Abstract
Two fully blocked and one partially blocked photosystem II nuclear mutants have been selected in Zea mays. The fully blocked mutants lack photosystem II activity, variable fluorescence, the light-inducible C-550 signal, the high potential form of cytochrome b-559, and most or all of the low potential form of the cytochrome. The block in these mutants may primarily affect the reducing side of photosystem II, inasmuch as chloroplasts isolated from both mutants exhibit an elevated F695 fluorescence emission peak. The partially blocked mutant exhibits partial photosystem II activity and a reduction, but not the total loss of the variable fluorescence yield, the C-550 signal, and the high potential form of cytochrome b-559. Lamellae isolated from the fully blocked mutants are greatly deficient for a major lamellar polypeptide with an apparent molecular weight of 32,000 daltons, whereas lamellae from the partially blocked mutant show the partial loss of this same polypeptide, suggesting that the 32,000 dalton polypeptide is necessary for the proper function of photosystem II.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber W., Trebst A., Harth E. On a new inhibitor of photosynthetic electron-transport in isolated chloroplasts. Z Naturforsch B. 1970 Oct;25(10):1157–1159. doi: 10.1515/znb-1970-1018. [DOI] [PubMed] [Google Scholar]
- Fork D. C., Heber U. W. Studies on electron-transport reactions of photosynthesis in plastome mutants of oenothera. Plant Physiol. 1968 Apr;43(4):606–612. doi: 10.1104/pp.43.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Coen D. M., Rich A., Bogorad L. Membrane proteins synthesized but not processed by isolated maize chloroplasts. J Cell Biol. 1978 Sep;78(3):734–746. doi: 10.1083/jcb.78.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebanier A. E., Steinback K. E., Bogorad L. Comparison of the Molecular Weights of Proteins Synthesized by Isolated Chloroplasts with Those Which Appear during Greening in Zea mays. Plant Physiol. 1979 Mar;63(3):436–439. doi: 10.1104/pp.63.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U., Boardman N. K., Anderson J. M. Cytochrome b-563 redox changes in intact CO-fixing spinach chloroplasts and in developing pea chloroplasts. Biochim Biophys Acta. 1976 Feb 16;423(2):275–292. doi: 10.1016/0005-2728(76)90185-7. [DOI] [PubMed] [Google Scholar]
- Henningsen K. W., Boardman N. K. Development of Photochemical Activity and the Appearance of the High Potential Form of Cytochrome b-559 in Greening Barley Seedlings. Plant Physiol. 1973 Jun;51(6):1117–1126. doi: 10.1104/pp.51.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann F. Chloroplast lamellar proteins of the plastid mutant en: viridis-1 of Antirrhinum majus having impaired photosystem II. Exp Cell Res. 1972 Feb;70(2):452–453. doi: 10.1016/0014-4827(72)90162-0. [DOI] [PubMed] [Google Scholar]
- Homann P. H. Fluorescence properties of chloroplasts from manganese deficient and mutant tobacco. Biochim Biophys Acta. 1968 Nov 26;162(4):545–554. doi: 10.1016/0005-2728(68)90062-5. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Burton W. G., Duram H. A. Membrane polypeptides associated with photochemical systems. Nat New Biol. 1972 Jun 7;237(75):176–177. doi: 10.1038/newbio237176a0. [DOI] [PubMed] [Google Scholar]
- Levine R. P., Gorman D. S. Photosynthetic electron transport chain of Chlamydomonas reinhardi. 3. Light-induced absorbance changes in chloroplast fragments of the wild type and mutant strains. Plant Physiol. 1966 Oct;41(8):1293–1300. doi: 10.1104/pp.41.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
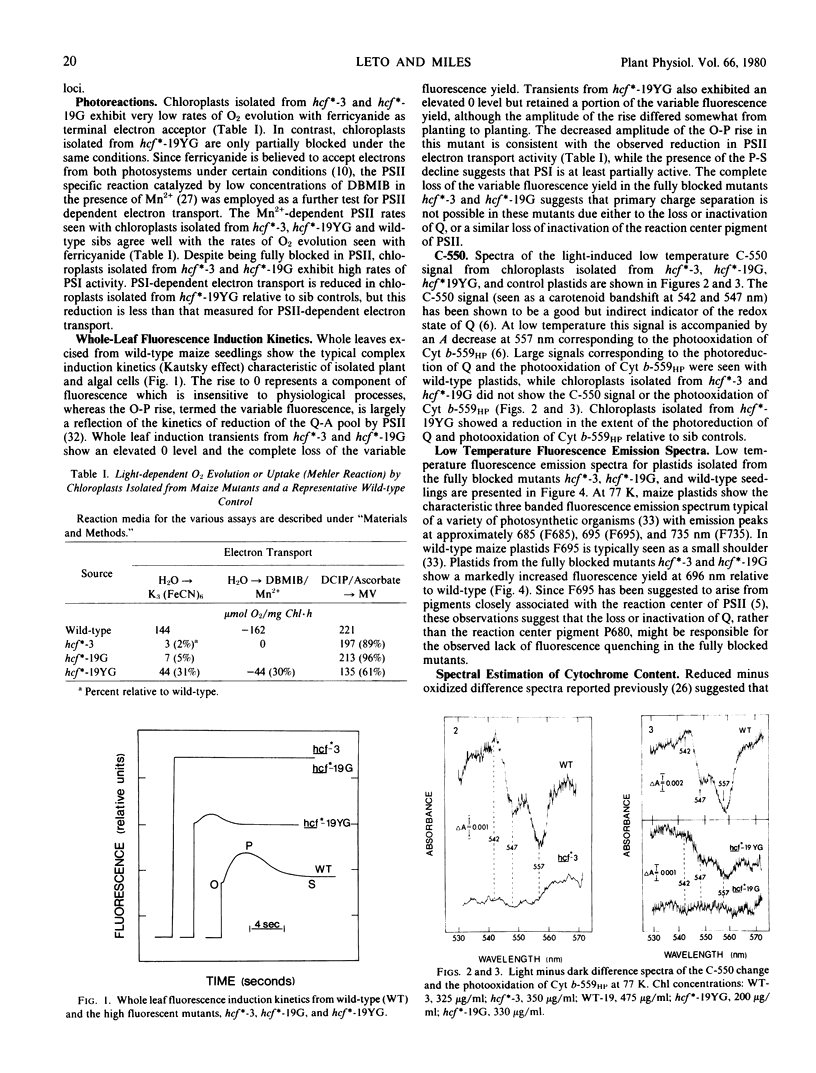
- Miles C. D., Daniel D. J. Chloroplast Reactions of Photosynthetic Mutants in Zea mays. Plant Physiol. 1974 Apr;53(4):589–595. doi: 10.1104/pp.53.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C. D. Manganese stimulation of oxygen consumption in chloroplasts with dibromothymoquinone. FEBS Lett. 1976 Jan 15;61(2):251–254. doi: 10.1016/0014-5793(76)81050-2. [DOI] [PubMed] [Google Scholar]
- Newman P. J., Sherman L. A. Isolation and characterization of photosystem I and II membrane particles from the blue-green alga, Synechococcus cedrorum. Biochim Biophys Acta. 1978 Aug 8;503(2):343–361. doi: 10.1016/0005-2728(78)90193-7. [DOI] [PubMed] [Google Scholar]
- Rijgersberg C. P., Amesz J., Thielen A. P., Swager J. A. Fluorescence emission spectra of chloroplasts and subchloroplast preparations at low temperature. Biochim Biophys Acta. 1979 Mar 15;545(3):473–482. doi: 10.1016/0005-2728(79)90156-7. [DOI] [PubMed] [Google Scholar]
- Russell G. K., Lyman H., Heath R. L. Absence of fluorescence quenching in a photosynthetic mutant of Euglena gracilis. Plant Physiol. 1969 Jun;44(6):929–931. doi: 10.1104/pp.44.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwelitz F. D., Dilley R. A., Crane F. L. Biochemical and Biophysical Characteristics of a Photosynthetic Mutant of Euglena gracilis Blocked in Photosystem II. Plant Physiol. 1972 Jul;50(1):161–165. doi: 10.1104/pp.50.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]