Abstract
Perinatal choline supplementation has produced several benefits in rodent models, from improved learning and memory to protection from the behavioral effects of fetal alcohol exposure. We have shown that supplemented choline through gestation and lactation produces long-term improvement in deficient sensory inhibition in DBA/2 mice which models a similar deficit in schizophrenia patients. The present study extends that research by feeding normal or supplemented choline diets to DBA/2 mice carrying the null mutation for the α7 nicotinic receptor gene (Chrna7). DBA/2 mice heterozygotic for Chrna7 were bred together. Dams were placed on supplemented (5 gm/kg diet) or normal (1.1 gm/kg diet) choline at mating and remained on the specific diet until offspring weaning. Thereafter, offspring were fed standard rodent chow. Adult offspring were assessed for sensory inhibition. Brains were obtained to ascertain hippocampal α7 nicotinic receptor levels. Choline-supplemented mice heterozygotic or null-mutant for Chrna7 failed to show improvement in sensory inhibition. Only wildtype choline-supplemented mice showed improvement with the effect solely through a decrease in test amplitude. This supports the hypothesis that gestational-choline supplementation is acting through the α7 nicotinic receptor to improve sensory inhibition. Although there was a significant gene-dose-related change in hippocampal α7 receptor numbers, binding studies did not reveal any choline-dose-related change in binding in any hippocampal region, the interaction being driven by a significant genotype main effect (wildtype>heterozygote>null mutant). These data parallel a human study wherein the offspring of pregnant women receiving choline supplementation during gestation, showed better sensory inhibition than offspring of women on placebo.
Keywords: DBA/2 mice, sensory gating, sensory inhibition, Chrna7 null mutation, gestational choline supplementation
1. Introduction
DBA/2 mice have been used extensively as a model for the sensory inhibition deficits observed in schizophrenia patients (Dinklo et al 2011; Hashimoto et al 2005; Kohlhaas et al 2011; Ng et al 2007; O’Neill et al 2003; Radek et al 2006; 2012; Simosky et al 2001; 2008; Singer et al 2009; Stevens et al 1996; 1997, 1998; 2010; Wildeboer and Stevens 2009). Deficient sensory inhibition is defined as the inability to inhibit the electrophysiological response to repeated auditory stimuli (Adler et al 1998). It is measured in a paired stimulus paradigm in which 2 identical sounds (clicks) are presented at short interval (0.5 sec) and the electrophysiological responses to both stimuli are compared (Adler et al 1998; Baker et al 1990). This deficit has been related to poor attentional focusing and thus cognitive problems (Martin and Freedman 2007; Olincy and Freedman 2012), and to sensory flooding (Venables 1964; 1992) in schizophrenia patients. DBA/2 mice not only demonstrate the deficit in sensory inhibition, they show reduced numbers of hippocampal α7 nicotinic receptors (Stevens et al 1996), as is seen in schizophrenia patients (Freedman et al 1995); the reduction presumably related to mutations in the proximal promoter region for the α7 nicotinic receptor gene in both humans (Leonard et al 2002) and DBA/2 mice (Stitzel et al 1996). Stimulation of these receptors with nicotine or agonists selective for the α7 receptor subtype improves sensory inhibition in both humans (Adler et al 1993; Olincy et al 2006) and DBA/2 mice (Stevens and Wear 1997; Stevens et al 1998).
While nicotinic agonists (including those selective for the α7 nicotinic receptor) are being explored as potential therapeutics for schizophrenia (Zhang et al 2012; Waldo et al 2012; Smith et al 2006; 2009; Freedman et al 2008; Olincy et al 2006; Deutsch et al 2008; Harris et al 2004; Myers et al 2004), these would only treat symptoms, not the root cause of the reduced levels of hippocampal α7 nicotinic receptors. Since schizophrenia is now considered to have its origins, at least in part, during development (for reviews see Schlotz and Phillips 2009; Markham and Keonig 2011) an ameliorative approach during development could correct the deficit, permanently. Studies have shown that these receptors do not appear in the DBA/2 mouse hippocampus until developmental day E16 as compared to E13 in C3H mice (Adams 2003), a strain of mouse with normal sensory inhibition (Stevens et al 1996), while acetylcholine (as evidenced by the presence of choline acetyltransferase) appears E14 to E18 in mice (Abreu-Villaça et al 2011). Thus, choline, a selective agonist for the α7 nicotinic receptor (Albuquerque et al 1998; Alkondon et al 1999; Fayuk and Yakel 2004), may stimulate α7 nicotinic receptors during early development, prior to the availability of endogenous acetylcholine. In a previous study, we gestated DBA/2 mice on a diet containing supplemented choline (5 gm/kg diet). At weaning, the offspring were placed on a diet containing normal choline levels (1.1 gm/kg diet). At adulthood the offspring mice were assessed for sensory inhibition in a paradigm utilizing auditory evoked potential recording from the hippocampal CA3 region of anesthetized mice (Stevens et al 1996; 1997; 1998; 2008). Offspring gestated on the supplemented choline diet showed normal sensory inhibition, while those gestated on a diet containing normal choline levels displayed deficient sensory inhibition. There was also a concurrent significant increase in hippocampal α7 nicotinic receptors in the mice gestated on supplemented choline (Stevens et al 2008). These data suggest that improvement in sensory inhibition in DBA/2 mice gestated on supplemented choline may be due to activation of α7 nicotinic receptors by the choline. In a recent pilot study, pregnant women were randomly assigned to a choline supplement or placebo from 17.2 to 52 weeks of pregnancy. The infants were assessed for sensory inhibition at gestational ages 33 and 89 days. Infants whose mothers received the choline supplementation had significantly better sensory inhibition at the first recording session than infants whose mothers were on placebo. By the second recording session, both groups showed normal sensory inhibition (Ross et al 2013), thus suggesting that sensory inhibition can also be improved in human offspring.
As noted, choline is a selective agonist for the α7 nicotinic receptor, but it is also an essential nutrient, membrane component, and methyl group donor (Blusztajn 1998; Zeisel 2000, Ziesel and Blusztajn 1994) which is readily absorbed in the gut from dietary sources (Li and Vance. 2008; Zeisel 2000). Studies have shown that the requirements for choline are particularly high during fetal development, when new cell membranes are being rapidly produced (Zeisel 2013; Zeisel and Blusztajn 1994) and there is a growing sentiment that the current recommended choline intake during pregnancy should be increased (Yan et al 2012). With the multiple systems reliant on choline, the salutary effects of choline supplementation during gestation may or may not be attributable to its affinity for α7 nicotinic receptors. To assess this, we obtained DBA/2 mice heterozygote for the α7 nicotinic receptor gene null mutation (Chrna7) from the Institute for Behavioral Genetics (University of Colorado, Boulder, CO) and bred them on normal (1.1 g/kg) or supplemented (5 g/kg) diet (Dyets, Bethlehem, PA) and assessed sensory inhibition at adulthood for offspring of all 3 genotypes (−/−, −/+ and +/+) and both sexes. If the presence of Chrna7, producing functional α7 nicotinic receptors is a requirement of gestational-choline-supplementation-related improvement in sensory inhibition, mice null-mutant for this receptor should not show improvement with gestational choline supplementation while wildtype should have improvement commensurate with that seen with parental DBA/2 mice gestated on supplemented choline. Since DBA/2 mice are reduced in hippocampal α7 nicotinic receptors initially (Stevens et al 1996), the heterozygotes, which should show an even greater reduction, may or may show improvement. To ascertain levels of hippocampal α7 receptors in the genetically altered mice, as well as determine any choline-related changes, we performed binding studies with 125I-α-bungarotoxin.
2. Results
2.1 Sensory inhibition
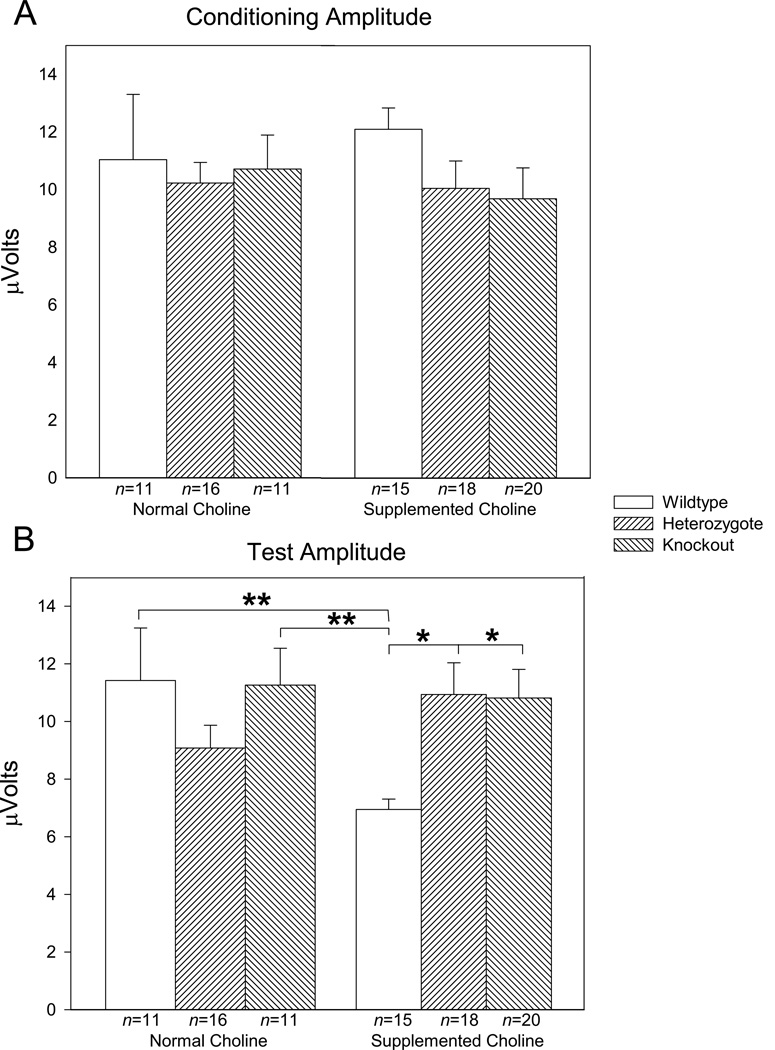
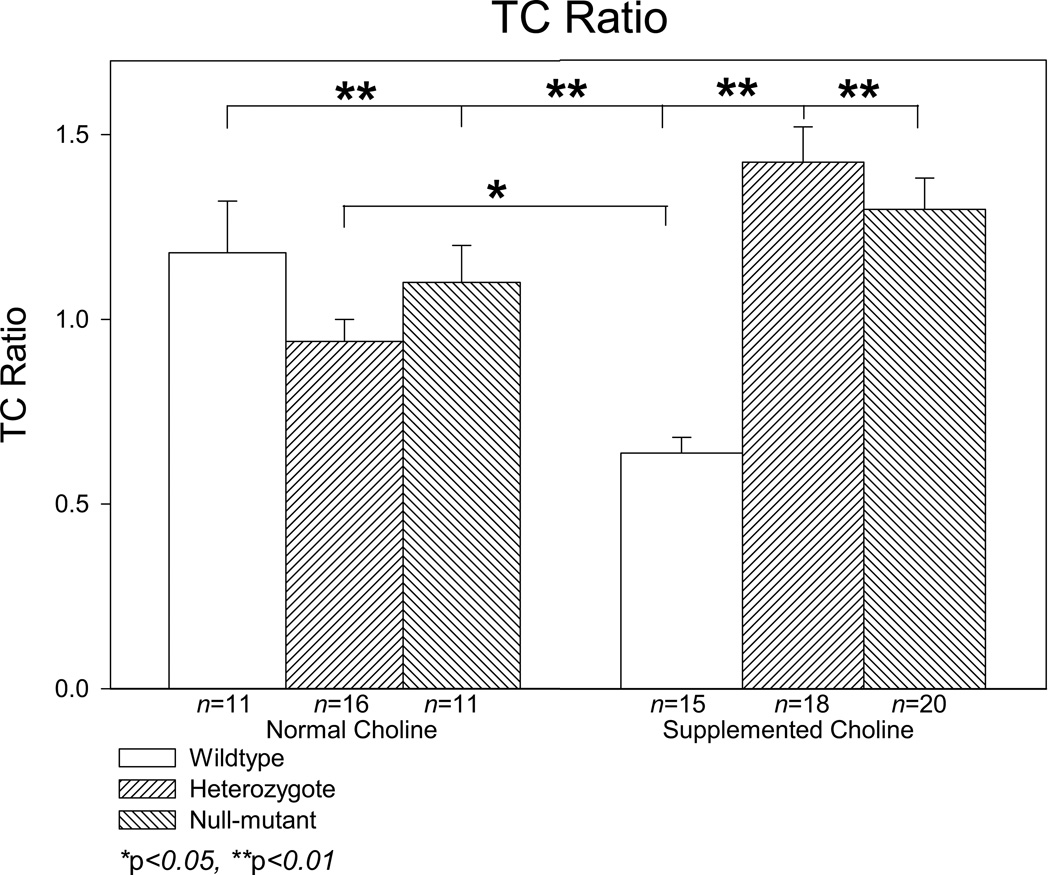
The offspring mice from matings of DBA/2 mice heterozygotic for Chrna7, gestated on normal (1.1 gm/kg) or supplemented (5 mg/kg) choline diet, were assessed for sensory inhibition parameters (conditioning amplitude, test amplitude, TC ratio). MANOVA for all parameters tested failed to show significant effects of sex [Conditioning amplitude F(1,78)=6.544, p=0.063, Test amplitude F(1,78)=2.009, p=0.160; TC ratio F(1,78)=1.507, p=0.223] so data were collapsed across sex and reanalyzed. Assessment of conditioning amplitude failed to show significant main effects for diet or genotype, or for the interaction of diet with genotype (Diet: F(1,83)=0.002, P=0.961; Genotype: F(2,84)=0.889, p=0.411; Diet × Genotype: F(2,84)=0.360, p=0.699) (Figures 1, 2A). Assessment of test amplitude, likewise, failed to show significant main effects for diet or genotype (Diet: F(1,83)=1.337, p=0.251; Genotype: F(2,84)=1.299, p=0.278), however there was a significant interaction of diet and genotype (F(2,84)=4.257, p=0.017) (Figures 1, 2B). A posteriori analysis revealed that wildtype mice gestated on supplemented choline showed significantly lower test amplitudes than any other group with exception of the normal choline gestated heterozygote mice. Analysis for TC ratio showed no main effect for diet (F1,84)=1.538, p=0.218), but a significant main effect for genotype (F(2,84)=4.776, p=0.011) and a significant interaction between diet and genotype (F(2,84)=12.200, p<0.001) (Figures 1, 3).
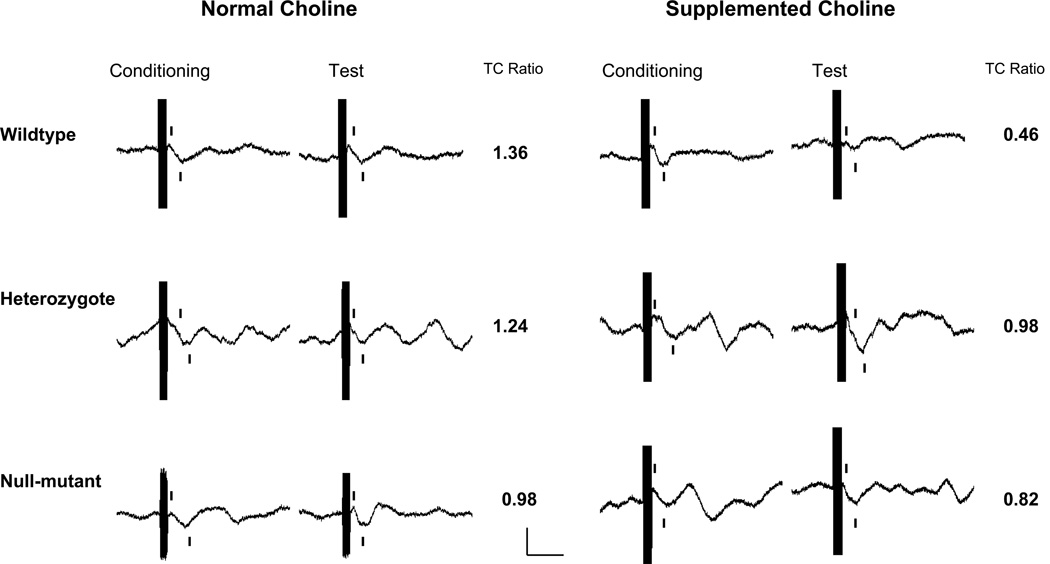
Figure 1.
Representative wave forms for DBA/2 α7 nicotinic receptor null mutation mice, gestated on a normal choline diet (1.1 g/kg diet) or supplemented choline diet (5 g/kg diet). Only the wildtype mice gestated on the supplemented choline diet showed normalized sensor inhibition, which was produced through a reduction in the amplitude of the test response. The large black line is an audio stimulus artifact, tick marks denote the wave of interest, calibration 20 µV, 40 msec.
Figure 2.
Averaged (A) conditioning and (B) test amplitudes for DBA/2 α7 nicotinic receptor null mutants gestated on normal (1.1 g/kg) or supplemented (5 g/kg) diet. Data are averaged over 12 records taken at 5 minute intervals. Conditioning amplitude did not vary significantly across the 3 genotypes or for the level of gestational choline. However, there were significant differences dependent upon genotype and gestational choline level for the test amplitude. Wildtype mice gestated on supplemented choline showed significantly lower test amplitudes as compared to all other groups except normal choline heterozygote mice. Data are mean ± SEM; group n’s—Wildype normal choline – 11; Heterozygote normal choline – 16; Null mutant normal choline – 10; Wildtype supplemented choline – 15; Heterozygote supplemented choline – 18; Null mutant supplemented choline – 20.
Figure 3.
Averaged TC ratio for DBA/2 α7 nicotinic receptor null mutants gestated on normal (1.1 g/kg) or supplemented (5 g/kg) diet. Data are averaged over 12 records taken at 5 minute intervals. Wildtype mice gestated on supplemented choline showed lower TC ratios than all other groups. Data are mean ± SEM; group n’s—Wildype normal choline – 11; Heterozygote normal choline – 16; Null mutant normal choline – 10; Wildtype supplemented choline – 15; Heterozygote supplemented choline – 18; Null mutant supplemented choline – 20.
2.2 α-Bungarotoxin binding
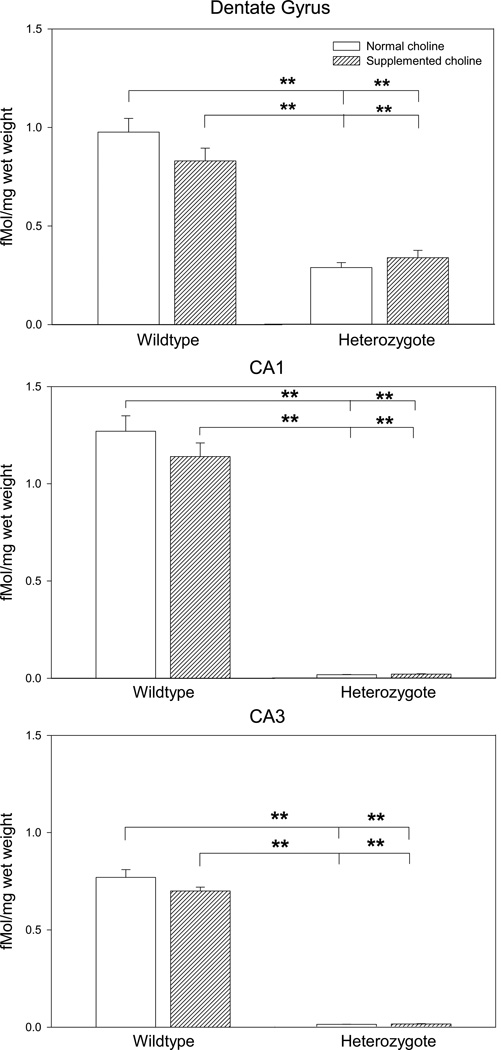
All three genotypes were assessed for α7 nicotinic receptor levels in the hippocampus. Analysis showed no significant binding for Chrna7 homozygous null mutant mice, thus, these mice were not included in the remaining analyses. ANOVAs for wildtype and heterozygote mice were performed for levels of I125 α-bungarotoxin in 3 primary regions of the hippocampus, dentate gyrus, CA1 and CA3. In all three regions, there were no significant effects of sex [Dentate gyrus F(1,3)=0.148, p=0.703; CA1 F(1,3)=0.767, p=0.386; CA3 F(1,3)=0.249, p=0.621] so for all analyses, the data were collapsed across sex. In the dentate gyrus, there was a main effect of genotype (F(1,43)=297.666, p<0.001) with wildtype having significantly greater levels of α7 nicotinic receptors than heterozygotes. There was no effect of diet (F(1,43)=6.754, p=0.059) but there was a significant interaction between genotype and diet (F(1,43)=8.800, p=0.005). Fisher’s LSD revealed that both wildtype normal and supplemented choline were significantly greater than heterozygote normal or supplemented choline (Figure 4). In a similar manner, CA1 showed a significant main effect of genotype (F(1,43)=256.241, p<0.001), with wildtype mice having greater levels of α7 nicotinic receptors than heterozygote mice, no effect of diet (F(1,43)=1.063, p=0.308) and a trend for an interaction of genotype and gestational diet (F(1,43)=4.003, p=0.051). Again, Fisher’s LSD showed both normal and choline supplementation in wildtype mice had greater α7 receptor levels than either heterozygote group (Figure 4). Finally, assessment of binding in the CA3 region of hippocampus produced a significant main effect of genotype (F(1,43)=379.049, p<0.001), no effect of diet (F1,43)=0.621, p=0.435 and a significant interaction of genotype and gestational diet (F)1,43)=4.326, p=0.044). As was seen in the other 2 hippocampal regions, wildtype mice, whether gestated on normal or supplemented choline levels, had greater levels of α7 nicotinic receptors than either heterozygote group (Figure 4).
Figure 4.
α7 nicotinic receptor levels in 3 regions of hippocampus, as reflected by I125-α-bungarotoxin binding, for DBA/2 wildtype and α7 nicotinic receptor heterozygote mice, gestated on normal (1.1 mg/kg) and supplemented (5 mg/kg) choline diet. In all cases, there was no significant effect of sex, so the data are collapsed across sex. In all regions, regardless of choline level in the dam’s diet, wildtype mice had greater levels of α7 nicotinic receptors. Across choline levels, within a genotype, there were no significant differences in binding levels. Data are mean ± SEM, n=12 per group. **p<0.001, Fisher’s LSD.
3. Discussion
Elevated levels of dietary choline during gestation and lactation, produced long-term (into adulthood) improvement in sensory inhibition in DBA/2 mice wildtype for the Chrna7, while having no effect on heterozygote or null mutant mice, nor were there differences related to the sex of the mouse. While sex trended towards significance for test amplitude, previous studies of DBA/2 mice have not shown sex differences (Stevens et al 2008). The improvement in sensory inhibition was solely due to a significant reduction in test amplitude in the supplemented wildtype mice as compared to the wildtype gestated on normal choline levels. These data are in concert with the findings of a similar study using DBA/2 mice (Stevens et al 2008) in which parental DBA/2 mice gestated on supplemented choline levels had significantly improved sensory inhibition, through a reduction in test amplitude, than offspring gestated on normal choline levels. The improvement in TC ratio, solely through a reduction in the test amplitude, implicates selective stimulation of α7 nicotinic receptors (Stevens and Wear 1997; Stevens et al 1998) as does the lack of effect of choline supplementation on homozygous mice lacking Chrna7. The failure of gestational choline supplementation to improve sensory inhibition in the heterozygote mice may be related to the significant reduction in α7 receptors when only a single copy of the gene is present (66–77% depending upon region and level of gestational choline). Parental DBA/2 mice have a deficit in sensory inhibition with only a 30% reduction in hippocampal α7 nicotinic receptor numbers (Stevens et al 1996). The significant reduction below this level with heterozygotes may have been just too great a deficit to overcome. While no significant increases in α7 receptor levels were observed with gestational choline supplementation in the present study, a small but significant increase was observed in the study using DBA/2 parental mice to which the improvement in sensory inhibition was attributed (Stevens et al 2008). The lack of increase in α7 levels in the present study would explain the lack of improvement in sensory inhibition in the heterozygote mice.
As noted, gestational choline supplementation failed in the present study to increase hippocampal α7 nicotinic receptor numbers in either the wildtype or heterozygote mice, over levels seen in the normal choline groups. This is contrary to the earlier study with parental DBA/2 on supplemented choline. It is possible that this is due to the fact that the wildtype mice in the present study are not genetically identical to the parental mice. The DBA/2 mice carrying the α7 nicotinic null mutation were bred from the original null mutant strain which was generated in the customary fashion by inserting a modified 129Svev cell into a C57Bl/6 mouse blastomere. Thus, there are potentially “passenger genes” originating from both the 129Svev and C57Bl/6 mouse now present in the DBA/2 α7 null mutation strain. These “passenger genes” may have altered the capacity of the DBA/2 α7 gene to increase protein levels in the adult supplemented offspring. The data are also contrary to the effects of chronic nicotine administration in which upregulation of all subtypes of nicotinic receptors have been observed (for review see Gentry and Lukas, 2002). Nicotine has been shown to induce a long-term, stable desensitization state in α7 nicotinic receptors (Papke et al 2009) where as choline induces rapid desensitization of α7 nicotinic receptors, but the activatable state is relatively quickly restablished (Papke et al 1996). Thus, the long-term stable desensitization induced by nicotine may cause cells to produce additional receptors to compensate for the lack of available receptors for activation, whereas choline-induced desensitization, being of shorter duration, does not.
The improvement in sensory inhibition in wildtype mice with choline supplementation is also on concert with a human study in which pregnant women, with normal pregnancies, were administered either 900 mg of choline daily (as phosphotidylcholine) or placebo from ~17.2 weeks of pregnancy, through delivery. Oral choline, whether delivered in the diet or as a supplement, is readily absorbed from the gut (Li and Vance 2008; Zeisel 2000) Choline-supplemented infants continued choline supplementation (100 mg phosphotidylcholine) through 52 weeks post the mother’s last menstrual period (infant age approximately 3 months). Sensory inhibition was tested in both groups of infants at 3 months age. A greater percentage of infants receiving choline supplementation had TC ratios in the normal range than infants whose mother’s were on placebo (76% versus 43%) (Ross et al 2013). Further, the study indicated that choline supplementation was safe and tolerable (Ross et al 2013).
While choline is a direct agonist of α7 nicotinic receptors (Albuquerque et al 1998; Alkondon et al 1999; Fayuk and Yakel 2004), it is also a methyl group donor (Zeisel 2013), a critical component of cell membranes (as phosphotidylcholine) (Hollenbeck 2012; Zeisel 2006) and forms part of the neurotransmitter acetylcholine (Cohen and Wurtman 1976). The inability of gestationally supplemented choline to improve sensory inhibition in the homozygous Chrna7 null mutant mice, while being successful in the wildtype mice, strongly suggests an α7 nicotinic receptor mechanism. However, additional mechanism(s) related to choline’s other biological roles, working in tandem with α7 receptor stimulation, cannot be ruled out. For example, choline levels have been shown to alter gene expression, primarily through DNA methylation, both developmentally (for review see Blusztajn and Mellot 2012; Derbyshire, 2007; Zeisel 2001; 2012) and in mature brain (for review see Anderson et al 2012; Blusztajn and Mellot 2012). Altered expression in genes other than the Chrna7 could alter the function of α7 nicotinic receptors. Further research is needed to clarify this issue.
Perinatal choline supplementation has been shown to have numerous beneficial effects in rodent studies. These include improved learning and memory (Meck et al 1988; Tees 1999 a;b), even into old age; (Meck and Williams 2003; Glenn et al 2008); protection from the detrimental effects of prenatal alcohol exposure (Thomas et al 2004; Monk et al 2012); improvement in behavioral deficits associated with a model of Rett’s syndrome (Nag and Berger-Sweeney 2007); improved cognitive function in a model of Down’s syndrome (Moon et al 2010); and antidepressant-like effects in adult female rats (Glenn et al 2012) as well as improving both cognitive deficits and sensory inhibition in mouse models of schizophrenia (Corriveau and Glenn 2012; Stevens et al 2008, respectively). The present study extends these studies by linking improvement in sensory inhibition to stimulation of α7 nicotinic receptors.
In conclusion, gestational choline supplementation in DBA/2 mice carrying the Chrna7 null mutation produced improved sensory inhibition in the wildtype mice only, without increasing hippocampal α7 receptor levels in either the wildtype or heterozygote mice. These data point to a pivotal role for α7 nicotinic receptors in the deficit amelioration and corroborate a similar studies with DBA/2 parental mice and women with normal pregnancies. In both cases, gestational choline supplementation improved sensory inhibition in supplemented offspring (Stevens et al 2008; Ross et al 2013, respectively).
4. Experimental Procedures
4.1 Animals
Initial DBA/2 mice carrying the null mutation for the α7 nicotinic receptor gene (Chrna7) were obtained from the Institute for Behavioral Genetics breeding colony at the University of Colorado, Boulder. These mice were derived by mating α7 mixed background (129 × C57Bl/6) mice with DBA/2 mice (both sexes were used). The resultant progeny were +/+ and +/− at the α7 locus. The heterozygote mice were back-crossed with DBA/2 mice. The α7 heterozygote offspring were used to generate the next generation. This process of back-crossing with DBA/2 parental mice was continued for 10 generations. DBA/2 α7 heterozygote mice were transferred to the animal colony at the Veterans Affairs Medical Center where they were bred on normal (1.1 gm/kg) or supplemented (5 gm/kg) choline diet (Dyets, Bethlehem, PA). They were housed in standard shoe box caging with water continuously available; lights cycled at 12 hours (lights on at 6 AM). Dams were placed on the respective diet at the time the sires were placed in the cage and remained on the diet through parturition and weaning of the offspring. At weaning, tail snips were obtained for genotyping at the α7 nicotinic gene locus (Transnetyx, Cordova, TN), and offspring were separated by litter/sex, and placed on standard rodent chow (Teklad, Harlan, Indianapolis, ID) until testing. The paradigm resulted initially in 12 groups of offspring mice, 2 sexes (male, female), 3 genotypes [wildtype, null mutant, heterozygote], 2 diet groups (normal choline, supplemented choline). Ultimately, the sexes were combined to yield only 6 groups, 3 genotypes and 2 diet groups. Final group sizes were as follows: Wildtype normal choline—11; Heterozygotes normal choline—16; Null mutant normal choline—10; Wildtype supplemented choline—15; Heterozygotes supplemented choline—18; Null mutant supplemented choline—20. These mice were taken from a total of 25 litters with a maximum of 4 mice taken from a single litter, but representing all 3 genotypes.
4.2 Sensory inhibition testing
Offspring mice (22–30 gm, ages ~9–14 weeks for males and 11–16 weeks for females) from all genotypes, both sexes and both diet groups were anesthetized with chloral hydrate (400 mg/kg, ip) and pyrazole (400 mg/kg, ip) to retard the metabolism of the chloral hydrate. The mouse was placed into the Cunningham mouse adaptor (Braintree Scientific, Braintree, MA) for a stereotaxic instrument and maintained at 37° C with a heating pad. The scalp was incised and retracted. A burr hole was opened over the dorsal hippocampus [1.8 mm posterior to bregma, 2.7 mm lateral to midline (Paxinos and Franklin 2004)] for the recording electrode and a second hole opened over the contralteral cortex rostral to bregma for the reference electrode. A Teflon-coated, stainless steel wire recording electrode (127 µM diameter) was lowered to the pyramidal cell layer of hippocampal area CA3 (−1.5–1.8 mm below dura). Final recording position was determined by the presence of complex spiking patterns typical of pyramidal cells (Miller and Freedman 1995). An identical electrode was placed on the anterior cortex to act as reference. Miniature earphones attached to hollow ear bars, placed at the externalization of the aural canal, delivered the computer-generated auditory stimuli. EEG responses to paired click stimuli (3000 Hz, 10 ms, 70 dB SPL, presented 0.5 sec apart, with 9 sec between pairs) were amplified 1000 times with bandpass filtering at 1–500 Hz and led to a computer for storage and analysis. Data were collected and analyzed using SciWorks (DataWave, Loveland CO) acquisition and analysis program. The responses to 16 pairs of stimuli were collected and averaged at 5-minute intervals. The maximum negativity between 20 and 60 msec after the stimulus (N40) was selected and measured relative to the preceding positivity (P20). This composite component has been shown to be less variable than either component (P20 or N40) alone (Hashimoto et al 2005). Three parameters were measured per record: conditioning amplitude—the magnitude of the response to the first stimulus, test amplitude—the magnitude of the response to the second stimulus, and TC ratio—the ratio of the test amplitude/conditioning amplitude, which is a measure of the level of inhibition (Stevens et al 1996). A TC ratio of 0.5 or less is evidence of normal sensory inhibition (Freedman et al 1983). 10 records were taken per mouse. The three most similar records in terms of latency and amplitude magnitude, were averaged to obtain characteristic parameters for each mouse.
4.3 Autoradiography
Mice were decapitated while deeply anesthetized, the brain dissected out, frozen on dry ice snow and stored at −70° C until sectioned. Sequential cryostat sections (12 µM) through the dorsal hippocampus were cut, thaw-mounted onto gelatin-coated slides and stored at −70° C before binding with I125 α-bungarotoxin. The sections, subdivided into total- and non-specific binding groups, were incubated in a solution containing 20 mM Tris-HCl, 120 mM NaCl and 2 mg/ml bovine serum albumin (TBS/BSA, pH 7.4) for 30 min at room temperature. Non-specific binding was determined by adding 50 nM α-bungarotoxin (αBTX) Tocris, Minneapolis, MN) to the TBS/BSA buffer. The two sets of tissue were incubated in TBS/BSA buffer containing I125 αBTX (5 nM, specific activity 2000 Ci/mmol; Perkin Elmer, Waltham, MA) at 37° C for 3 hr. After incubation with the labeled ligand, the tissue was rinsed in TBA/BSA buffer for 5 min, in TBS without BSA for 15 min and in PBS for 5 min, all at 37° C. Subsequently, the slides were dipped briefly in distilled water, dried quickly under a stream of cool air and exposed to Super Resolution Phosphor Storage Screens (Perkin-Elmer Life Sciences, Boston, MA). A set of I125 tissue paste standards was included on each screen for calibration. Following a two-week exposure each screen was scanned using a Cyclone Phosphoimager (Perkin-Elmer Life Sciences). Signal intensity was measured with the OptiQuant imaging software (Perkin-Elmer) and was a linear function of I125 content of the standards (r>0.99). Signal intensity (fMol ligand bound/mg wet tissue weight) was calculated using the I125 standard curve for each screen and the known specific activity of I125-α-bungarotoxin.
4.4 Statistics
Sensory inhibition data were analyzed by a 4 factor repeated measures MANOVA, with genotype and sex as fixed factors, diet as the between subject variable, and recording time as the repeated measure. Fisher’s LSD a posteriori analyses were performed where appropriate. Binding data were analyzed by mixed model 3 factor ANOVA with genotype and sex as fixed factors and diet as the between subject variable, again with Fisher’s LSD a posteriori analyses performed where appropriate.
Highlights.
Perinatal choline supplementation improves sensory inhibition in DBA/2 Chrna7 +/+ mice
Choline supplementation does not improve sensory inhibition in Chrna7 +/− or −/− mice
Choline supplementation does not alter hippocampal α7 nicotinic receptor levels
Chrna7 +/− mice have fewer hippocampal α7 nicotinic receptors than +/+ mice, and −/− have none
Acknowledgements
The research was supported by a P30 grant (DA022462 to Michael Marks), P50 grant (MH086383 to Robert Freedman) and VA Merit Reviews to KES and CEA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abreu-Villaça Y, Filgueiras CC, Manhães AC. Developmental aspects of the cholinergic system. ehav Brain Res. 2011;221:367–378. doi: 10.1016/j.bbr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- Adams CE. Comparison of alpha7 nicotinic acetylcholine receptor development in the hippocampal formation of C3H and DBA/2 mice. Brain Res Dev Brain Res. 2003;143:137–149. doi: 10.1016/s0165-3806(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pang K, Gerhardt G, Rose GM. Modulation of the gating of auditory evoked potentials by norepinephrine: pharmacological evidence obtained using a selective neurotoxin. Biol Psychiat. 1988;24:179–190. doi: 10.1016/0006-3223(88)90273-9. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Braga MF, Alkondon M. Contribution of nicotinic receptors to the function of synapses in the central nervous system: the action of choline as a selective agonist of alpha7 receptors. J Physiology. 1998;92:309–316. doi: 10.1016/s0928-4257(98)80039-9. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson OS, Sant KE, Dolinoy DC. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J Nutr Biochem. 2012;23:853–859. doi: 10.1016/j.jnutbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NJ, Staunton M, Adler LE, Gerhardt GA, Drebing C, Waldo M, Nagamoto H, Freedman R. Sensory gating deficits in psychiatric inpatients: relation to catecholamine metabolites in different diagnostic groups. Biol Psychiat. 1990;27:519–528. doi: 10.1016/0006-3223(90)90443-6. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK. Choline, a vital amine. Science. 1998;281:794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Blusztajn JK, Mellott TJ. Choline nutrition programs brain development via DNA and histone methylation. Cent Nerv Syst Agents Med Chem. 2012;12:82–94. doi: 10.2174/187152412800792706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau JA, Glenn MJ. Postnatal choline levels mediate cognitive deficits in a rat model of schizophrenia. Pharmacol Biochem Behav. 2010;102:60–68. doi: 10.1016/j.pbb.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire E. A review of maternal nutrition and fetal gene expression. Br J Nurs. 2007;16:820–822. doi: 10.12968/bjon.2007.16.13.24250. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Schwartz BL, Schooler NR, Rosse RB, Mastropaolo J, Gaskins B. First administration of cytidine diphosphocholine and galantamine in schizophrenia: a sustained alpha7 nicotinic agonist strategy. Clin Neuropharmacol. 2008;31:34–39. doi: 10.1097/wnf.0b013e31806462ba. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol Pharmacol. 2004;66:658–666. doi: 10.1124/mol.104.000042. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adle RLE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiat. 1983;18:537–551. [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiat. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Adams RS, McClurg L. Supplemental dietary choline during development exerts antidepressant-like effects in adult female rats. Brain Res. 2012;1443:52–63. doi: 10.1016/j.brainres.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn MJ, Kirby ED, Gibson EM, Wont-Goodrich SJ, Mellott TJ, Blusztajn JK, Williams CL. Age-related declines in exploratory behavior and markers of hippocampal plasticity are attenuated by prenatal choline supplementation in rats. Brain Res. 2008;1237:110–123. doi: 10.1016/j.brainres.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacol. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Iyo M, Freedman R, Stevens KE. Tropisetron improves deficient inhibitory auditory processing in DBA/2 mice: role of alpha 7 nicotinic acetylcholine receptors. Psychopharmacol. 2005;183:13–19. doi: 10.1007/s00213-005-0142-0. [DOI] [PubMed] [Google Scholar]
- Kohlhaas KL, Bitner RS, Gopalakrishnan M, Rueter LE. Effects of α7 nicotinic acetylcholine receptor agonists on antipsychotic efficacy in a preclinical mouse model of psychosis. Psychopharmacol. 2012;220:823–833. doi: 10.1007/s00213-011-2535-6. [DOI] [PubMed] [Google Scholar]
- Li Z, Vance D. Phosphotidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacol. 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- Meck WH, Smith RA, Wklliams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;24:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Miller CL, Freedman R. The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neurosci. 1995;69:371–381. doi: 10.1016/0306-4522(95)00249-i. [DOI] [PubMed] [Google Scholar]
- Monk BR, Leslie FM, Thomas JD. The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt. Hippocampus. 2012;22:1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Chen M, Gandhy SU, Strawderman M, Levitsky DA, Maclean KN, Strupp BJ. Perinatal choline supplementation improves cognitive functioning and emotional regulation in the Ts65Dn mouse model of Down syndrome. Behav Neurosci. 2010;214:346–361. doi: 10.1037/a0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, Thaker GK. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacol. 2004;174:334–340. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- Nag N, Berger-Sweeney JE. Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiol Dis. 2007;26:473–480. doi: 10.1016/j.nbd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Olincy A, Freedman R. Nicotinic mechanisms in the treatment of psychotic disorders: a focus on the α7 nicotinic receptor. Handb Exp Pharmacol. 2012;213:211–232. doi: 10.1007/978-3-642-25758-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiellom P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines andnicotine. J Pharmacol Exp Ther. 2009;329:791–807. doi: 10.1124/jpet.108.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Radek RJ, Miner HM, Bratcher NA, Decke,r MW, Gopalakrishnan M, Bitner RS. Alpha4beta2 nicotinic receptor stimulation contributes to the effects of nicotine in the DBA/2 mouse model of sensory gating. Psychopharmacol. 2006;187:47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;223:905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Singer P, Feldon J, Yee BK. Are DBA/2 mice associated with schizophrenia-like endophenotypes? A behavioural contrast with C57BL/6 mice. Psychopharmacol. 2009;206:677–698. doi: 10.1007/s00213-009-1568-6. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Davis JM, Cornwell J, Noth K, Gupta S, Sershen H, Lajtha A. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophr Res. 2009;110:149–155. doi: 10.1016/j.schres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, Khan A. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006 Mar;31(3):637–643. doi: 10.1038/sj.npp.1300881. 2006. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Adams CE, Yonchek J, Hickel C, Danielson J, Kisley MA. Permanent improvement in deficient sensory inhibition in DBA/2 mice with increased perinatal choline. Psychopharmacol. 2008;198:413–420. doi: 10.1007/s00213-008-1170-3. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Genetic correlation of hippocampal auditory evoked response and α-bungarotoxin binding in inbred mouse strains. Neuropsychopharmacol. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir V, Freedman R. Selective α7 nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacol. 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Wear KD. Normalizing effects of nicotine and a novel nicotinic agonist on hippocampal auditory gating in two animals models. Pharmacol Biochem Behav. 1997;57:869–874. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- Stitzel JA, Farnham DA, Collins AC. Linkage of strain-specific nicotinic receptor alpha 7 subunit restriction fragment length polymorphisms with levels of alpha-bungarotoxin binding in brain. Brain Res, Molecular Brain Res. 1996;43:30–40. doi: 10.1016/s0169-328x(96)00149-0. [DOI] [PubMed] [Google Scholar]
- Tees RC. The influences of sex, rearing environment, and neonatal choline dietary supplementation on spatial and nonspatial learning and memory in adult rats. Dev Psychobiol. 1999a;35:328–342. doi: 10.1002/(sici)1098-2302(199912)35:4<328::aid-dev7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav Brain Res. 1999b;105:173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:34–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Waldo MC, Woodward L, Adler LE. Varenicline and P50 auditory gating in medicated schizophrenic patients: a pilot study. Psychiat Res. 2010;175:179–180. doi: 10.1016/j.psychres.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables PH. Input dysfunction in schizophrenia. Prog Exp Pers Res. 1964;72:1–42. [PubMed] [Google Scholar]
- Venables PH. Hippocampal function and schizophrenia. Experimental psychological evidence. Ann NY Acad Sci. 1992;658:111–127. doi: 10.1111/j.1749-6632.1992.tb22841.x. [DOI] [PubMed] [Google Scholar]
- Yan J, Jiang Z, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Stabler SP, Allen RH, Caudill MA. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr. 2012;95:1060–1071. doi: 10.3945/ajcn.111.022772. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. Choline: An essential nutrient for humans. Nutrition. 2000;16:669–767. doi: 10.1016/s0899-9007(00)00349-x. [DOI] [PubMed] [Google Scholar]
- Zeisel SH. The supply of choline is important for fetal progenitor cells. Semin Cell Dev Biol. 2011;22:624–628. doi: 10.1016/j.semcdb.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutat Res. 2012;733:34–38. doi: 10.1016/j.mrfmmm.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH. Nutrition in pregnancy: the argument for including a source of choline. Intl J Women’s Health. 2013;5:193–199. doi: 10.2147/IJWH.S36610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Blusztajn JK. Choline and human nutrition. Ann Rev Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Liu L, Liu S, Hong X, Chen, da C, Xiu MH, Yang FD, Zhang Z, Zhang X, Kosten TA, Kosten TR. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry. 2012;169:974–981. doi: 10.1176/appi.ajp.2012.11081289. [DOI] [PubMed] [Google Scholar]