Abstract
Alpha-synuclein (α-syn) is central to the pathogenesis of Parkinson disease (PD). Gene duplications, triplications and point mutations in SNCA1, the gene encoding α-syn, cause autosomal dominant forms of PD. Aggregated and post-translationally modified forms of α-syn are present in Lewy bodies and Lewy neurites in both sporadic and familial PD, and recent work has emphasized the prion-like ability of aggregated α-syn to produce spreading pathology. Accumulation of abnormal forms of α-syn is a trigger for PD, but recent evidence suggests that much of the downstream neurodegeneration may result from inflammatory responses. Components of both the innate and adaptive immune systems are activated in PD, and influencing interactions between innate and adaptive immune components has been shown to modify the pathological process in animal models of PD. Understanding the relationship between α-syn and subsequent inflammation may reveal novel targets for neuroprotective interventions. In this review, we examine the role of α-syn and modified forms of this protein in the initiation of innate and adaptive immune responses.
Structure of α-synuclein
Alpha-synuclein (α-syn) is a small 14kDa (140 amino acid) highly charged, presynaptic protein with a propensity to aggregate into oligomers of varying morphology [1–4]. It has the ability to reversibly associate with lipid vesicles based on its conformation and is commonly thought to have a role in pre-synaptic vesicle trafficking, although the precise mechanism is unknown[5, 6]. Soluble monomeric α-syn is thought to have little tertiary structure, folding as a random coil [7–9], but work by Bartels, et al. has suggested that stably folded soluble tetramers may be a native conformation in cells [10].
α-Syn has three domains: the N-terminal domain (aa 1–65), the non-amyloid-β component of plaques (NAC) domain (aa 66–95), and the C terminal domain (aa 96–140) [11]. The highly conserved N-terminal domain is composed of two amphipathic α-helices that allow for the reversible association with lipid membranes [12, 13]. The NAC domain is unique to α-syn, as it is not present in the two other members of the synuclein protein family, β-synuclein and γ-synuclein [14]. This domain was first discovered as the non-amyloid component of amyloid-β plaques in Alzheimer disease, and allows for fibrillization of α-syn through its ability to adopt a β-sheet conformation [15]. The highly variable acidic C-terminal domain differs in length and composition between species, contains many of the sites available for post-translational modifications of α-syn, and mediates many of α-syn’s protein:protein and SNARE complex chaperone interactions [16–22].
Genetics of α-synuclein in Parkinson Disease
Genetic variations in SNCA1, the gene encoding α-syn, cause familial Parkinson disease (PD) and are risk factors for sporadic PD. Rare missense point mutations in the N-terminal α-helices of α-syn, (A53T, A30P, E46K, but also the newly described H50Q, G51D and A53E) cause autosomal dominant familial PD and PD-like syndromes, presumably by directly altering the folding or clearance of the protein [23–28]. Additionally, gene multiplications of SNCA1 can be causative of familial PD in an α-syn dose-dependent manner. Patients with a gene dosage of ~1.5, or three copies of SNCA1, have a disease presentation similar to that of sporadic late-onset PD, while patients with a gene dosage of ~2.0, or four copies of SNCA1, tend to develop severe early-onset PD with extensive dementia and non-motor features [29–32]. These increased gene dosages lead to increased abundance of α-syn that can be measured directly in blood and other tissues [33]. Furthermore, increased α-syn mRNA and protein expression associates with an increased risk of sporadic late-onset PD [34–37]. A well-characterized mechanism for increasing α-syn mRNA and protein expression is increased length of the dinucleotide repeat polymorphism in the microsatellite Rep1 in the promoter region of SNCA1, which has been shown to be associated with an increased risk of PD [37, 38]. Together these data suggest that high levels of α-syn induce neurotoxicity sufficient to produce human PD.
α-Synuclein Pathology in Parkinson Disease
Accumulation of neuronal α-syn aggregates is thought to be a key process in PD pathogenesis and is promoted by the presence of high levels of α-syn [39, 40]. α-Syn aggregates are found throughout the substantia nigra pars compacta (SNpc) in PD, but can also be found in other neurons throughout the central nervous system (CNS) and peripheral nervous system (PNS). Regions affected in early PD include the spinal cord, medulla, olfactory bulbs, and autonomic nervous system; cortical involvement is seen in later-stage PD [41, 42]. While α-syn in is typically mostly soluble in normal brain, in PD the α-syn inclusions are detergent-insoluble [43, 44]. The largest and most highly organized forms of α-syn aggregate are Lewy bodies which can be either dense round bodies with a central core and peripheral halo, typically found inside the dopaminergic neurons of the substantia nigra, or a diffuse, less compact structures more typically found in the cortex [45]. These structures also involve the incorporation of ubiquitin as well as almost 300 other cellular proteins to the final structure [46]. Lewy bodies are most frequently found in the cell body, but can occasionally be found in the neurites [47]. Other neuritic pathology can be found including dystrophic Lewy neurites and smaller dot-like structures [45, 48]. These α-syn inclusions contain post-translationally modified α-syn, including phosphorylated and ubiquitinated forms [49].
Innate Immune System in Parkinson Disease
Microglia are the resident immune cell of the brain, and as such, function as the primary contributor towards innate immunity in the CNS [50]. They have important roles in exploring the cellular environment, phagocytosis, antigen processing and presentation, and production of cytokines and chemokines [50]. Microglia, while morphologically and functionally similar to circulating monocytes and tissue macrophages, derive from a different lineage, originating from the yolk sac and migrating into the brain early in development [51, 52]. Additionally, microglia express a distinct, transforming growth factor-β (TGF-β) dependent, gene signature from monocytes and macrophages both at baseline and when activated [53]. Maintenance of the microglial population does not require hematopoiesis [54].
Recent studies have highlighted the invasion of circulating monocytes into the CNS in pathological conditions, where they make unique and important contributions to inflammation and neural injury [55–59]. It is important to note that until recently, the techniques used to study activation of CNS microglia could not distinguish between resident brain microglia and other monocytic invaders. The “microglia” described in many earlier reports may actually represent a mixed population of resident brain microglia and invading peripheral monocytes that have differential effects on disease state.
The earliest evidence for the involvement of the immune system in PD pathogenesis came from the observation of activated microglia surrounding degenerating dopaminergic neurons in the SNpc of PD post-mortem brains [60]. In PD, the degree of microglial activation as assessed by staining for CD68 and human leukocyte antigen-DR (HLA-DR) is directly correlated with α-syn load in post-mortem brains, suggesting that α-syn may activate the innate immune system directly [61]. The presence of neuromelanin inside of activated microglia indicates that diseased dopaminergic neurons in the nigra are likely being phagocytosed, thereby allowing pathogenic α-syn to undergo processing and potential antigen presentation [62]. Furthermore, activated microglia in the midbrain and striatum can be seen in PD patients undergoing positron emission tomography (PET) imaging with [11C](R)-PK11195, a ligand for the peripheral benzodiazepine receptor, which is upregulated in activated microglia [63]. An increase in midbrain [11C](R)-PK11195 binding is inversely correlated to these patients’ motor function and the presence of the dopamine transporter in the striatum; in other words, activation of microglia is directly correlated to PD severity both clinically and pathologically [64]. Additionally, evidence from multiple studies suggests that some non-steroidal anti-inflammatory drugs (NSAIDs), particularly ibuprofen, may reduce the risk of developing PD [65–67]; however, this result is inconsistent. For example, a population cohort study of Danish older adults with severe osteoarthritis that have typically very high use of NSAIDs did not show any reduction in PD incidence [68]. It is possible then that the use of NSAIDs besides ibuprofen altered the magnitude of the effect, or that the elder age in which anti-inflammatory therapy was initiated was not sufficient to prevent the development of PD.
Activation of innate immunity leads to production of soluble mediators of the immune system, including chemokines, cytokines and the complement system, and these are found in increased abundance in the PD post-mortem brain. Pro-inflammatory cytokines and chemokines, particularly tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and interferon- γ (IFN-γ), are upregulated in both post-mortem brain and cerebrospinal fluid (CSF) from PD patients [69–72]. Cytokine and chemokine expression are also upregulated in peripheral blood mononuclear cells (PBMCs) in PD patients; monocyte chemoattractant protein (MCP-1), (chemokine CC-motif ligand 3) CCL3, CCL5, IFN-γ, IL-1β, IL-8 and TNF-α expression levels in PBMCs at baseline or stimulated with lipopolysaccharide (LPS) correlate with motor function as assessed by United Parkinson’s Disease Rating scale (UPDRS) III and Hoehn and Yahr (H&Y) stage [73]. Complement system components C3d, C4d, C7, and C9 have been found in association with degenerating neurons and α-syn inclusions in PD brain [74], and in the case of C1q, in association with activated microglia surrounding degenerating neurons [62]; some evidence also suggests that C1q, iC3b (an activated form of C3) and C9 are upregulated in PD patient brains [75, 76].
Genetic factors related to innate immunity predispose to PD. In 2010, the first noncoding single nucleotide polymorphism (SNP) in HLA-DR conferring risk for sporadic PD was found through a genome wide association study in late-onset idiopathic PD [77]. Other studies have confirmed that the HLA-DR region is associated with PD risk [78–86], and at least one study has suggested that there may be multiple risk alleles within the HLA-DR region [87]. Additional SNPs in other signaling pathways associated with immune system activation, including TNF-α, TNFR, IL-1α and β, IL-1RA, IL-2, IL-6, IL-8, IL-10, IL-17A, IL-18, IFN-γ, IFN-γR2, intercellular adhesion molecule-1 (ICAM-1), MCP-1, fractalkine (CX3CR1), CCR2, CCR3, CCR5, nuclear factor kappa light chain enhancer of B-cells (NFκB) and TGF-β, have been studied in PD patients with regards to both risk of developing disease and age of onset [88–115]. Many of these studies are small scale or show conflicting results between ethnic backgrounds; however, in Holmans 2013, pathway analysis in two independent genome wide association studies (GWAS) revealed a significant association between PD diagnosis and SNPs in pathways encoding cytokine-mediated signaling and regulation of leukocyte/lymphocyte activity [116].
Innate Immune System in Animal Models of Parkinson Disease
The innate immune system has a pathogenic role in both toxin-based and α-syn-based animal models of PD. Studies have shown extensive accumulation of activated microglia in the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) [117, 118], 6-OHDA (6-hydroxydopamine) [119], and rotenone toxin models of PD [120]. There is also significant microglial activation in α-syn-overexpression models of PD. In the mouse AAV-SYN model, which uses an adeno-associated virus (AAV) to overexpress α-syn via stereotaxic injection into the SNpc, there is prominent accumulation of ionized calcium-binding adaptor molecule 1(Iba1)+, CD11b+, CD68+ and major histocompatibility complex II (MHC-II)+ microglia in the SNpc starting as early as four weeks post-injection and extending to six months post-injection [121–123]. In the rat AAV-SYN model, there is microglial activation in the striatum along with cytokine expression, particularly TNF-α, IL-1β and IFN-γ [124]. In the Thy1-α-syn overexpressing mice which overexpresses α-syn under the thymocyte antigen 1 (Thy1) promoter, there is Iba1+ microgliosis exhibited in the striatum starting as early as one month of age, and extending to 14 months of age. At 14 months of age, there were also prominent MHC-II+ microglia in the striatum. In the substantia nigra, activated Iba1+ microglia were present at 6 months of age; this activation receded by 14 months of age. In the cortex, activated Iba1+ microglia were only present at 14 months of age. There was no activation in the cerebellum [125]. These results show that microglial activation is triggered asynchronously in the Thy1-α-syn mice and therefore, may reflect differences between regions of the nigrostriatal system in both the events leading to immune system activation and the maintenance of an immunological reaction in response to α-syn overexpression [125].
Suppressing microglial activation can inhibit neurodegeneration. In both 6-OHDA and MPTP mouse models of PD, dopaminergic neurodegeneration is ameliorated by the administration of minocycline, a tetracycline derivative that inhibits microglial activation [126, 127]. Furthermore, in PD animal models, cytokines are instrumental in mediating dopaminergic neuron loss. In both LPS and 6-OHDA rat models of PD, inhibition of TNF-α signaling has been shown to ameliorate both neuroinflammation and dopaminergic cell loss [128–130]. In an LPS mouse model, IL-1 knockout mice were protected from neuroinflammation and loss of motor function as assessed by rotarod [131]. Interestingly, TNF knockouts did not show improved motor function; however, this study did not directly measure neuron loss [131]. In mice given a single intraperitoneal injection of a sub-neurotoxic dose of MPTP, knocking out TNF-α led to a partial reduction of microglial activation, while knocking out IFN-γ was sufficient to completely ameliorate microglial activation [132]. No studies have been completed in α-syn models on the role of particular cytokines in neurodegeneration.
Activation of Innate Immunity by α-synuclein
Microglia are intimately involved with maintaining their microenvironment’s homeostasis [50]. When stimulated, microglia can alter their morphology, chemotactically move towards areas of inflammation, phagocytose pathogens or cellular debris, release pro-inflammatory or anti-inflammatory cytokines, release chemokines, and present antigen to T-lymphocytes [50].
Microglia have the ability to phagocytose extracellular aggregated α-syn from their environment and target it to the light chain 3B (LC3B)+ autophagosome for degradation [133]. Additionally, the Fc-γ Receptor (FcγR) is required for uptake of extracellular aggregated α-syn and engagement of downstream NFκB-dependent signaling cascades, including chemokine production [133]. FcγR−/− mice are protected from the neuroinflammation and neurodegeneration after AAV-mediated α-syn overexpression, suggesting that phagocytosis of aggregated α-syn into microglia is important for inducing an immune response that leads to neurodegeneration [122].
Although the evidence for phagocytosis of α-syn by microglia is clear, there is conflicting data as to whether α-syn itself can directly activate microglia and macrophages. Several studies suggest that α-syn can elicit the production of pro-inflammatory cytokines in microglia or monocytes. Treatment of BV2 cells or primary microglia with aggregated α-syn led to the production of the pro-inflammatory cytokines, TNF-α and IL-1β [134, 135]. Treatment of primary microglia with aggregated nitrated α-syn also increased production of TNF-α, IL-1β, and additionally, MCP-1, and IFN-γ [136]. Treatment of THP-1 cells, a human monocytic cell line, with monomeric wild-type α-syn led to the secretion of IL-1β; additionally, monomeric A53T mutant α-syn led to the secretion of both TNF-α and IL-1β [137]. Furthermore, treating THP-1s with IFN-γ in addition to monomeric α-syn potentiated pro-inflammatory cytokine secretion [137]. In Codolo et al (2013), human monocytes were found to transcribe pro-IL-1β and produce increased reactive oxygen species (ROS) production in response to both monomeric and fibrillar α-syn; however, only aggregated α-syn treatment allowed for the release of mature IL-1β [138]. Taken together, these studies suggest that modified α-syn leads to microglial or monocytic activation, with the production of the corresponding pro-inflammatory cytokines.
On the other hand, several studies have failed to report cytokine upregulation in microglia in response to α-syn despite other α-syn-mediated effects. For example, upregulation of superoxide and ROS was observed in response to phagocytosis of aggregated α-syn in rat primary microglia without a change in TNF-α production [139]. Similarly, Cao 2012 showed that treating murine primary microglia with aggregated α-syn led to a limited NF-κB-dependent chemokine response with detectable increases of IL-1α, IP-10, CCL-3, CCL-4, chemokine CXC motif ligand 2 (CXCL2), and MCP-1, but not TNF-α or IL-6 [133]. Furthermore, FcγR−/− microglia did not exhibit this cytokine and chemokine response to aggregated α-syn, supporting this idea that phagocytosis of α-syn is required [133]. Although microglia treated with aggregated α-syn increased antigen processing and MHC-II expression, a robust MHC-II-dependent cytokine response to aggregated α-syn was only induced in primary microglia by co-culturing them with T-cells [123].
Several different receptor systems have been implicated in the uptake of α-syn into microglia and macrophages. In addition to FcR-mediated phagocytosis, toll-like receptor (TLR) distribution is modulated in response to α-syn treatment [140]. In murine primary microglia pretreated with wild-type oligomeric α-syn, TLR-2 is upregulated, while TLR-3 and 7 are downregulated [140]. Furthermore, TLR2 deficient (−/−) microglia do not express TNF-α or IL-1β in response to treatment with conditioned media from SH-SY5Y cells overexpressing α-syn [141]. Complement and its receptors, particularly C3 and CR3, could be involved in the uptake of α-syn into microglia. C3 and CR3 are involved in the phagocytosis and lysosomal targeting of fibrillar amyloid-β, which facilitates its clearance [142]. No similar studies have been conducted with α-syn. Finally, exosomal transport of α-syn is known to occur in SH-SY5Y cells in a calcium-dependent manner, and can be upregulated in states of lysosomal dysfunction [143, 144]. Furthermore, α-syn oligomers have been found on both the inside and outside of the exosome [145], and there is an increased amount of α-syn in plasma exosomes in PD patients [146]. Exosomal transport and reuptake is hypothesized to be a mechanism of transferring toxic species of α-syn between neurons in synucleinopathies like PD [147]. It is likely that oligomeric α-syn from neurons could be transmitted to microglia in the same manner.
Several biological factors may contribute to the observed experimental variability in the response of isolated microglia and macrophages to α-syn. The activation state of microglia is regulated differently from their monocytic counterparts due in part to their contact with neurons via the CD200/CD200R and CX3CL1/CX3CR1 ligand/receptor interactions, and this may contribute to some of the difference between microglia and monocytes, and between in vitro and in vivo settings [148]. Microglia and macrophages also have different programs for activation, depending on the stimulus to which they are responding [149]. Typically associated with infectious pathogens, “classical” M1 activation is characterized by the expression of pro-inflammatory cytokines, TNF-α, IL-1, and IL-6, and expression of iNOS [149]. “Alternative” M2 activation is characterized by expression of IL-10 and arginase 1 and is typically associated with a tissue repair response [149]. While these two cellular programs illustrate how polarized microglia can be in response to particular stimuli, in reality, microglia can express phenotypes along this spectrum, and may only be partially activated in response to α-syn [149].
An additional source of variability in these studies likely comes from the conformation of α-syn used for treatment of microglia and monocytes. Aggregated α-syn can exist in a wide variety of shapes, each with potentially different toxicity to neurons and potentially different effects on microglia [4, 150–154]. Most experiments are conducted with α-syn aggregates formed in vitro, and depending on the details of the method used to prepare them may contain a variety of structures in varying concentrations with varying molecular properties. Further, the relationship between these artificially produced conformers of α-syn and the aggregated forms present in human PD is still uncertain.
Adaptive Immune System in Parkinson Disease
The adaptive immune system is characterized by specific responses to foreign proteins acting as antigens, and requires the recognition of antigens by T-cells and the subsequent activation of B-cells to differentiate and produce antibodies [155]. In PD patient post-mortem brain, there is a 10-fold greater infiltration of CD4+ and CD8+ T-lymphocytes into the SNpc of PD patients compared to age-matched controls [156]. In peripheral blood, abnormalities in the lymphocytes of PD patients are found as well. In addition to peripheral innate immune dysfunction, as evidenced by increased neutrophils and natural killer (NK) cells, there are decreased numbers of both T and B-lymphocytes. CD4+ T-cells are particularly reduced in the blood when compared to CD8+ T-cells, which are unchanged [157–159]. The CD4+ reduction is correlated with UPDRS III performance in PD patients [158]. Characterization of CD4+ peripheral T-cells from PD patients shows that they are likely Th1 cells, as the ratio of IFN-γ:IL-4-producing cells is increased [157]. Furthermore, various surface markers are altered and correlate with disease state as assessed by UPDRS III [160]. The CD45RO+ subset is increased and positively correlated with UPDRS III, while the CD45RA+ subset is decreased; FasL is increased; CD31 is decreased and negatively correlated with UPDRS III; α4β7 integrin is decreased and negatively correlated with UPDRS III [160]. These results suggest that peripheral T-lymphocytes in PD are activated, effector/memory cells with a Th1 phenotype. Furthermore, these T-cells are likely undergoing activation-induced Fas-mediated apoptosis, leading to their decrease in number [160]. Decreases in α4β7 integrin on CD4+ T-cells could signal a relative increase in brain-homing function or an active immune response in the gut sequestering CD4+ α4β7+ T-cells from peripheral blood [160]. Indeed, T-cell protein expression may be used as a biomarker for PD in the future; a panel of 13 proteins expressed in T-lymphocytes was quantified by multiple reaction monitoring and validated as PD-specific in a small blinded cohort [161].
An interesting subset of T-cells, the γδ T-cell, is found upregulated in PD CSF [162]. These T-lymphocytes have both innate and adaptive functions and a different antigen repertoire than their CD4+ and CD8+ counterparts, as their immunological memory is independent of MHC-I or MHC-II [163]. Rather, they are activated by a variety of proteins, including ones expressing pathogen and damage associated molecular patterns and non-peptide phospho-antigens typically upregulated under cellular stress [163].
B-cells have not yet been observed in PD patient brain; however, immunoglobulins (Ig) are plentiful. IgG, but not IgM, is deposited in the SNpc on dopaminergic neurons in PD patient post-mortem brain, and it colocalizes with α-syn aggregates [164]. IgG staining is predominantly IgG1, though there is some IgG3 and less IgG2 [164]. IgG staining is positively correlated to the number of activated monocytes and extent of neurodegeneration, suggesting a possible role in pathogenesis [164]. Additionally, FcγRI and FcγRIII, receptors for the IgG, were found on activated monocytes and lymphocytes, respectively, in the SNpc of PD patient brain, but not in visual cortex of PD patients or in age-matched control SNpc [164]. Indeed, the IgG seen in PD brain is likely neurotoxic; IgG isolated from PD patient brain stereotaxically injected into rat brain is sufficient to induce specific neurodegeneration of 35% of SN neurons only 4 weeks post-injection [165]
Adaptive Immune System in Models of Parkinson Disease
Increased numbers of T-cells are found in both toxin models and α-syn models of PD and appear to play specific roles in neurodegeneration [121, 125, 166]. Indeed, in an MPTP mouse model of PD, CD4−/− animals showed attenuation of neurodegeneration, whereas CD8a−/− animals had no effect on neurodegeneration [156]. In passive transfer studies into Rag1−/− mice, it was further discovered that CD4+ T-cells acted in a FasL-dependent, IFN-γ-independent manner to mediate MPTP toxicity to dopaminergic neurons [156]. These results conflict with a study in IFN-γ −/− mice that show reduced dopaminergic toxicity in an MPTP model [167]. In vitro work suggests that dopaminergic toxicity may depend on the effects of IFN-γ on microglia in neurodegeneration. When microglia lacked an IFN-γ receptor, neurons did not die in response to rotenone and exogenous IFN-γ; however, when neurons lacked an IFN-γ receptor, they were still vulnerable, suggesting that microglia necessarily mediates their dopaminergic neurotoxicity via IFN-γ, as suggested previously [167]. T-cells are not the only source of IFN-γ in the CNS; IFN-γ is made in response to TNF-α in astrocytes [168], and microglia themselves can produce IFN-γ in response to infection, LPS or particular cytokines [169]. Recent work by Cebrian et al. shows that treatment of primary microglia with neuromelanin, α-syn, nitrated α-syn, and mutated α-syn led to increased microglial expression of IFN-γ [170]. In this study, IFN-γ expression was able to induce surface MHC-I expression and antigen processing in catecholaminergic neurons, allowing them to be selectively targeted for degeneration in vitro by CD8+ T-cells [170].
Work by Sanchez-Guajardo et al shows that microglial phenotype and T-cell infiltration in the rat AAV-SYN model of PD are dependent on whether the α-syn transgene injected is sufficient to cause cell loss by 4 weeks post-injection, or whether the transgene only leads to a decrease in striatal TH+ fiber density without a corresponding cell loss up to 15 weeks post-injection. Rats injected with the AAV-SYN that leads to a decrease in striatal TH+ fiber density show an increase in MHCII+ microglia in both the substantia nigra and striatum, whereas rats injected with the AAV-SYN that leads to cell loss show an increase in CD68+ and MHCII+ microglia that they describe as being similar in appearance to peripherally-derived macrophages [171]. Additionally, rats injected with the AAV-SYN that leads to cell loss show increased infiltration of both CD4+ and CD8+ T-cells into the substantia nigra 8 weeks post-injection, whereas rats injected with the AAV-SYN that leads to striatal fiber loss do not [171]. They hypothesize that the differences between these two AAV-SYN viruses is the expression level of α-syn per cell [171]. Thus, it is interesting to note that the type and degree of both the innate and adaptive immune responses change in vivo depending on the degree of expression of α-syn, thereby suggesting that α-syn itself drives the inflammatory response.
Role of α-synuclein in Activation of Adaptive Immunity
The evidence for activation of adaptive immunity in PD is clear, but a critical question is the nature of the antigens responsible. This raises an obvious and important question: is α-syn the source of the antigens that trigger adaptive immune responses in PD?
There is certainly evidence for the ability of modified forms of α-syn to modulate adaptive responses in animal model systems. In the MPTP model of PD, adoptive transfer of T-cells from mice immunized to the nitrated C-terminus of α-syn to mice administered MPTP leads to increased neurodegeneration [172]. Additionally, when T-cells responsive to the nitrated C-terminus of α-syn were polarized to the Th1, Th2 and Th17 subtypes prior to adoptive transfer, both pro-inflammatory Th1 and Th17 subtypes were found to enhance neurodegeneration in response to MPTP, with Th17 cells having a greater effect than Th1 cells[173]. Cells of the Th2 subtype had no effect. Furthermore, adoptive transfer of a Treg-enriched population protected the mice from neurodegeneration in response to MPTP [173]. These results show that changing which T-cell subsets exhibit an α-syn-induced immune response can also dramatically change the extent of dopaminergic neurodegeneration.
α-Synuclein as a Potential Self-antigen in Parkinson Disease
If a peptide from α-syn is indeed a self-antigen triggering T-cell activation via MHC-II, the degree of the subsequent immune response is likely controlled by the precise conformation of α-syn undergoing antigen processing. The conformation of α-syn in PD is dependent on both aggregation and on post-translational modifications, including phosphorylation at S129 and other residues, ubiquitination of lysines, nitration of tyrosines, and various truncations at the C-terminus [49, 174–176]. Not only are these post-translational modifications of α-syn more frequently found in PD brain and models of PD than in control brain, but they also affect α-syn’s toxicity, oligomerization, and immunogenicity [177–185]. For example, S129 phosphorylation is a post-translational modification of α-syn that affects its toxicity in animal models [186]. Less than 4% of total α-syn is phosphorylated at S129 in normal human brain, but around 90% of α-syn in Lewy bodies is phosphorylated at S129 [49, 187]. Dephosphorylating S129 by increasing the activity of phosphoprotein phosphatase 2A led to fewer α-syn aggregates, reduced inflammation and improved motor behavior in the Thy1-α-syn overexpressing mouse model of PD [188]. It has been shown previously that T-cells in the CNS have the ability to distinguish between de-phosphorylated and phosphorylated self-antigens [189, 190]. Particularly in the case of αB-crystallin, a putative self-antigen in multiple sclerosis, stress-induced phosphorylation at S45 is included within the dominant peptide expressed in the MHC-I cleft, and the phosphate group within the antigen comes into direct contact with the T-cell receptor [189]. Similar to the phosphorylation of S45 in αB-crystallin, the phosphorylation of α-syn at S129 is relatively rare and induced in disease [49, 187]. Thus, these epitopes may not exist in the thymus when negative selection occurs, and therefore may be erroneously recognized as foreign antigens [191].
Another way to change the antigenicity of a protein is by changing how it is degraded in the lysosome and processed for antigen presentation [191]. For example, in Rasmussen’s encephalitis, the glutamate receptor subunit 3 (GluR3) is presented as an antigen only when not glycosylated [192]. Glycosylation of GluR3 occurs at a Granzyme B cleavage site, and when glycosylated, the protease is unable to access its cleavage site during antigen processing [192]. Similarly, it is known that aggregated forms of α-syn are resistant to chaperone-mediated autophagy [193]. This would thereby significantly alter the repertoire of peptides produced that could be presented as antigen [191]. C-terminally truncated forms of α-syn and the C-terminal fragments are some of the conformations produced due to incomplete α-syn degradation [177, 194–204]. Furthermore, C-terminal truncation mutants of α-syn have been identified in Lewy bodies and whole PD brain [202, 205, 206] and are known to increase α-syn aggregation and neuropathology in both cell culture systems and animal models [207–215]. In order to directly address whether modifying C-terminal truncation leads to improved outcomes in vivo, Games, et al. passively immunized mice to α-syn using antibodies designed to bind C-terminal fragments and prevent C-terminal cleavage [178]. Administration of these antibodies ameliorated aggregation, cell loss, and loss of motor function in the Thy1-α-syn model of PD [178]. It is therefore possible that these toxic C-terminal fragments and the truncated α-syn itself are some of the newly exposed antigens induced by altered α-syn processing in the lysosome.
Finally, conformation of α-syn could modify antigen presentation by changing the affinity of α-syn peptides for binding MHC-II [191]. In mice containing the HLA-DRB1*0401 MHC-II molecule associated with rheumatoid arthritis, citrullinated peptides have an increased affinity for binding MHC-II compared to their wild-type counterparts; this increased affinity is sufficient to produce activated CD4+ T-cells sensitized to citrullinated proteins [216]. Similarly, sensitization of CD4+ T-cells to nitro-tyrosine residues has been observed directly in mice in a residue-specific and MHC-II haplotype-specific manner [217]. Nitration of tyrosine residues occurs during both mitochondrial dysfunction and inflammation [218, 219]. α-Syn can be nitrated at its tyrosine residues[179], and is present in its nitrated form in disease models and human PD[220, 221]; presumably nitration of α-syn arises from activation of inducible nitric oxide synthase and production of reactive nitrogen species [222]. Direct administration of nitrated α-syn to the substantia nigra of rats leads to dose-dependent nigral degeneration and behavioral deficits, suggesting that the effect of nitrated α-syn is sufficient alone to induce degeneration [180]. Work reviewed above shows that immunization to nitrated α-syn modifies disease course in a T-cell subset specific manner in an MPTP model of PD [172, 173]. However, whether similar immunity can be induced to other modified forms of α-syn is yet unknown.
Alpha-synuclein-driven Inflammation in the Peripheral Nervous System
Although usually considered to be a brain disease, increasing evidence suggests that PD involves extracranial neurons as well, and may even begin in non-CNS neurons. Based on pathological studies of sporadic PD, Braak et al have proposed that α-syn aggregation and Lewy pathology initiates in the enteric nervous system [41, 42]. In this hypothesis, oligomeric α-syn propagates in a prion-like manner upwards via the vagus nerve to the brainstem, into the nigra and beyond to the cortex [223]. Indeed, α-syn aggregation can be found in the myenteric and submucosal plexuses throughout the entire enteric nervous system from the esophagus to the rectum in PD patients [223–227]. There is increased intestinal permeability to sucralose in PD patients, and this correlates with increased E. coli, 3-nitro-tyrosine and α-syn staining in the intestinal mucosa [228]. Given the intensity of the α-syn pathology, it is not surprising that inflammation is active in the gut as well. In ascending colon biopsies from PD patients, TNF-α, IL-1β, IL-6, IFN-γ and Sox10 mRNA were upregulated in PD patients and correlated with disease duration [229].
Although defining macrophage activation status, particularly in relation to enteric neurons with α-syn accumulation, in human intestinal biopsies in PD has not been done, two classes of macrophages surround α-syn+ enteric neurons in aged rats, CD163+ phagocytic macrophages laden with α-syn, and other classically activated MHC-II+ macrophages, also capable of phagocytizing α-syn, but not in the same quantities [230]. Although neither set of macrophages was preferentially found around α-syn accumulations in aged rats, there were classically activated MHC-II+ macrophages surrounding and invading ganglions with neurons expressing large amounts of α-syn, and circulating monocytes were also seen entering the area [230]. Together these results suggest the possibility that the macrophages of the gut initiate the adaptive immune response to α-syn.
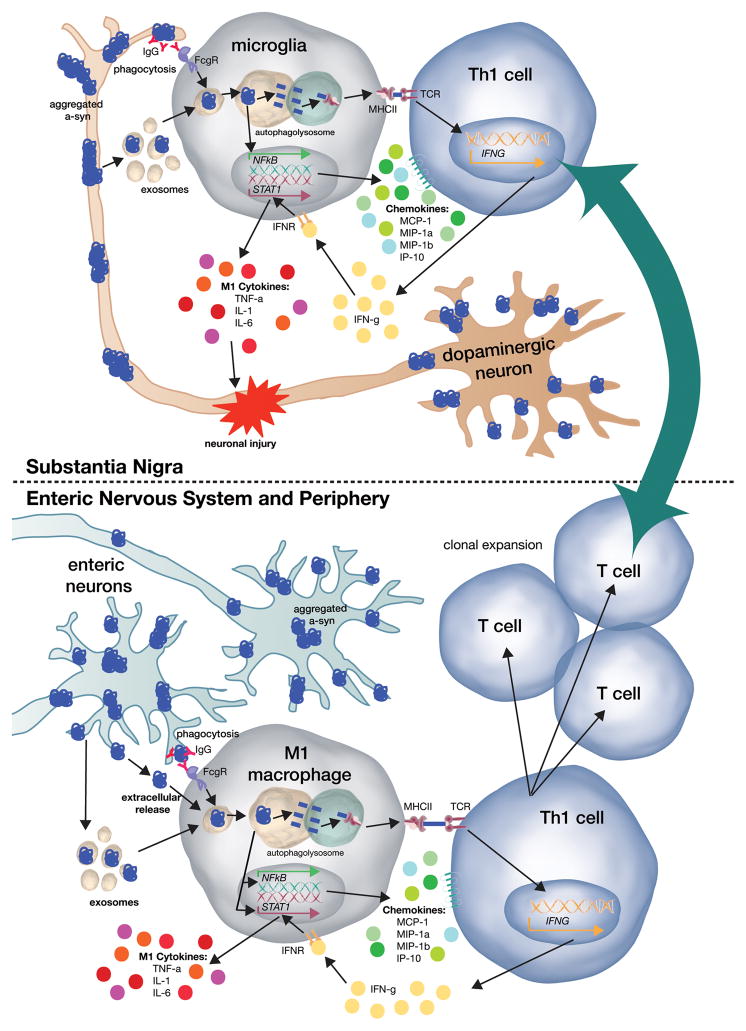
These observations lead to the question of whether α-syn pathology in the PNS may precede and even trigger the pathological and inflammatory changes in the brain which are characteristic of PD. Is it possible that abnormal forms of α-syn in the periphery trigger a systemic adaptive immune response which in turn promotes CNS innate and adaptive responses? Viewed this way, abnormal forms of α-syn in the PNS could induce a specific, systemic, immunological memory that enhances CNS inflammation. This memory could then be engaged everywhere that abnormal forms of α-syn are present (Figure 1). A self-perpetuating cycle of α-syn aggregation, nitration and oxidation of α-syn, inflammation and neurodegeneration may then occur.
Figure 1. Model for α-synuclein-induced immune system activation in PD.
α-Synuclein (α-syn) aggregates and other pathological conformations of α-syn form in the dopaminergic neurons of the substantia nigra during PD. Neighboring microglia take up this α-syn by either phagocytosis or fusion of α-syn-containing exosomes. Once inside the microglia, aggregated α-syn is targeted to the autophagolysosome, where it can be processed into peptides and loaded onto the MHC-II complex. The α-syn-loaded MHC-II traffics to the cell membrane, allowing for antigen presentation to CD4+ T-cells. Additionally, when α-syn enters the microglia, it induces NFκB-dependent expression of chemokines, which allows for migration of white blood cells, including T-cells, towards the site of injury. Once CD4+ T-cells enter the CNS, they are able to bind MHC-II via their T-cell receptor and thus, begin to release IFN-γ. IFN-γ then binds to its receptor on microglia, thereby inducing STAT-1-mediated pro-inflammatory cytokine expression. These cytokines then injure dopaminergic neurons and induce cell death.
A similar process may occur in the enteric nervous system. Enteric neurons develop α-syn aggregates, and can release them to resident macrophages in one of three ways. First, α-syn is regularly phagocytosed by resident gut macrophages. Secondly, α-syn can be exocytosed, and finally, α-syn can be exosomally released. When primed M1-polarized macrophages take up aggregated α-syn, it is also targeted to the autophagolysosome, where it can be processed into peptides and loaded onto MHCII. In addition, α-syn then induces expression of both pro-inflammatory cytokines and chemokines. The CD4+ T-cell that binds to α-syn-loaded MHC-II will then activate and clonally expand. These T-cells would then be directing a body-wide specific immune response to α-syn and will respond quickly throughout the CNS as α-syn pathology spreads.
Immune system function in mice lacking α-synuclein
A few roles for native α-syn have been discovered within immune cells themselves by examining α-syn knockout mice. α-Syn null microglia have a ramified structure with increased vacuoles compared to wildtype [231]. They have increased production of TNF and IL-6 in response to LPS stimulation, but demonstrate a decreased capacity for phagocytosis [231]. Additionally, α-syn has recently been shown to be involved in B-cell lymphopoiesis [232]. In α-syn knockout mice, B-cells were decreased about four fold in bone marrow [232]. Spleen and lymph node architecture was compromised; with reduced follicle size in the spleen, and reduced number of follicles in lymph nodes [232]. Furthermore, the quantity of circulating IgG was reduced in α-syn knockout mice compared to wildtype, whereas the amount of circulating IgM remained the same [232]. In response to a T-cell dependent antigen, antigen-specific IgG1 and IgG2b was not produced; only IgM was, suggesting a deficit in class-switching in the absence of α-syn [232]. While these results show a potential role for native α-syn in antigen presenting cells, it is unclear to what extent these activities are active in α-syn-based PD models or PD itself. They may be relevant to some proposed treatment strategies that would seek to reduce α-syn expression in PD patients.
Antigen Presentation in PD
Antigen presentation of α-syn in PD would involve antigen processing and presentation by MHC-II complexes to receptive T-cell populations. A model for how α-syn induces both an innate and adaptive immune response in PD is presented in Figure 1. All of the components required for this model are present in the PD brain: monocytes phagocytose α-syn aggregates, express MHC-II, and can interact with CD4+ T-cells [123, 133, 138]. It is possible that antigen presentation also occurs in the enteric nervous system; intestinal macrophages can and do phagocytose α-syn aggregates, express MHC-II and can also interact with CD4+ T-cells [230]. In either case, aggregated or modified α-syn is likely the antigen responsible, but proof that this is the case in human PD is still lacking. Determining which peptides from α-syn can be loaded into MHC-II, and whether this process is modified by fibrillization or post-translational modifications typically seen in human PD is an important question. Detailed studies of the adaptive immune response and the use of new sequence-based techniques to explore the nature of the T-cell repertoire in PD will be necessary to shed light on the precise mechanism of an α-syn-specific adaptive immune response.
Conclusion
Multiple lines of evidence implicate activation of both innate and adaptive immune systems in PD. This inflammatory response plays an essential role in neurodegeneration. The evidence reviewed here implicates α-syn itself as the primary trigger of the immune response in PD. While modification of α-syn by nitration elicits a stronger immune response than aggregation alone, it is likely that aggregation is sufficient to induce the inflammation seen in PD. This concept has implications for both the prevention and treatment of PD. In the prodromal phase, thought to exist for at least a decade before motor symptoms appear, therapies that prevent the formation of altered α-syn may delay or even prevent the onset of symptoms. Clinical trials using antibodies targeting α-syn are already in development to test this hypothesis. A particularly interesting approach is the two AFFITOPE vaccines already at stage one clinical trials that attempt to halt α-syn aggregation by using six-amino-acid peptides in the α-syn sequence to direct an immune response towards eliminating α-syn itself in the PD brain. It will be interesting to see how this active vaccination therapeutic strategy interacts with both the neuroinflammation and neurodegeneration seen in PD. As seen with Alzheimer’s disease trials targeting amyloid-β, one difficulty with this strategy will be detecting PD early enough to prevent disease progression. After the onset of motor symptoms and the spread of α-syn pathology, the inflammatory response to α-syn continues to be a driver of neurodegeneration. In this phase, strategies to reduce α-syn aggregation may not be as useful since propagation of pathogenic α-syn has already occurred, but therapies directly targeting neuroinflammation may continue to be useful in halting progression of disease. The realization that abnormal forms of α-syn leads to robust neuroinflammatory responses that involve both CNS and peripheral immune cells and potentiate neurodegeneration offers a novel set of drug targets in PD, some of which may exist in the periphery in addition to the brain.
Acknowledgments
The authors would like to thank Dr. Ashley S. Harms for a critical reading of the manuscript and Drs. Luk Cox and Idoya Lahortiga from Somersault 18:24 for use of their Library of Science and Medical Illustrations (http://www.somersault1824.com/resources/) in the making of the figure. Funding support from the Parkinson Association of Alabama and the National Institute of Neurological Disorders and Stroke via F31-NS076017 was also greatly appreciated.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 2.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 4.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 5.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 6.Bellani S, Sousa VL, Ronzitti G, Valtorta F, Meldolesi J, Chieregatti E. The regulation of synaptic function by alpha-synuclein. Commun Integr Biol. 2010;3:106–109. doi: 10.4161/cib.3.2.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 8.Burre J, Vivona S, Diao J, Sharma M, Brunger AT, Sudhof TC. Properties of native brain alpha-synuclein. Nature. 2013;498:E4–6. doi: 10.1038/nature12125. discussion E6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 10.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 13.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 14.George JM. The synucleins. Genome Biol. 2002;3:REVIEWS3002. doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 16.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J Biol Chem. 2000;275:390–397. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- 18.Jensen PH, Islam K, Kenney J, Nielsen MS, Power J, Gai WP. Microtubule-associated protein 1B is a component of cortical Lewy bodies and binds alpha-synuclein filaments. J Biol Chem. 2000;275:21500–21507. doi: 10.1074/jbc.M000099200. [DOI] [PubMed] [Google Scholar]
- 19.Kim TD, Paik SR, Yang CH. Structural and functional implications of C-terminal regions of alpha-synuclein. Biochemistry. 2002;41:13782–13790. doi: 10.1021/bi026284c. [DOI] [PubMed] [Google Scholar]
- 20.Kim TD, Paik SR, Yang CH, Kim J. Structural changes in alpha-synuclein affect its chaperone-like activity in vitro. Protein Sci. 2000;9:2489–2496. doi: 10.1110/ps.9.12.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SM, Jung HY, Kim TD, Park JH, Yang CH, Kim J. Distinct roles of the N-terminal-binding domain and the C-terminal-solubilizing domain of alpha-synuclein, a molecular chaperone. J Biol Chem. 2002;277:28512–28520. doi: 10.1074/jbc.M111971200. [DOI] [PubMed] [Google Scholar]
- 22.Souza JM, Giasson BI, Lee VM, Ischiropoulos H. Chaperone-like activity of synucleins. FEBS Lett. 2000;474:116–119. doi: 10.1016/s0014-5793(00)01563-5. [DOI] [PubMed] [Google Scholar]
- 23.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 24.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 25.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 26.Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, Aasly JO, Rajput A, Rajput AH, Jon Stoessl A, Farrer MJ. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord. 2013;28:811–813. doi: 10.1002/mds.25421. [DOI] [PubMed] [Google Scholar]
- 27.Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, Pieri L, Madiona K, Durr A, Melki R, Verny C, Brice A French Parkinson’s Disease Genetics Study G. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. 2013;73:459–471. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- 28.Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, Tienari PJ, Poyhonen M, Paetau A. A novel alpha-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson’s disease-type pathology. Neurobiol Aging. 2014;35:2180 e2181–2185. doi: 10.1016/j.neurobiolaging.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 29.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 30.Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 31.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 32.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 33.Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Singleton AB. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004;62:1835–1838. doi: 10.1212/01.wnl.0000127517.33208.f4. [DOI] [PubMed] [Google Scholar]
- 34.Chiba-Falek O, Lopez GJ, Nussbaum RL. Levels of alpha-synuclein mRNA in sporadic Parkinson disease patients. Mov Disord. 2006;21:1703–1708. doi: 10.1002/mds.21007. [DOI] [PubMed] [Google Scholar]
- 35.Kingsbury AE, Daniel SE, Sangha H, Eisen S, Lees AJ, Foster OJ. Alteration in alpha-synuclein mRNA expression in Parkinson’s disease. Mov Disord. 2004;19:162–170. doi: 10.1002/mds.10683. [DOI] [PubMed] [Google Scholar]
- 36.Hansson O, Hall S, Ohrfelt A, Zetterberg H, Blennow K, Minthon L, Nagga K, Londos E, Varghese S, Majbour NK, Al-Hayani A, El-Agnaf OM. Levels of cerebrospinal fluid alpha-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res Ther. 2014;6:25. doi: 10.1186/alzrt255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuchs J, Tichopad A, Golub Y, Munz M, Schweitzer KJ, Wolf B, Berg D, Mueller JC, Gasser T. Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J. 2008;22:1327–1334. doi: 10.1096/fj.07-9348com. [DOI] [PubMed] [Google Scholar]
- 38.Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, Hulihan MM, Aasly JO, Ashizawa T, Chartier-Harlin MC, Checkoway H, Ferrarese C, Hadjigeorgiou G, Hattori N, Kawakami H, Lambert JC, Lynch T, Mellick GD, Papapetropoulos S, Parsian A, Quattrone A, Riess O, Tan EK, Van Broeckhoven C Genetic Epidemiology of Parkinson’s Disease C. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- 39.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 40.Zach S, Bueler H, Hengerer B, Gillardon F. Predominant neuritic pathology induced by viral overexpression of alpha-synuclein in cell culture. Cell Mol Neurobiol. 2007;27:505–515. doi: 10.1007/s10571-007-9141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages) J Neurol. 2002;249(Suppl 3):III/1–5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 42.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 43.Cantuti-Castelvetri I, Klucken J, Ingelsson M, Ramasamy K, McLean PJ, Frosch MP, Hyman BT, Standaert DG. Alpha-synuclein and chaperones in dementia with Lewy bodies. J Neuropathol Exp Neurol. 2005;64:1058–1066. doi: 10.1097/01.jnen.0000190063.90440.69. [DOI] [PubMed] [Google Scholar]
- 44.Campbell BC, McLean CA, Culvenor JG, Gai WP, Blumbergs PC, Jakala P, Beyreuther K, Masters CL, Li QX. The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J Neurochem. 2001;76:87–96. doi: 10.1046/j.1471-4159.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- 45.Irizarry MC, Growdon W, Gomez-Isla T, Newell K, George JM, Clayton DF, Hyman BT. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson’s disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Leverenz JB, Umar I, Wang Q, Montine TJ, McMillan PJ, Tsuang DW, Jin J, Pan C, Shin J, Zhu D, Zhang J. Proteomic identification of novel proteins in cortical lewy bodies. Brain Pathol. 2007;17:139–145. doi: 10.1111/j.1750-3639.2007.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I. Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 48.Braak H, Sandmann-Keil D, Gai W, Braak E. Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by alpha-synuclein immunocytochemistry. Neurosci Lett. 1999;265:67–69. doi: 10.1016/s0304-3940(99)00208-6. [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM, Trojanowski JQ, Mann D, Iwatsubo T. Phosphorylated alpha-synuclein is ubiquitinated in alpha-synucleinopathy lesions. J Biol Chem. 2002;277:49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- 50.Katsumoto A, Lu H, Miranda AS, Ransohoff RM. Ontogeny and Functions of Central Nervous System Macrophages. J Immunol. 2014;193:2615–2621. doi: 10.4049/jimmunol.1400716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 53.Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 55.Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, Hayakawa Y, Tatezawa R, Inui A, Fujimiya M. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PLoS One. 2013;8:e81744. doi: 10.1371/journal.pone.0081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM, Huang AY, Silver J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol. 2014;254:109–120. doi: 10.1016/j.expneurol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 61.Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Depboylu C, Schafer MK, Arias-Carrion O, Oertel WH, Weihe E, Hoglinger GU. Possible involvement of complement factor C1q in the clearance of extracellular neuromelanin from the substantia nigra in Parkinson disease. J Neuropathol Exp Neurol. 2011;70:125–132. doi: 10.1097/NEN.0b013e31820805b9. [DOI] [PubMed] [Google Scholar]
- 63.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 65.Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76:863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology. 2010;74:995–1002. doi: 10.1212/WNL.0b013e3181d5a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samii A, Etminan M, Wiens MO, Jafari S. NSAID use and the risk of Parkinson’s disease: systematic review and meta-analysis of observational studies. Drugs Aging. 2009;26:769–779. doi: 10.2165/11316780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 68.Rugbjerg K, Friis S, Jorgensen TL, Ritz B, Korbo L, Olsen JH. Risk for Parkinson’s disease among patients with osteoarthritis: a Danish cohort study. Mov Disord. 2010;25:2355–2360. doi: 10.1002/mds.23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 70.Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 71.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 72.Brodacki B, Staszewski J, Toczylowska B, Kozlowska E, Drela N, Chalimoniuk M, Stepien A. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci Lett. 2008;441:158–162. doi: 10.1016/j.neulet.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 73.Reale M, Iarlori C, Thomas A, Gambi D, Perfetti B, Di Nicola M, Onofrj M. Peripheral cytokines profile in Parkinson’s disease. Brain Behav Immun. 2009;23:55–63. doi: 10.1016/j.bbi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 74.Yamada T, McGeer PL, McGeer EG. Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992;84:100–104. doi: 10.1007/BF00427222. [DOI] [PubMed] [Google Scholar]
- 75.Loeffler DA, Camp DM, Conant SB. Complement activation in the Parkinson’s disease substantia nigra: an immunocytochemical study. J Neuroinflammation. 2006;3:29. doi: 10.1186/1742-2094-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10(Suppl 1):S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nature genetics. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ahmed I, Tamouza R, Delord M, Krishnamoorthy R, Tzourio C, Mulot C, Nacfer M, Lambert JC, Beaune P, Laurent-Puig P, Loriot MA, Charron D, Elbaz A. Association between Parkinson’s disease and the HLA-DRB1 locus. Mov Disord. 2012;27:1104–1110. doi: 10.1002/mds.25035. [DOI] [PubMed] [Google Scholar]
- 79.Jamshidi J, Movafagh A, Emamalizadeh B, Zare Bidoki A, Manafi A, Ghasemi Firouzabadi S, Shahidi GA, Kazeminasab S, Petramfar P, Fazeli A, Motallebi M, Mortazavi-Tabatabaei SA, Kowsari A, Jafarian Z, Darvish H. HLA-DRA is associated with Parkinson’s disease in Iranian population. Int J Immunogenet. 2014 doi: 10.1111/iji.12151. [DOI] [PubMed] [Google Scholar]
- 80.Puschmann A, Verbeeck C, Heckman MG, Soto-Ortolaza AI, Lynch T, Jasinska-Myga B, Opala G, Krygowska-Wajs A, Barcikowska M, Uitti RJ, Wszolek ZK, Ross OA. Human leukocyte antigen variation and Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:376–378. doi: 10.1016/j.parkreldis.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ran C, Willows T, Sydow O, Johansson A, Soderkvist P, Dizdar N, Ahmadi A, Olson L, Belin AC. The HLA-DRA variation rs3129882 is not associated with Parkinson’s disease in Sweden. Parkinsonism Relat Disord. 2013;19:701–702. doi: 10.1016/j.parkreldis.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Wissemann WT, Hill-Burns EM, Zabetian CP, Factor SA, Patsopoulos N, Hoglund B, Holcomb C, Donahue RJ, Thomson G, Erlich H, Payami H. Association of Parkinson disease with structural and regulatory variants in the HLA region. Am J Hum Genet. 2013;93:984–993. doi: 10.1016/j.ajhg.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao Y, Gopalai AA, Ahmad-Annuar A, Teng EW, Prakash KM, Tan LC, Au WL, Li HH, Lim SY, Lim SK, Chong YB, Tan LP, Ibrahim NM, Tan EK. Association of HLA locus variant in Parkinson’s disease. Clin Genet. 2013;84:501–504. doi: 10.1111/cge.12024. [DOI] [PubMed] [Google Scholar]
- 84.Guo Y, Deng X, Zheng W, Xu H, Song Z, Liang H, Lei J, Jiang X, Luo Z, Deng H. HLA rs3129882 variant in Chinese Han patients with late-onset sporadic Parkinson disease. Neurosci Lett. 2011;501:185–187. doi: 10.1016/j.neulet.2011.05.245. [DOI] [PubMed] [Google Scholar]
- 85.Chiang HL, Lee-Chen GJ, Chen CM, Chen YC, Lee CM, Liao MH, Wu YR. Genetic analysis of HLA-DRA region variation in Taiwanese Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:391–393. doi: 10.1016/j.parkreldis.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 86.Sun C, Wei L, Luo F, Li Y, Li J, Zhu F, Kang P, Xu R, Xiao L, Liu Z, Xu P. HLA-DRB1 alleles are associated with the susceptibility to sporadic Parkinson’s disease in Chinese Han population. PLoS One. 2012;7:e48594. doi: 10.1371/journal.pone.0048594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hill-Burns EM, Factor SA, Zabetian CP, Thomson G, Payami H. Evidence for more than one Parkinson’s disease-associated variant within the HLA region. PLoS One. 2011;6:e27109. doi: 10.1371/journal.pone.0027109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arman A, Isik N, Coker A, Candan F, Becit KS, List EO. Association between sporadic Parkinson disease and interleukin-1 beta −511 gene polymorphisms in the Turkish population. Eur Cytokine Netw. 2010;21:116–121. doi: 10.1684/ecn.2010.0186. [DOI] [PubMed] [Google Scholar]
- 89.Bialecka M, Klodowska-Duda G, Kurzawski M, Slawek J, Gorzkowska A, Opala G, Bialecki P, Sagan L, Drozdzik M. Interleukin-10 (IL10) and tumor necrosis factor alpha (TNF) gene polymorphisms in Parkinson’s disease patients. Parkinsonism Relat Disord. 2008;14:636–640. doi: 10.1016/j.parkreldis.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Chu K, Zhou X, Luo BY. Cytokine gene polymorphisms and Parkinson’s disease: a meta-analysis. Can J Neurol Sci. 2012;39:58–64. doi: 10.1017/s0317167100012695. [DOI] [PubMed] [Google Scholar]
- 91.Dai D, Lin P, Wang Y, Zhou X, Tao J, Jiang D, Zhou H, Ru P, Pan G, Li J, Zhang Y, Yin H, Duan S. Association of and polymorphisms with Parkinson’s disease: A meta-analysis of 15 genetic association studies. Biomed Rep. 2014;2:713–718. doi: 10.3892/br.2014.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dodel RC, Lohmuller F, Du Y, Eastwood B, Gocke P, Oertel WH, Gasser T. A polymorphism in the intronic region of the IL-1alpha gene and the risk for Parkinson’s disease. Neurology. 2001;56:982–983. doi: 10.1212/wnl.56.7.982. [DOI] [PubMed] [Google Scholar]
- 93.Hakansson A, Westberg L, Nilsson S, Buervenich S, Carmine A, Holmberg B, Sydow O, Olson L, Johnels B, Eriksson E, Nissbrandt H. Investigation of genes coding for inflammatory components in Parkinson’s disease. Mov Disord. 2005;20:569–573. doi: 10.1002/mds.20378. [DOI] [PubMed] [Google Scholar]
- 94.Huerta C, Alvarez V, Mata IF, Coto E, Ribacoba R, Martinez C, Blazquez M, Guisasola LM, Salvador C, Lahoz CH, Pena J. Chemokines (RANTES and MCP-1) and chemokine-receptors (CCR2 and CCR5) gene polymorphisms in Alzheimer’s and Parkinson’s disease. Neurosci Lett. 2004;370:151–154. doi: 10.1016/j.neulet.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 95.Infante J, Garcia-Gorostiaga I, Sanchez-Juan P, Sanchez-Quintana C, Gurpegui JL, Rodriguez-Rodriguez E, Mateo I, Berciano J, Combarros O. Inflammation-related genes and the risk of Parkinson’s disease: a multilocus approach. Eur J Neurol. 2008;15:431–433. doi: 10.1111/j.1468-1331.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- 96.Kalinderi K, Bostantjopoulou S, Katsarou Z, Clarimon J, Fidani L. Lack of association between CX3CR1 V249I and T280M polymorphisms and risk of Parkinson’s disease in a Greek population. Genet Test Mol Biomarkers. 2012;16:974–977. doi: 10.1089/gtmb.2011.0330. [DOI] [PubMed] [Google Scholar]
- 97.Kruger R, Hardt C, Tschentscher F, Jackel S, Kuhn W, Muller T, Werner J, Woitalla D, Berg D, Kuhnl N, Fuchs GA, Santos EJ, Przuntek H, Epplen JT, Schols L, Riess O. Genetic analysis of immunomodulating factors in sporadic Parkinson’s disease. J Neural Transm. 2000;107:553–562. doi: 10.1007/s007020070078. [DOI] [PubMed] [Google Scholar]
- 98.Liu GJ, Feng RN, Luo C, Bi S. Lack of association between interleukin-1 alpha, beta polymorphisms and Parkinson’s disease. Neurosci Lett. 2010;480:158–161. doi: 10.1016/j.neulet.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 99.Mattila KM, Rinne JO, Lehtimaki T, Roytta M, Ahonen JP, Hurme M. Association of an interleukin 1B gene polymorphism (−511) with Parkinson’s disease in Finnish patients. J Med Genet. 2002;39:400–402. doi: 10.1136/jmg.39.6.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McGeer PL, Yasojima K, McGeer EG. Association of interleukin-1 beta polymorphisms with idiopathic Parkinson’s disease. Neurosci Lett. 2002;326:67–69. doi: 10.1016/s0304-3940(02)00300-2. [DOI] [PubMed] [Google Scholar]
- 101.Moller JC, Depboylu C, Kolsch H, Lohmuller F, Bandmann O, Gocke P, Du Y, Paus S, Wullner U, Gasser T, Oertel WH, Klockgether T, Dodel RC. Lack of association between the interleukin-1 alpha (−889) polymorphism and early-onset Parkinson’s disease. Neurosci Lett. 2004;359:195–197. doi: 10.1016/j.neulet.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 102.Nie K, Zhang Y, Gan R, Wang L, Zhao J, Huang Z, Tang H, Wang L. Polymorphisms in immune/inflammatory cytokine genes are related to Parkinson’s disease with cognitive impairment in the Han Chinese population. Neurosci Lett. 2013;541:111–115. doi: 10.1016/j.neulet.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 103.Nishimura M, Kuno S, Kaji R, Yasuno K, Kawakami H. Glutathione-S-transferase-1 and interleukin-1beta gene polymorphisms in Japanese patients with Parkinson’s disease. Mov Disord. 2005;20:901–902. doi: 10.1002/mds.20477. [DOI] [PubMed] [Google Scholar]
- 104.Nishimura M, Kuno S, Mizuta I, Ohta M, Maruyama H, Kaji R, Kawakami H. Influence of monocyte chemoattractant protein 1 gene polymorphism on age at onset of sporadic Parkinson’s disease. Mov Disord. 2003;18:953–955. doi: 10.1002/mds.10462. [DOI] [PubMed] [Google Scholar]
- 105.Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kaji R, Kuno S. Tumor necrosis factor gene polymorphisms in patients with sporadic Parkinson’s disease. Neurosci Lett. 2001;311:1–4. doi: 10.1016/s0304-3940(01)02111-5. [DOI] [PubMed] [Google Scholar]
- 106.Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kuno S. Influence of interleukin-1beta gene polymorphisms on age-at-onset of sporadic Parkinson’s disease. Neurosci Lett. 2000;284:73–76. doi: 10.1016/s0304-3940(00)00991-5. [DOI] [PubMed] [Google Scholar]
- 107.Pascale E, Passarelli E, Purcaro C, Vestri AR, Fakeri A, Guglielmi R, Passarelli F, Meco G. Lack of association between IL-1beta, TNF-alpha, and IL-10 gene polymorphisms and sporadic Parkinson’s disease in an Italian cohort. Acta Neurol Scand. 2011;124:176–181. doi: 10.1111/j.1600-0404.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 108.Ross OA, O’Neill C, Rea IM, Lynch T, Gosal D, Wallace A, Curran MD, Middleton D, Gibson JM. Functional promoter region polymorphism of the proinflammatory chemokine IL-8 gene associates with Parkinson’s disease in the Irish. Hum Immunol. 2004;65:340–346. doi: 10.1016/j.humimm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 109.Schulte T, Schols L, Muller T, Woitalla D, Berger K, Kruger R. Polymorphisms in the interleukin-1 alpha and beta genes and the risk for Parkinson’s disease. Neurosci Lett. 2002;326:70–72. doi: 10.1016/s0304-3940(02)00301-4. [DOI] [PubMed] [Google Scholar]
- 110.Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- 111.Wintermeyer P, Riess O, Schols L, Przuntek H, Miterski B, Epplen JT, Kruger R. Mutation analysis and association studies of nuclear factor-kappaB1 in sporadic Parkinson’s disease patients. J Neural Transm. 2002;109:1181–1188. doi: 10.1007/s00702-001-0688-x. [DOI] [PubMed] [Google Scholar]
- 112.Wu YR, Chen CM, Hwang JC, Chen ST, Feng IH, Hsu HC, Liu CN, Liu YT, Lai YY, Huang HJ, Lee-Chen GJ. Interleukin-1 alpha polymorphism has influence on late-onset sporadic Parkinson’s disease in Taiwan. J Neural Transm. 2007;114:1173–1177. doi: 10.1007/s00702-007-0726-4. [DOI] [PubMed] [Google Scholar]
- 113.Wu YR, Feng IH, Lyu RK, Chang KH, Lin YY, Chan H, Hu FJ, Lee-Chen GJ, Chen CM. Tumor necrosis factor-alpha promoter polymorphism is associated with the risk of Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:300–304. doi: 10.1002/ajmg.b.30435. [DOI] [PubMed] [Google Scholar]
- 114.Xu X, Li D, He Q, Gao J, Chen B, Xie A. Interleukin-18 promoter polymorphisms and risk of Parkinson’s disease in a Han Chinese population. Brain Res. 2011;1381:90–94. doi: 10.1016/j.brainres.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 115.Zhou YT, Yang JF, Zhang YL, Wang XY, Chan P. Protective role of interlekin-1 alpha gene polymorphism in Chinese Han population with sporadic Parkinson’s disease. Neurosci Lett. 2008;445:23–25. doi: 10.1016/j.neulet.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 116.Holmans P, Moskvina V, Jones L, Sharma M, Vedernikov A, Buchel F, Saad M, Bras JM, Bettella F, Nicolaou N, Simon-Sanchez J, Mittag F, Gibbs JR, Schulte C, Durr A, Guerreiro R, Hernandez D, Brice A, Stefansson H, Majamaa K, Gasser T, Heutink P, Wood NW, Martinez M, Singleton AB, Nalls MA, Hardy J, Morris HR, Williams NM International Parkinson’s Disease Genomics C. A pathway-based analysis provides additional support for an immune-related genetic susceptibility to Parkinson’s disease. Hum Mol Genet. 2013;22:1039–1049. doi: 10.1093/hmg/dds492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 118.McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 119.Akiyama H, McGeer PL. Microglial response to 6-hydroxydopamine-induced substantia nigra lesions. Brain Res. 1989;489:247–253. doi: 10.1016/0006-8993(89)90857-3. [DOI] [PubMed] [Google Scholar]
- 120.Sherer TB, Betarbet R, Kim JH, Greenamyre JT. Selective microglial activation in the rat rotenone model of Parkinson’s disease. Neurosci Lett. 2003;341:87–90. doi: 10.1016/s0304-3940(03)00172-1. [DOI] [PubMed] [Google Scholar]
- 121.Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao S, Theodore S, Standaert DG. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol Neurodegener. 2010;5:42. doi: 10.1186/1750-1326-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33:9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet MF. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol. 2012;237:318–334. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.He Y, Appel S, Le W. Minocycline inhibits microglial activation and protects nigral cells after 6-hydroxydopamine injection into mouse striatum. Brain Res. 2001;909:187–193. doi: 10.1016/s0006-8993(01)02681-6. [DOI] [PubMed] [Google Scholar]
- 128.Harms AS, Barnum CJ, Ruhn KA, Varghese S, Trevino I, Blesch A, Tansey MG. Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson’s disease. Mol Ther. 2011;19:46–52. doi: 10.1038/mt.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A, Tansey MG. Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tanaka S, Ishii A, Ohtaki H, Shioda S, Yoshida T, Numazawa S. Activation of microglia induces symptoms of Parkinson’s disease in wild-type, but not in IL-1 knockout mice. J Neuroinflammation. 2013;10:143. doi: 10.1186/1742-2094-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barcia C, Ros CM, Annese V, Gomez A, Ros-Bernal F, Aguado-Llera D, Martinez-Pagan ME, de Pablos V, Fernandez-Villalba E, Herrero MT. IFN-gamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2012;3:e379. doi: 10.1038/cddis.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao S, Standaert DG, Harms AS. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J Neuroinflammation. 2012;9:259. doi: 10.1186/1742-2094-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Couch Y, Alvarez-Erviti L, Sibson NR, Wood MJ, Anthony DC. The acute inflammatory response to intranigral alpha-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J Neuroinflammation. 2011;8:166. doi: 10.1186/1742-2094-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, Junn E, Kim HS. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 136.Reynolds AD, Glanzer JG, Kadiu I, Ricardo-Dukelow M, Chaudhuri A, Ciborowski P, Cerny R, Gelman B, Thomas MP, Mosley RL, Gendelman HE. Nitrated alpha-synuclein-activated microglial profiling for Parkinson’s disease. J Neurochem. 2008;104:1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 137.Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 138.Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de Bernard M. Triggering of inflammasome by aggregated alpha-synuclein, an inflammatory response in synucleinopathies. PLoS One. 2013;8:e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 140.Roodveldt C, Labrador-Garrido A, Gonzalez-Rey E, Lachaud CC, Guilliams T, Fernandez-Montesinos R, Benitez-Rondan A, Robledo G, Hmadcha A, Delgado M, Dobson CM, Pozo D. Preconditioning of microglia by alpha-synuclein strongly affects the response induced by toll-like receptor (TLR) stimulation. PLoS One. 2013;8:e79160. doi: 10.1371/journal.pone.0079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fu H, Liu B, Frost JL, Hong S, Jin M, Ostaszewski B, Shankar GM, Costantino IM, Carroll MC, Mayadas TN, Lemere CA. Complement component C3 and complement receptor type 3 contribute to the phagocytosis and clearance of fibrillar Abeta by microglia. Glia. 2012;60:993–1003. doi: 10.1002/glia.22331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Banks WA, Zhang J. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128:639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Russo I, Bubacco L, Greggio E. Exosomes-associated neurodegeneration and progression of Parkinson’s disease. Am J Neurodegener Dis. 2012;1:217–225. [PMC free article] [PubMed] [Google Scholar]
- 148.Kierdorf K, Prinz M. Factors regulating microglia activation. Front Cell Neurosci. 2013;7:44. doi: 10.3389/fncel.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Bockmann A, Meier BH, Melki R. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ding TT, Lee SJ, Rochet JC, Lansbury PT., Jr Annular alpha-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry. 2002;41:10209–10217. doi: 10.1021/bi020139h. [DOI] [PubMed] [Google Scholar]
- 153.Kayed R, Pensalfini A, Margol L, Sokolov Y, Sarsoza F, Head E, Hall J, Glabe C. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J Biol Chem. 2009;284:4230–4237. doi: 10.1074/jbc.M808591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 155.Krogsgaard M, Davis MM. How T cells ‘see’ antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 156.Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. The Journal of clinical investigation. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11:493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 158.Niwa F, Kuriyama N, Nakagawa M, Imanishi J. Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson’s disease. Geriatr Gerontol Int. 2012;12:102–107. doi: 10.1111/j.1447-0594.2011.00740.x. [DOI] [PubMed] [Google Scholar]
- 159.Stevens CH, Rowe D, Morel-Kopp MC, Orr C, Russell T, Ranola M, Ward C, Halliday GM. Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol. 2012;252:95–99. doi: 10.1016/j.jneuroim.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 160.Saunders JA, Estes KA, Kosloski LM, Allen HE, Dempsey KM, Torres-Russotto DR, Meza JL, Santamaria PM, Bertoni JM, Murman DL, Ali HH, Standaert DG, Mosley RL, Gendelman HE. CD4+ regulatory and effector/memory T cell subsets profile motor dysfunction in Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7:927–938. doi: 10.1007/s11481-012-9402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Alberio T, McMahon K, Cuccurullo M, Gethings LA, Lawless C, Zibetti M, Lopiano L, Vissers JP, Fasano M. Verification of a Parkinson’s disease protein signature in T-lymphocytes by multiple reaction monitoring. J Proteome Res. 2014;13:3554–3561. doi: 10.1021/pr401142p. [DOI] [PubMed] [Google Scholar]
- 162.Fiszer U, Mix E, Fredrikson S, Kostulas V, Olsson T, Link H. gamma delta+ T cells are increased in patients with Parkinson’s disease. J Neurol Sci. 1994;121:39–45. doi: 10.1016/0022-510x(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 163.Ferreira LM. Gammadelta T cells: innately adaptive immune cells? Int Rev Immunol. 2013;32:223–248. doi: 10.3109/08830185.2013.783831. [DOI] [PubMed] [Google Scholar]
- 164.Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM. A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain: a journal of neurology. 2005;128:2665–2674. doi: 10.1093/brain/awh625. [DOI] [PubMed] [Google Scholar]
- 165.Chen S, Le WD, Xie WJ, Alexianu ME, Engelhardt JI, Siklos L, Appel SH. Experimental destruction of substantia nigra initiated by Parkinson disease immunoglobulins. Arch Neurol. 1998;55:1075–1080. doi: 10.1001/archneur.55.8.1075. [DOI] [PubMed] [Google Scholar]
- 166.Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. MHC class II positive microglia and lymphocytic infiltration are present in the substantia nigra and striatum in mouse model of Parkinson’s disease. Acta Neurobiol Exp (Wars) 1999;59:1–8. doi: 10.55782/ane-1999-1289. [DOI] [PubMed] [Google Scholar]
- 167.Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, Anisman H, Hayley S, Park DS. Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci. 2007;27:3328–3337. doi: 10.1523/JNEUROSCI.5321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xiao BG, Link H. IFN-gamma production of adult rat astrocytes triggered by TNF-alpha. Neuroreport. 1998;9:1487–1490. doi: 10.1097/00001756-199805110-00044. [DOI] [PubMed] [Google Scholar]
- 169.De Simone R, Levi G, Aloisi F. Interferon gamma gene expression in rat central nervous system glial cells. Cytokine. 1998;10:418–422. doi: 10.1006/cyto.1997.0314. [DOI] [PubMed] [Google Scholar]
- 170.Cebrian C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, Zecca L, Loike JD, Sulzer D. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun. 2014;5:3633. doi: 10.1038/ncomms4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One. 2008;3:e1376. doi: 10.1371/journal.pone.0001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J Immunol. 2010;184:2261–2271. doi: 10.4049/jimmunol.0901852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Games D, Seubert P, Rockenstein E, Patrick C, Trejo M, Ubhi K, Ettle B, Ghassemiam M, Barbour R, Schenk D, Nuber S, Masliah E. Axonopathy in an alpha-synuclein transgenic model of Lewy body disease is associated with extensive accumulation of C-terminal-truncated alpha-synuclein. Am J Pathol. 2013;182:940–953. doi: 10.1016/j.ajpath.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Good PF, Hsu A, Werner P, Perl DP, Olanow CW. Protein nitration in Parkinson’s disease. J Neuropathol Exp Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 176.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 177.Lewis KA, Yaeger A, DeMartino GN, Thomas PJ. Accelerated formation of alpha-synuclein oligomers by concerted action of the 20S proteasome and familial Parkinson mutations. J Bioenerg Biomembr. 2010;42:85–95. doi: 10.1007/s10863-009-9258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Games D, Valera E, Spencer B, Rockenstein E, Mante M, Adame A, Patrick C, Ubhi K, Nuber S, Sacayon P, Zago W, Seubert P, Barbour R, Schenk D, Masliah E. Reducing C-terminal-truncated alpha-synuclein by immunotherapy attenuates neurodegeneration and propagation in Parkinson’s disease-like models. J Neurosci. 2014;34:9441–9454. doi: 10.1523/JNEUROSCI.5314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Uversky VN, Yamin G, Munishkina LA, Karymov MA, Millett IS, Doniach S, Lyubchenko YL, Fink AL. Effects of nitration on the structure and aggregation of alpha-synuclein. Brain Res Mol Brain Res. 2005;134:84–102. doi: 10.1016/j.molbrainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 180.Yu Z, Xu X, Xiang Z, Zhou J, Zhang Z, Hu C, He C. Nitrated alpha-synuclein induces the loss of dopaminergic neurons in the substantia nigra of rats. PLoS One. 2010;5:e9956. doi: 10.1371/journal.pone.0009956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meier F, Abeywardana T, Dhall A, Marotta NP, Varkey J, Langen R, Chatterjee C, Pratt MR. Semisynthetic, site-specific ubiquitin modification of alpha-synuclein reveals differential effects on aggregation. J Am Chem Soc. 2012;134:5468–5471. doi: 10.1021/ja300094r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Davies SE, Hallett PJ, Moens T, Smith G, Mangano E, Kim HT, Goldberg AL, Liu JL, Isacson O, Tofaris GK. Enhanced ubiquitin-dependent degradation by Nedd4 protects against alpha-synuclein accumulation and toxicity in animal models of Parkinson’s disease. Neurobiol Dis. 2014;64:79–87. doi: 10.1016/j.nbd.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, Lansbury PT, Jr, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. J Biol Chem. 2008;283:16895–16905. doi: 10.1074/jbc.M800747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schreurs S, Gerard M, Derua R, Waelkens E, Taymans JM, Baekelandt V, Engelborghs Y. In vitro phosphorylation does not influence the aggregation kinetics of WT alpha-synuclein in contrast to its phosphorylation mutants. Int J Mol Sci. 2014;15:1040–1067. doi: 10.3390/ijms15011040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oueslati A, Paleologou KE, Schneider BL, Aebischer P, Lashuel HA. Mimicking phosphorylation at serine 87 inhibits the aggregation of human alpha-synuclein and protects against its toxicity in a rat model of Parkinson’s disease. J Neurosci. 2012;32:1536–1544. doi: 10.1523/JNEUROSCI.3784-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, Mandel RJ, Muzyczka N. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci U S A. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 188.Lee KW, Chen W, Junn E, Im JY, Grosso H, Sonsalla PK, Feng X, Ray N, Fernandez JR, Chao Y, Masliah E, Voronkov M, Braithwaite SP, Stock JB, Mouradian MM. Enhanced phosphatase activity attenuates alpha-synucleinopathy in a mouse model. J Neurosci. 2011;31:6963–6971. doi: 10.1523/JNEUROSCI.6513-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.van Stipdonk MJ, Willems AA, Amor S, Persoon-Deen C, Travers PJ, Boog CJ, van Noort JM. T cells discriminate between differentially phosphorylated forms of alphaB-crystallin, a major central nervous system myelin antigen. Int Immunol. 1998;10:943–950. doi: 10.1093/intimm/10.7.943. [DOI] [PubMed] [Google Scholar]
- 190.Utz PJ, Hottelet M, Schur PH, Anderson P. Proteins phosphorylated during stress-induced apoptosis are common targets for autoantibody production in patients with systemic lupus erythematosus. J Exp Med. 1997;185:843–854. doi: 10.1084/jem.185.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Anderton SM. Post-translational modifications of self antigens: implications for autoimmunity. Curr Opin Immunol. 2004;16:753–758. doi: 10.1016/j.coi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 192.Gahring L, Carlson NG, Meyer EL, Rogers SW. Granzyme B proteolysis of a neuronal glutamate receptor generates an autoantigen and is modulated by glycosylation. J Immunol. 2001;166:1433–1438. doi: 10.4049/jimmunol.166.3.1433. [DOI] [PubMed] [Google Scholar]
- 193.Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC. alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol. 2010;190:1023–1037. doi: 10.1083/jcb.201003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Iwata A, Maruyama M, Akagi T, Hashikawa T, Kanazawa I, Tsuji S, Nukina N. Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Hum Mol Genet. 2003;12:2625–2635. doi: 10.1093/hmg/ddg283. [DOI] [PubMed] [Google Scholar]
- 195.Kasai T, Tokuda T, Yamaguchi N, Watanabe Y, Kametani F, Nakagawa M, Mizuno T. Cleavage of normal and pathological forms of alpha-synuclein by neurosin in vitro. Neurosci Lett. 2008;436:52–56. doi: 10.1016/j.neulet.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 196.Choi DH, Kim YJ, Kim YG, Joh TH, Beal MF, Kim YS. Role of matrix metalloproteinase 3-mediated alpha-synuclein cleavage in dopaminergic cell death. J Biol Chem. 2011;286:14168–14177. doi: 10.1074/jbc.M111.222430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Takahashi M, Ko LW, Kulathingal J, Jiang P, Sevlever D, Yen SH. Oxidative stress-induced phosphorylation, degradation and aggregation of alpha-synuclein are linked to upregulated CK2 and cathepsin D. Eur J Neurosci. 2007;26:863–874. doi: 10.1111/j.1460-9568.2007.05736.x. [DOI] [PubMed] [Google Scholar]
- 199.Mishizen-Eberz AJ, Guttmann RP, Giasson BI, Day GA, 3rd, Hodara R, Ischiropoulos H, Lee VM, Trojanowski JQ, Lynch DR. Distinct cleavage patterns of normal and pathologic forms of alpha-synuclein by calpain I in vitro. J Neurochem. 2003;86:836–847. doi: 10.1046/j.1471-4159.2003.01878.x. [DOI] [PubMed] [Google Scholar]
- 200.Mishizen-Eberz AJ, Norris EH, Giasson BI, Hodara R, Ischiropoulos H, Lee VM, Trojanowski JQ, Lynch DR. Cleavage of alpha-synuclein by calpain: potential role in degradation of fibrillized and nitrated species of alpha-synuclein. Biochemistry. 2005;44:7818–7829. doi: 10.1021/bi047846q. [DOI] [PubMed] [Google Scholar]
- 201.Liu CW, Corboy MJ, DeMartino GN, Thomas PJ. Endoproteolytic activity of the proteasome. Science. 2003;299:408–411. doi: 10.1126/science.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liu CW, Giasson BI, Lewis KA, Lee VM, Demartino GN, Thomas PJ. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: implications for pathogenesis of Parkinson disease. J Biol Chem. 2005;280:22670–22678. doi: 10.1074/jbc.M501508200. [DOI] [PubMed] [Google Scholar]
- 203.Tofaris GK, Layfield R, Spillantini MG. alpha-synuclein metabolism and aggregation is linked to ubiquitin-independent degradation by the proteasome. FEBS Lett. 2001;509:22–26. doi: 10.1016/s0014-5793(01)03115-5. [DOI] [PubMed] [Google Scholar]
- 204.Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274:33855–33858. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- 205.Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ. Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem. 2006;281:29739–29752. doi: 10.1074/jbc.M600933200. [DOI] [PubMed] [Google Scholar]
- 206.Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, Dawson TM, Jakala P, Hartmann T, Price DL, Lee MK. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc Natl Acad Sci U S A. 2005;102:2162–2167. doi: 10.1073/pnas.0406976102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 208.Levitan K, Chereau D, Cohen SI, Knowles TP, Dobson CM, Fink AL, Anderson JP, Goldstein JM, Millhauser GL. Conserved C-terminal charge exerts a profound influence on the aggregation rate of alpha-synuclein. J Mol Biol. 2011;411:329–333. doi: 10.1016/j.jmb.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Murray IV, Giasson BI, Quinn SM, Koppaka V, Axelsen PH, Ischiropoulos H, Trojanowski JQ, Lee VM. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530–8540. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- 210.Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proc Natl Acad Sci U S A. 2000;97:4897–4902. doi: 10.1073/pnas.97.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ulusoy A, Febbraro F, Jensen PH, Kirik D, Romero-Ramos M. Co-expression of C-terminal truncated alpha-synuclein enhances full-length alpha-synuclein-induced pathology. Eur J Neurosci. 2010;32:409–422. doi: 10.1111/j.1460-9568.2010.07284.x. [DOI] [PubMed] [Google Scholar]
- 212.Daher JP, Ying M, Banerjee R, McDonald RS, Hahn MD, Yang L, Flint Beal M, Thomas B, Dawson VL, Dawson TM, Moore DJ. Conditional transgenic mice expressing C-terminally truncated human alpha-synuclein (alphaSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Mol Neurodegener. 2009;4:34. doi: 10.1186/1750-1326-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. J Neurosci. 2006;26:3942–3950. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wakamatsu M, Ishii A, Iwata S, Sakagami J, Ukai Y, Ono M, Kanbe D, Muramatsu S, Kobayashi K, Iwatsubo T, Yoshimoto M. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol Aging. 2008;29:574–585. doi: 10.1016/j.neurobiolaging.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 216.Hill JA, Southwood S, Sette A, Jevnikar AM, Bell DA, Cairns E. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J Immunol. 2003;171:538–541. doi: 10.4049/jimmunol.171.2.538. [DOI] [PubMed] [Google Scholar]
- 217.Gauba V, Grunewald J, Gorney V, Deaton LM, Kang M, Bursulaya B, Ou W, Lerner RA, Schmedt C, Geierstanger BH, Schultz PG, Ramirez-Montagut T. Loss of CD4 T-cell-dependent tolerance to proteins with modified amino acids. Proc Natl Acad Sci U S A. 2011;108:12821–12826. doi: 10.1073/pnas.1110042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ahsan H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol. 2013;74:1392–1399. doi: 10.1016/j.humimm.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 219.Schulz JB, Matthews RT, Klockgether T, Dichgans J, Beal MF. The role of mitochondrial dysfunction and neuronal nitric oxide in animal models of neurodegenerative diseases. Mol Cell Biochem. 1997;174:193–197. [PubMed] [Google Scholar]
- 220.Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, Gollomp SM, Ischiropoulos H, Lee VM, Trojanowski JQ. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Choi DY, Zhang J, Bing G. Aging enhances the neuroinflammatory response and alpha-synuclein nitration in rats. Neurobiol Aging. 2010;31:1649–1653. doi: 10.1016/j.neurobiolaging.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 222.Iravani MM, Kashefi K, Mander P, Rose S, Jenner P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience. 2002;110:49–58. doi: 10.1016/s0306-4522(01)00562-0. [DOI] [PubMed] [Google Scholar]
- 223.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 224.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG Arizona Parkinson’s Disease C. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 226.Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F. Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv Neurol. 1993;60:609–612. [PubMed] [Google Scholar]
- 227.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 228.Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, Estes JD, Dodiya HB, Keshavarzian A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Devos D, Lebouvier T, Lardeux B, Biraud M, Rouaud T, Pouclet H, Coron E, Bruley des Varannes S, Naveilhan P, Nguyen JM, Neunlist M, Derkinderen P. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 230.Phillips RJ, Billingsley CN, Powley TL. Macrophages are unsuccessful in clearing aggregated alpha-synuclein from the gastrointestinal tract of healthy aged Fischer 344 rats. Anat Rec (Hoboken) 2013;296:654–669. doi: 10.1002/ar.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Austin SA, Floden AM, Murphy EJ, Combs CK. Alpha-synuclein expression modulates microglial activation phenotype. J Neurosci. 2006;26:10558–10563. doi: 10.1523/JNEUROSCI.1799-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Xiao W, Shameli A, Harding CV, Meyerson HJ, Maitta RW. Late stages of hematopoiesis and B cell lymphopoiesis are regulated by alpha-synuclein, a key player in Parkinson’s disease. Immunobiology. 2014 doi: 10.1016/j.imbio.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]