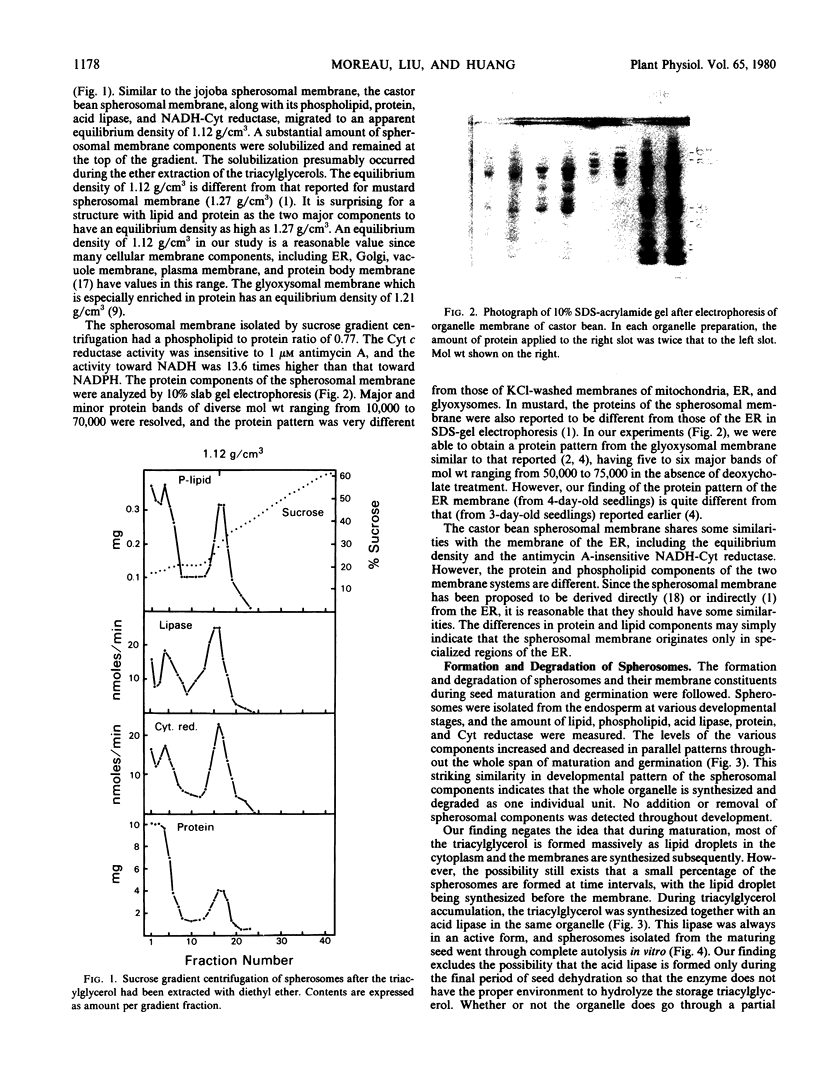
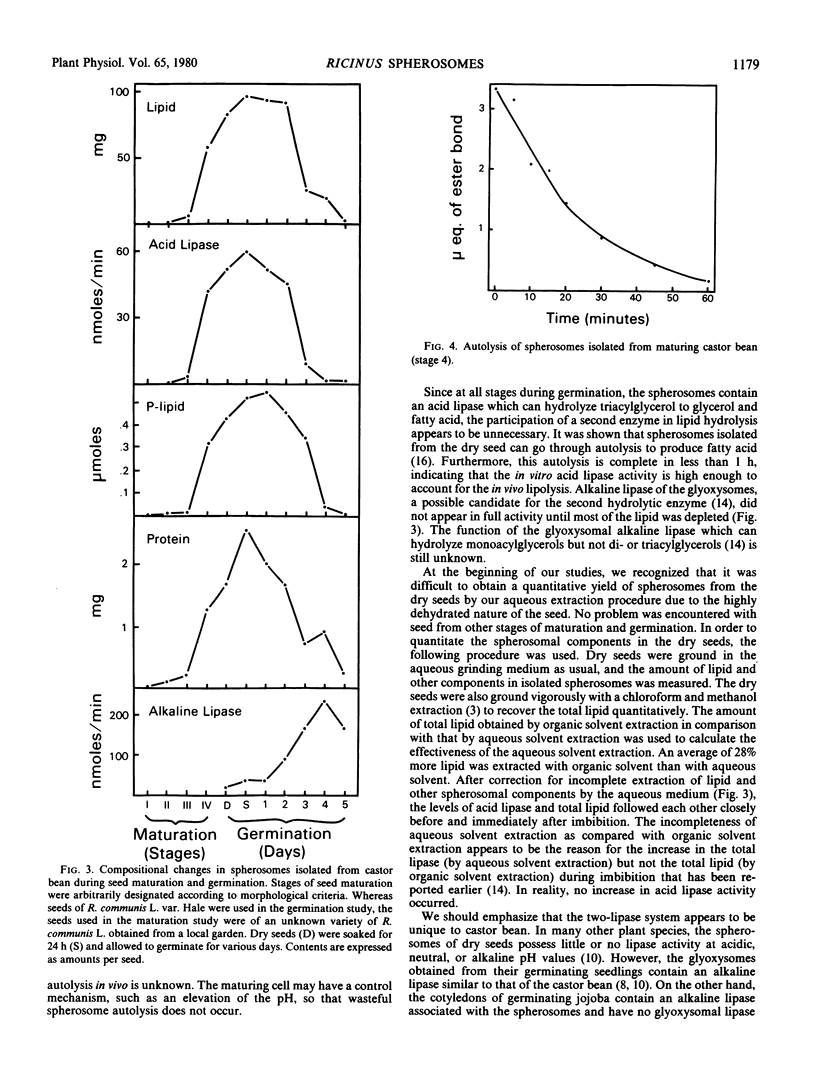
The membrane components of the castor bean spherosomes were characterized. The storage triacylglycerols of isolated spherosomes were extracted with diethyl ether, and the membrane was isolated by sucrose gradient centrifugation. It had an apparent equilibrium density of 1.12 grams per cubic centimeter, and possessed an antimycin A-insensitive NADH cytochrome c reductase and an acid lipase. Phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol in roughly equal amounts were the major phospholipids. The membrane proteins were resolved into several major and minor protein bands of molecular weights ranging from 10,000 to 70,000 by acrylamide gel electrophoresis, and the protein pattern in the gel was different from those of the endoplasmic reticulum, mitochondrial, and glyoxysomal membranes.
The varying amounts of spherosomal components in the seed were followed throughout seed maturation and germination. A striking similarity existed in the developmental pattern of each of the spherosomal components. This finding suggests that the spherosome is synthesized and degraded as one individual unit. The spherosomes isolated from maturing seeds exhibited rapid hydrolysis of the storage lipid in vitro, thus raising the problem of cellular control in preventing in vivo autolysis of the spherosomes during seed maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bieglmayer C., Ruis H. Protein composition of the glyoxysomal membrane. FEBS Lett. 1974 Oct 1;47(1):53–55. doi: 10.1016/0014-5793(74)80424-2. [DOI] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Similarities in the polypeptide composition of glyoxysomal and endoplasmic-reticulum membranes from castor-bean endosperm. Biochem J. 1976 Feb 15;154(2):491–499. doi: 10.1042/bj1540491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P. Accumulation of free ricinoleic Acid in germinating castor bean endosperm. Plant Physiol. 1977 Jun;59(6):1064–1066. doi: 10.1104/pp.59.6.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson R. P. Membrane lipid metabolism in germinating castor bean endosperm. Plant Physiol. 1976 Apr;57(4):510–515. doi: 10.1104/pp.57.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H. Comparative studies of glyoxysomes from various Fatty seedlings. Plant Physiol. 1975 May;55(5):870–874. doi: 10.1104/pp.55.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moreau R. A., Huang A. H. Gluconeogenesis from storage wax in the cotyledons of jojoba seedlings. Plant Physiol. 1977 Aug;60(2):329–333. doi: 10.1104/pp.60.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORY R. L., ST ANGELO A. J., ALTSCHUL A. M. Castor bean lipase: action on its endogenous substrate. J Lipid Res. 1960 Apr;1:208–213. [PubMed] [Google Scholar]
- Ory R. L., Yatsu L. Y., Kircher H. W. Association of lipase activity with the spherosomes of Ricinus communis. Arch Biochem Biophys. 1968 Feb;123(2):255–264. doi: 10.1016/0003-9861(68)90132-x. [DOI] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Spherosome membranes: half unit-membranes. Plant Physiol. 1972 Jun;49(6):937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]



