Letter to the Editor
Histone deacetylase (HDAC) 6, a class-IIb HDAC, is the only HDAC with two functional deacetylase domains and a zinc finger motif. HDAC6 was initially described as tubulin deacetylase; however, it also deacetylates other non-histone proteins, including heat shock protein (Hsp90). Importantly, HDAC6 has an essential role in aggresomal protein degradation pathway, an alternative system to the proteasome for degradation of polyubiquitinated misfolded/unfolded proteins. Specifically, HDAC6 binds both polyubiquitinated proteins and dynein motors, thereby acting to recruit protein cargo to dynein motors for transport to aggresomes for lysosomal degradation 1. We have previously shown that inhibition of both proteasomal and aggresomal protein degradation using bortezomib (BTZ) to inhibit the proteasome and tubacin to inhibit HDAC6, respectively, induces accumulation of polyubiquitinated proteins and significant MM cell stress, followed by apoptosis 2. More recently, a hydroxamic acid HDAC6 selective inhibitor ACY-1215 combined with BTZ shows significant anti-MM activities in preclinical studies 3, which has been rapidly translated to phase I/II clinical studies in relapsed/refractory MM patients 4. Previous studies have also shown that the non-selective HDAC inhibitor vorinostat in combination with BTZ also triggers synergistic cytotoxicity in MM 5, which has provided the framework for combination clinical trials 6.
Carfilzomib (CFZ) is an epoxyketone proteasome inhibitor, which recently received accelerated FDA approval to treat relapsed refractory MM 7, 8. In this study, we asked whether HDAC6 inhibitors enhance anti-MM toxicity induced by CFZ. We also delineated differential molecular mechanisms that trigger synergistic MM toxicity triggered by CFZ in combination with either HDAC6 selective or class-I HDAC selective inhibitors ACY-1215 and MS275 (entinostat), respectively 9.
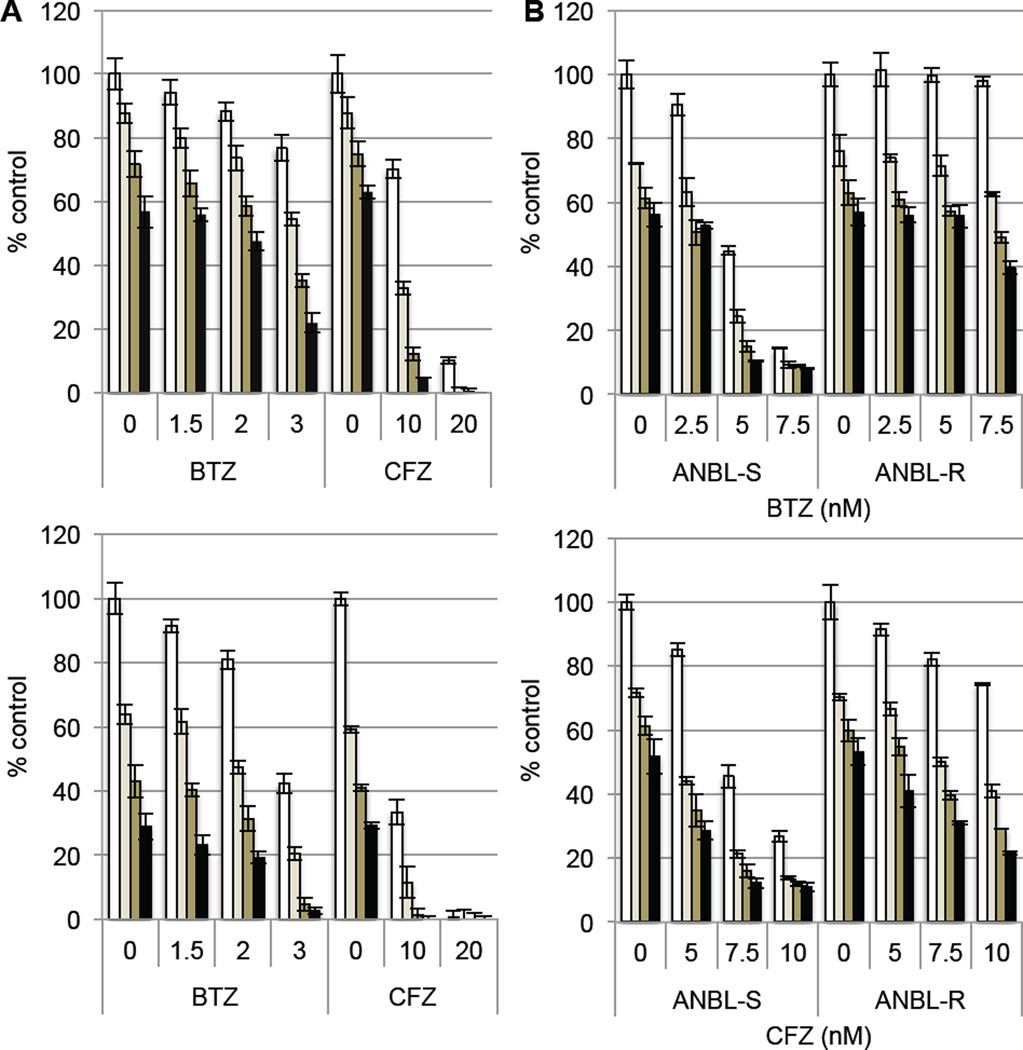
We first compared the combination effect of the HDAC6 selective inhibitor ACY-1215 with either BTZ or CFZ in MM cell lines. Synergistic cytotoxicity induced by CFZ with ACY-1215 was greater than BTZ with ACY-1215 in both RPMI8226 (Figure 1A, upper panel) and MM.1S cells (Figure 1A, lower panel). To further confirm the role of HDAC6 inhibition, we carried out similar combination cytotoxicity experiments using the chemically unrelated HDAC6 selective inhibitor tubastatin-A. Consistent with the ACY-1215 results, tubastatin-A synergistically enhanced both BTZ- and CFZ-induced cytotoxicity. Interestingly, synergistic cytotoxicity, based on combination index (CI) < 1, was more significant in RPMI8226 cells than MM.1S cells (Supplementary Figure 1). Although BTZ has remarkable clinical activity in MM, acquired resistance limits its long-term utility. We therefore next examined whether HDAC6 inhibition can overcome BTZ resistance in vitro using BTZ-resistant ANBL (ANBL-R) human MM cell line. Importantly, synergistic cytotoxicity of ACY-1215 with BTZ was observed even in ANBL-R cells. Similar to Figure 1A, this synergistic cytotoxicity was even more significant with CFZ than BTZ (Figure 1B). These results suggest that CFZ may have greater clinical activity in MM than BTZ when combined with HDAC6 inhibitors.
Figure 1. HDAC6 inhibition enhances proteasome inhibitor-induced cytotoxicity.
(A) RPMI8226 (upper panel) and MM.1S cells (lower panel) were treated with BTZ or CFZ in the presence of control media (□), and with 1 µM (■), 2 µM (■), or 3 µM (■) ACY-1215 for 48h. (B) BTZ-sensitive (S) and –resistant (R) ANBL cells were treated with BTZ (upper panel) or CFZ (lower panel) in the presence of control media (□), as well as with 1 µM (■), 2 µM (■), or 3 µM (■) ACY-1215 for 48h. Cell growth was assessed by MTT assay, and data represents mean ± SD from average of 2 (A) or 3 (B, C) independent experiments.
We and others have shown that non-selective HDAC inhibitors in combination with BTZ trigger synergistic cytotoxicity in MM 10, 11. These non-selective HDAC inhibitors block not only HDAC6 but also class-I HDAC activity 9, and whether synergistic cytotoxicity induced by non-selective HDAC inhibitors with BTZ is due solely to HDAC6 blockade remains unclear. Indeed, we have recently shown that both HDAC3 knockdown and a small molecule HDAC3 selective inhibitor BG45 trigger synergistic MM cytotoxicity in combination with BTZ 12. To address this question, we first examined the cytotoxicity of class-I HDAC selective inhibitor MS275 in combination with CFZ. MS275 with CFZ induced synergistic cytotoxicity in RPMI8226 cells and additive cytotoxicity in MM.1S cells (Supplementary Figure 2). Consistent with our studies, MS275 with BTZ also shows significant anti-tumor activities in other cancer cell types 13.
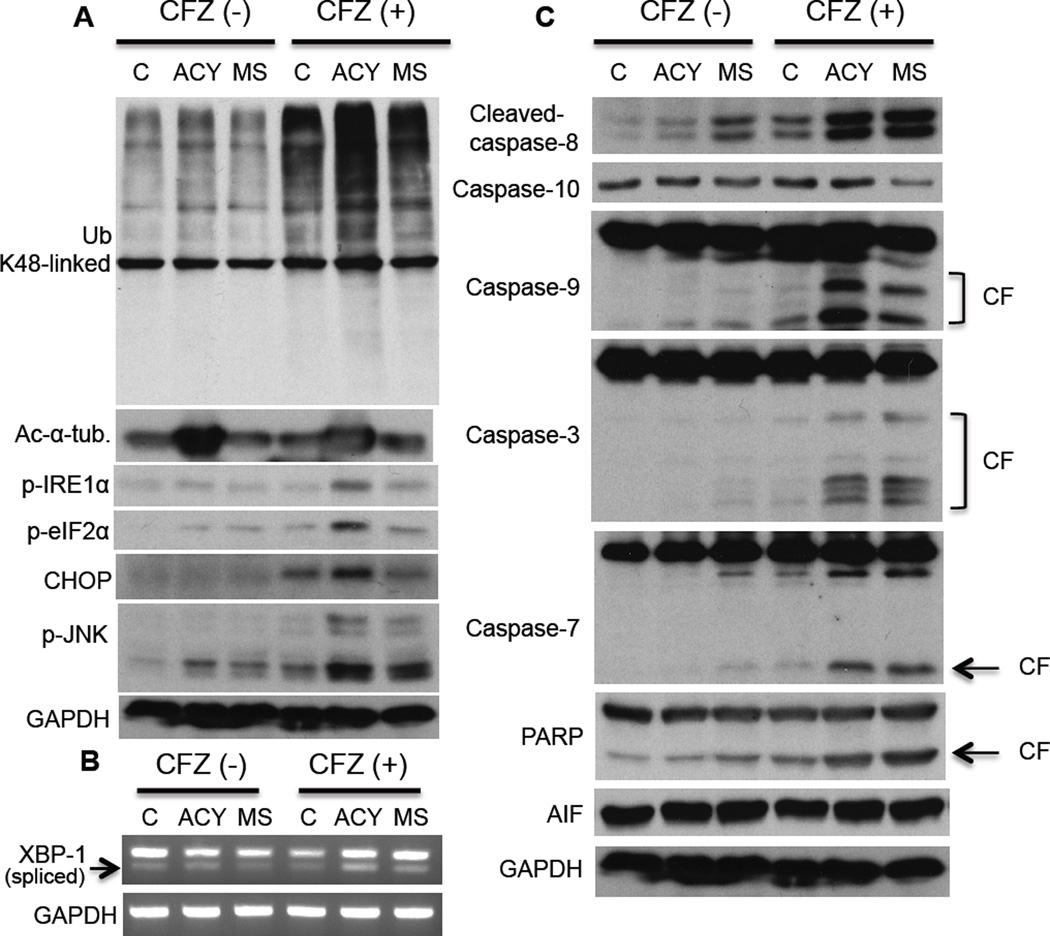
We next determined whether different molecular mechanisms mediate the synergistic MM cell cytotoxicity of HDAC6 versus class-I HDAC inhibition in combination with CFZ. We first defined the doses of ACY-1215 (2 µM) and MS275 (2 µM) with CFZ (10 nM), which induced equivalent cytotoxicity (35%) (Supplementary Figure 3), and used these culture conditions for subsequent experiments. We have shown that ACY-1215 with BTZ triggers accumulation of polyubiquitinated proteins associated with cell stress 3. Although not all polyubiquitinated proteins are degraded by the proteasome, recent studies have shown that proteins specifically linked with lysine (K)48 polyubiquitin are degraded by proteasomes 14. Our studies showed that CFZ induced accumulation of K48-linked polyubiquitinated proteins; and that ACY-1215, but not MS275, markedly enhanced this effect (Figure 2A). These results further confirm our previous observation showing involvement of HDAC6 in mediating protein degradation via aggresomes; conversely, that blocking HDAC6 increases proteasomal protein breakdown.
Figure 2. HDAC6 inhibitor combined with carfilzomib triggers ER stress followed by intrinsic apoptotic pathway.
RPMI8226 cells were treated with control media (C), ACY-1215 (ACY, 2 µM), or MS275 (MS, 2 µM), in the absence or presence of CFZ (10 nM) for 16h. (A and C) Cell lysates were subjected to Immunoblotting with indicated Abs; CF, cleaved fragment. (B) Total RNA was extracted; XBP1 and β-actin mRNA were evaluated by RT-PCR.
RPMI8226 was the most sensitive MM cell line to combined treatment of HDAC6 inhibitors with proteasome inhibitors. Moreover, our previous studies have shown that RPMI8226 cells constitutively express XBP-1 spliced form, a marker of unfolded protein response (UPR) upon endoplasmic reticulum (ER) stress 15. RPMI8226 cells are therefore under constitutive ER stress at physiological conditions. We consequently hypothesized that combining ACY-1215, but not MS275, with CFZ further enhanced ER stress by accumulating polyubiquitinated proteins, resulting in enhanced sensitivity to the combination treatment. Indeed, among MM cell lines, RPMI8226 cells are the most sensitive to ER stressor tunicamycin (Supplementary Figure 4). Importantly, we observed that downstream molecules of ER stress (p-IRE1α, p-eIF2α, p-JNK, CHOP) were significantly enhanced by combination of ACY-1215 with CFZ (Figure 2A). We further confirmed ER stress triggered by this combination treatment using qPCR to demonstrate enhanced XBP-1 splicing (Figure 2B). These results indicate that proteasome inhibition with HDAC6 inhibition, but not class-I HDAC inhibition, triggers accumulation of polyubiquitinated proteins followed by ER stress.
We further delineated downstream apoptotic pathway, in cells treated with these combinations. Caspases-3 and -7, hallmarks of caspase activation, were cleaved by treatment with ACY-1215 or MS275 in combination with CFZ; however, caspase-9 was more significantly cleaved by ACY-1215 than MS275 in RPMI8226 cells. In contrast, caspase-10 was cleaved by CFZ with MS275, but not ACY-1215. Apoptosis-inducing factor (AIF) contributing caspase-independent apoptosis was not induced (Figure 2C). Consistent with RPMI8226 cells, we observed enhanced CHOP and capase-9 cleavage by ACY-1215 with CFZ, as well caspase-8 cleavage by MS275 with CFZ in primary MM tumor cells (Supplementary Figure 5A). To confirm the significance of caspase cleavage (activation), we used selective caspase inhibitors in the combination treatments. As expected, caspase-9 inhibitor has more prominent inhibitory effect in cytotoxicity induced by ACY1215 with CFZ than MS275 with CFZ in RPMI8226 cells (Supplementary Figure 5B) and primary MM tumor cells (Supplementary Figure 5C and 5D).
In summary, we show that BTZ and CFZ trigger significant cytotoxicity in MM cells when combined with selective HDAC6 inhibitors (ACY-1215, tubastatin-A), and that more potent synergistic cytotoxicity of HDAC6 inhibitors was observed with CFZ than with BTZ. We also showed synergistic cytotoxicity triggered by combination CFZ with class-I HDAC inhibitor MS275. Importantly, HDAC6 inhibitor ACY-1215, but not MS275, triggered marked accumulation of K48-linked polyubiquitinated proteins and endoplasmic reticulum stress, followed by unfolded protein response. Moreover, CFZ combined with ACY-1215 predominantly triggered intrinsic apoptotic signaling, whereas CFZ with MS275 triggered the extrinsic apoptotic pathway. Our results therefore delineate distinct apoptotic pathways induced by CFZ with HDAC6 versus class-I HDAC inhibition, providing the preclinical framework for clinical evaluation of combination therapy to improve patient outcome in MM.
Materials and Methods
Cells
Dex-sensitive MM.1S and resistant MM.1R human MM cell lines were kindly provided by Dr. Steven Rosen (Northwestern University, Chicago, IL). U266, RPMI8226, and NCI-H929 (H929) human MM cells were obtained from American Type Culture Collection. OPM1 and OPM2 plasma cell leukemia cell lines were kindly provided by Dr. Edward Thompson (University of Texas Medical Branch, Galveston, TX). All cell lines were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS), 2 µM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Life Technologies, Grand Island, NY). ANBL6 bortezomib-sensitive (S) and -resistant (R) cells were kindly provided by Dr. Robert Orlowski (M.D. Anderson Cancer Center) and cultured in the presence of IL-6 (2.5 ng/ml) and bortezomib (5 ng/ml). Myeloma tumor cells were purified from the bone marrow aspirates from patients using the RosetteSep® negative selection system (Stem Cell Technologies, Vancouver, BC, Canada) after informed consent was obtained in accordance with the Declaration of Helsinki and approval by the Institutional Review Board of the Dana-Farber Cancer Institute.
Reagents and antibodies
HDAC6 selective inhibitor ACY-1215 was obtained from MedKoo Biosciences (Chapel Hill, NC). HDAC 6 selective inhibitor tubastatin-A, BTZ, CFZ, and entinostat (MS275) were obtained from Selleck Chemicals (Houston, TX). Anti-HDAC6, -α-tubulin, -GAPDH, -K48-linked-ubiquitin, -K63-linked-ubiquitin, -phospho-JNK, -CHOP, -caspase-3, - caspase-8, -caspase-9, -caspase-10, -PARP, -AIF, and -IRE1α Abs were purchased from Cell Signaling Technology (Danvers, MA). Anti-phospho-IRE1α Ab was obtained from Affinity Bioreagents (Golden, CO). Anti-acetylated α-tubulin was purchased from Sigma (St. Louis, MO). Inhibitors of caspase-8 (Z-IETD-FMK) and caspase-10 (Z-AEVD-FMK) were purchased from R&D Systems (Minneapolis, MN). Capse-9 inhibitor III (Ac-LEHD-CMK) was purchased from Calbiochem (San Diego, CA).
Cell growth assay
Cell growth was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) dye absorbance. Briefly, cells were pulsed with 10 µl of 5 mg/ml MTT to each well for the last 4 h of the cultures, followed by 100 µl isopropanol containing 0.04N HCl. Absorbance was measured at 570/630 nm using a spectrophotometer (Molecular Devices Corp., Sunnyvale CA). All experiments were performed in quadruplicate.
Immunoblotting
Whole cell lysates were obtained by cell lysis in TritonX-100 buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 5 mM EDTA, 5 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 5 µg/ml leupeptin, and 5 µg/ml aprotinin) and subjected to SDS-PAGE, transferred to nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA), and immunoblotted with specific Abs.
RNA extraction and RT-PCR
RNA was extracted using RNeasy Mini Kit (Invitrogen, Grand Island, NY) and measured by a Nanodrop spectrophotometer (Labtech International LTD., Uckfield, UK). cDNA was synthesized using the Superscript III First strand synthesis Kit (Invitrogen). To evaluate relative expression levels of XBP1u/XBP1s, RT-PCR analysis was performed using PCR SuperMix (Invitrogen). Human XBP1 primer sequences were as follows: 5′-CCTGGTTGCTGAAGAGGAGG-3′ and 5′-CCA TGGGGAGATGTTCTGGAG-3′. Murine XBP1 primer sequences were as follows: 5′-ACACGCTTGGGAATGGACAC-3′ and 5′-CCATGGGAAGATGTTCTGGG-3′. β-actin was used as a loading control, with primers as follows: 5′-GGGTCAGAAGGATTCCTATG-3′ and 5′-GGTCTCAAACATGATCTGGG-3. PCR products were analyzed on a 3.5% agarose gel.
Statistical analysis
The combination effect of HDAC inhibitors with proteasome inhibitors was analyzed by isobologram analysis using the CalcuSyn software program (Biosoft, Ferguson, MO) to determine whether the combination was additive or synergistic; a combination index (CI) < 1.0 indicates a synergistic effect.
Supplementary Material
Acknowledgements
This study was supported by the National Institute of Health Grants (SPORE-P50100707, P01 CA78378, R01 CA50947 (K.C.A.) and P50 CA086355 (R.M.). K.C.A. is an American Cancer Society Clinical Research Professor.
Footnotes
Conflict-of-Interest
T.H. and N.R. are consultants for Acetylon Pharmaceuticals. K.C.A. is a member of advisory committees for Onyx, Celgene, Gilead, and Sanofi-Aventis, and is a scientific founder of Acetylon and Oncopep. R.M. has financial interests in SHAPE Pharmaceuticals and Acetylon Pharmaceuticals. He is also the inventor on IP licensed to these two entities. RM's interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. P.G.R. is a member of advisory board for Celgene, Millennium, Johnson & Johnson, Novartis and Keryx. Other authors declare no competing financial interests.
Supplementary information is available at Leukemia's website (http://www.nature.com/leu).
References
- 1.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell. 2003 Dec 12;115:727–738. doi: 10.1016/s0092-8674(03)00939-5. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima H, Bradner JE, Wong JDC, Richardson P, Schreiber SL, et al. Small molecule inhibition of proteasome and aggresome function induces synergistic anti-tumor activity in multiple myeloma. Proc Natl Acad Sci USA. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119:2579–2589. doi: 10.1182/blood-2011-10-387365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raje N, Mahindra A, Vogl D, Voorhees P, W B, Hari P, et al. New drug partner for combination therapy in multiple myeloma (MM): development of ACY-1215, a selective histone deacetylase 6 inhibitor alone and in combination with bortezomib or lenalidomide. Haematologica. 2013;98(S1):320. [Google Scholar]
- 5.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci U S A. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimopoulos M, Jagannath S, Yoon S-S, Siegel D, Lonial S, Hajek R, et al. Vantage 088. Vorinostst in combination with bortezomib in patients with relapsed refractory multiple myeloma: results of a global randomized phase 3 trial. Blood. 2011;118:368–369. [Google Scholar]
- 7.Badros AZ, Vij R, Martin T, Zonder JA, Kunkel L, Wang Z, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia. 2013 doi: 10.1038/leu.2013.29. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakubowiak AJ, Siegel DS, Martin T, Wang M, Vij R, Lonial S, et al. Treatment outcomes in patients with relapsed and refractory multiple myeloma and high-risk cytogenetics receiving single-agent carfilzomib in the PX-171-003-A1 study. Leukemia. 2013 doi: 10.1038/leu.2013.152. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catley L, Weisberg E, Kiziltepe T, Tai YT, Hideshima T, Neri P, et al. Aggresome induction by proteasome inhibitor bortezomib and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108:3441–3449. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ocio EM, Vilanova D, Atadja P, Maiso P, Crusoe E, Fernandez-Lazaro D, et al. In vitro and in vivo rationale for the triple combination of panobinostat (LBH589) and dexamethasone with either bortezomib or lenalidomide in multiple myeloma. Haematologica. 2010;95:794–803. doi: 10.3324/haematol.2009.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minami J, Suzuki R, Mazitschek R, Gorgun G, Ghosh B, Cirstea D, et al. Histone deacetylase 3 (HDAC3) as a novel therapeutic target in multiple myeloma. Leukemia. 2013 doi: 10.1038/leu.2013.231. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baradari V, Hopfner M, Huether A, Schuppan D, Scherubl H. Histone deacetylase inhibitor MS-275 alone or combined with bortezomib or sorafenib exhibits strong antiproliferative action in human cholangiocarcinoma cells. World J Gastroenterol. 2007;13:4458–4466. doi: 10.3748/wjg.v13.i33.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigo-Brenni MC, Foster SA, Morgan DO. Catalysis of lysine 48-specific ubiquitin chain assembly by residues in E2 and ubiquitin. Molecular cell. 2010;39:548–559. doi: 10.1016/j.molcel.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.