Abstract
Alcohol use is a leading cause of preventable morbidity and mortality worldwide, with much of its negative impact as the result of alcoholic liver disease (ALD). ALD is a broad term that encompasses a spectrum of phenotypes ranging from simple steatosis to steatohepatitis, progressive fibrosis, cirrhosis, and hepatocellular carcinoma. The mechanisms underlying the development of these different disease stages are incompletely understood. Standard treatment of ALD, which includes abstinence, nutritional support, and corticosteroids, has not changed in the last 40 years despite continued poor outcomes. Novel therapies are therefore urgently needed. The development of such therapies has been hindered by inadequate resources for research and unsuitable animal models. However, recent developments in translational research have allowed for identification of new potential targets for therapy. These targets include: (i) CXC chemokines, (ii) IL-22/STAT3, (iii) TNF receptor superfamily, (iv) osteopontin, (v) gut microbiota and lipopolysaccharide (LPS), (vi) endocannabinoids, and (vii) inflammasomes. We review the natural history, risk factors, pathogenesis, and current treatments for ALD. We further discuss the findings of recent translational studies and potential therapeutic targets.
Keywords: alcoholic liver diseases, fibrosis, inflammation, translational research
Introduction
Alcohol consumption is a leading cause of global morbidity and mortality, with much of the burden resulting from alcoholic liver disease (ALD). Excessive alcohol intake can lead to liver damage through its direct action as a hepatotoxin1 as well as potentiation of other liver diseases including chronic viral hepatitis and non-alcoholic fatty liver disease (NAFLD).2–4
Despite the profound impact of ALD on public health, relatively few advances have been made in this field. The disease pathogenesis remains poorly understood, and medical treatment for ALD has not changed significantly in 40 years.5 This situation is in marked contrast to the considerable advances in the treatment of other liver diseases such as viral hepatitis. Impediments to more robust progress in the field of ALD include inadequate experimental models of disease, a lack of interest from pharmaceutical companies, and inadequate public funding of ALD research.
Here, we review the natural history of ALD and its clinical and pathologic characteristics. We also describe the current understanding of pathogenic mechanisms underlying this disease as well as potential new therapeutic targets.
Natural history and risk factors
ALD is a broad designation that encompasses a range of disorders including simple steatosis, inflammation, fibrosis, and cirrhosis. Steatosis, which is present in more than 90% of heavy drinkers, is asymptomatic and reversible with abstinence. However, with continued alcohol intake, hepatic inflammation and injury can occur, a condition known as alcoholic hepatitis (AH). The prognosis of AH is variable, with nearly 100% survival in mild cases as compared to high short-term mortality among the most severe cases.6,7 Various predictive models have been developed to aid in the assessment of prognosis and to guide treatment, including Maddrey’s discriminant function, the model for end-stage liver disease (MELD), the Glasgow score, the ABIC score, and the Lille model.7–11
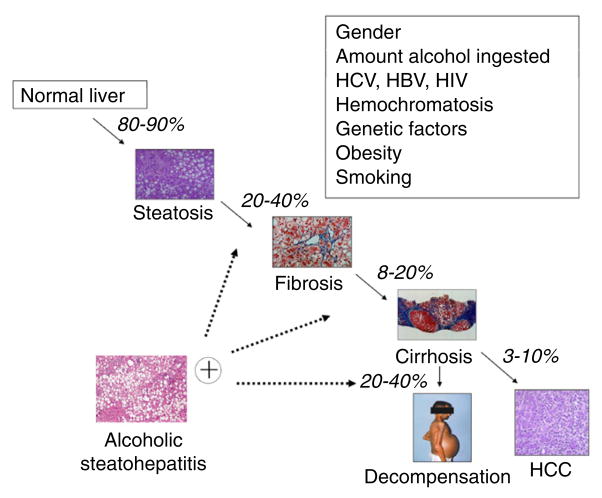
Patients with continued excessive alcohol consumption are at risk for the development of fibrosis and cirrhosis. Twenty to 40% of patients with steatosis will progress to fibrosis, of which 8–20% will develop cirrhosis.12,13 As in other liver diseases, patients with cirrhosis are at risk for hepatic decompensation (ascites, variceal bleeding, and encephalopathy) and hepatocellular carcinoma (HCC) (Fig. 1).
Figure 1.
Natural history of alcoholic liver disease and modifier factors. More than 80–90% of heavy drinkers develop fatty liver, but only up to 20–40% of this population develops more severe forms of alcoholic liver disease (ALD), including fibrosis, alcoholic hepatitis, cirrhosis, and hepatocarcinoma (HCC). Multiple other risk factors have been proposed to play a role in susceptibility to severe forms of ALD. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Although the most important risk factor for ALD is the absolute amount of alcohol intake, multiple other factors play a role in host susceptibility.14 Women are at greater risk of ALD, as are Mexican and black non-Hispanic Americans for reasons that are not well understood.15–17 Obesity may potentiate the hepatotoxic effects of alcohol, presumably through mechanisms similar to those that result in non-alcoholic steatohepatitis.18,19 Smoking and the pattern of alcohol use are also associated with the increased risk of ALD.14,20,21
Genetic factors are also important in host susceptibility to ALD. Polymorphisms in the genes encoding NFκB subunits, interleukin (IL)-1β and IL-1 receptor antagonists, IL-2, IL-6, and IL-10 may modify ALD progression.22 Genetic variation in components of lipopolysaccharide (LPS)-induced intracellular pathways, such as CD14 and toll-like receptor (TLR) 4, may also be associated with ALD.23 Variations in PNPLA3, which encodes patatin-like phospholipase domain-containing protein 3, strongly and reproducibly influence the progression of ALD.24–26 To date, there are no large-scale, well-designed, genome-wide association studies for ALD. Such a study will be vital in advancing the field of ALD and identifying new targets for therapy.
Pathogenesis
Steatosis is the first response of the liver to alcohol abuse. It is defined histologically as the deposition of fat in hepatocytes. Alcohol intake increases NADH/NAD+ in hepatocytes, thereby disrupting fatty acid oxidation and leading to steatosis development.27 It also increases fatty acid and triglyceride synthesis, enhances hepatic influx of free fatty acids from adipose tissue and chylomicrons from the intestinal mucosa, increases hepatic lipogenesis, decreases lipolysis, and damages mitochondria and microtubules, resulting in accumulation of very-low-density lipoprotein (VLDL).28–32
Alcohol upregulates lipogenic enzymes through upregulation of sterol regulatory element-binding protein 1c (SREBP-1c)33 and downregulation of peroxisome proliferator-activated receptor (PPAR)-α.34,35 In addition, alcohol downregulates adenosine monophosphate-activated protein kinase (AMPK). AMPK inactivates acetyl-CoA carboxylase, which, through its effects on malonyl-CoA and carnitine palmitoyltransferase 1, leads to reduced fatty acid synthesis and increased fatty acid oxidation, promoting steatosis.36,37
Steatohepatitis is characterized by steatosis, a superimposed inflammatory infiltrate of predominantly polymorphonuclear leukocytes and hepatocellular damage. In addition, biopsy specimens may show centrilobular hepatocyte ballooning, Mallory-Denk hyaline inclusions, and a “chicken wire”-like pattern of fibrosis.16 When the inflammation and hepatocellular injury are severe, the condition is termed AH.
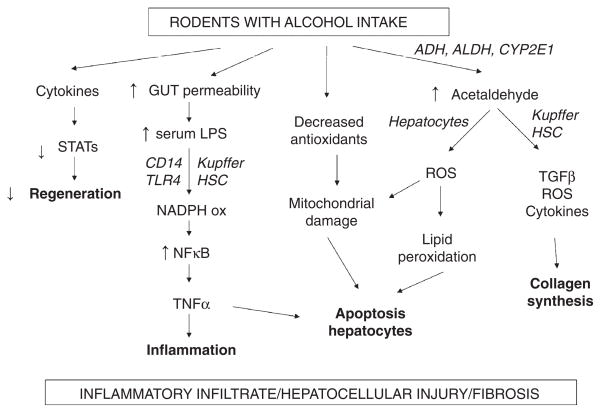
The pathogenesis of AH is complex and multifactorial (Fig. 2). In the liver, alcohol is metabolized primarily into acetaldehyde, which binds proteins and DNA, forming adducts that promote glutathione depletion, lipid peroxidation, and mitochondrial damage.38,39 These adducts also act as antigens that activate the adaptive immune response, leading to lymphocyte recruitment to the liver.40–42
Figure 2.
Pathogenic mechanisms of alcoholic liver disease proposed based in animal models evidence. Ethanol promotes the translocation of lipopolysaccharide from the gastrointestinal lumen to the portal vein, where it binds to the lipopolysaccharide-binding protein. In Kupffer cells, lipopolysaccharide binds to CD14, which combines with TLR4 activating multiple cytokine genes. The increase on inflammatory cytokine production in conjunction with a decrease in STATs expression reduces liver regeneration. Long-term alcohol consumption alters the intracellular balance of antioxidants with subsequent decrease in the release of mitochondrial cytochrome c and expression of Fas ligand, leading to hepatic apoptosis. Activated Kupffer cells and hepatocytes are suggested to be sources of free radicals (especially ROS), which are responsible for lipid peroxidation and further apoptotic damage. Activation of hepatic stellate cells also contributes to the production of cytokines, ROS and TGF-β exacerbating liver fibrosis. ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; CD14, CYP2E1, cytochrome P450 2E1; HSC, hepatic stellate cells; LPS, lipopolysaccharide; NADPH, nicotinamide adenine dinucleotide phosphate; NFκB, nuclear factor κB; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription; TGF-β, transforming growth factor β; TLR4, Toll-like receptor 4.
Alcohol use also increases gut permeability and translocation of bacterial products such as LPS into the portal circulation.43 In Kupffer cells, LPS activates the MyD88-independent signaling pathway through TLR4, resulting in the production of proinflammatory cytokines such as tumor necrosis factor (TNF)-α that contribute to hepatocellular damage.44–47 Interestingly, Kupffer cells also produce anti-inflammatory cytokines (IL-10) and hepatoprotective factors (IL-6) that reduce alcohol-induced hepatocellular damage.48–50 This protective pathway may be an explanation for 70% of heavy drinkers not developing severe forms of alcoholic liver injury.
The presence of a neutrophilic infiltrate, a key feature of alcoholic steatohepatitis, is likely instigated by a host of proinflammatory cytokines. Acetaldehyde, LPS, TNF-α, palmitic acid, and downregulation of proteasome functions stimulate the production of these cytokines.51–53 IL-17, one of the implicated cytokines, directly induces neutrophil recruitment and also stimulates hepatic stellate cells (HSCs) to produce IL-8 and CXCL1.54–57 In turn, IL-8 and CXCL1 also promote recruitment of neutrophils. Additional cytokines and chemokines, such as TNF-α, IL-1, osteopontin, CXCL4, CXCL5, and CXCL6, are upregulated and may also contribute to neutrophil recruitment during alcoholic liver injury.
Chronic alcohol use can result in fibrosis, which refers to the extracellular accumulation of collagen and other matrix proteins. Acetaldehyde plays a central role in fibrogenesis. It directly increases the expression of collagen in HSCs, and when combined with cellular components, produces various adducts that maintain HSC activation.58 HSCs can also be activated by neutrophils, damaged hepatocytes, and activated Kupffer cells through various pro-fibrogenic mediators including TGF-β, platelet-derived growth factor, IL-8, TNF-α, and reactive oxygen species (ROS).59,60 ROS decrease the action of metalloproteinases and up-regulate tissue inhibitor of metalloproteinases 1, resulting in collagen accumulation.61 They also stimulate HSC pro-fibrogenic signaling pathways such as ERK1, ERK2, phosphoinositide 3 kinase-Akt, and c-Jun N-terminal kinase (JNK).62,63 LPS is also involved in fibrogenesis. LPS activates TLR4 signaling in HSCs and sinusoidal endothelial cells, resulting in HSC activation and promotion of fibrogenesis through regulation of angiogenesis.64,65
Finally, alcohol consumption inhibits the anti-fibrotic action of natural killer (NK) cells.66 NK cells destroy activated HSCs and produce interferon (IFN)-γ which induces HSC cell cycle arrest and apoptosis.67–69 Such interference with the function of NK cells and IFN-γ may be an important component of both alcoholic fibrosis and alcohol promotion of fibrosis due to viral hepatitis.
Current treatment
Alcohol cessation is the mainstay of therapy for patients with all stages of ALD.70,71 In addition, abstinence is critical for patients who require liver transplantation because active alcohol use is, in general, a contraindication to transplant.72 Referral to formal rehabilitation programs is usually necessary to achieve abstinence. In addition, pharmacologic therapy with agents such as disulfiram, acamprosate, baclofen, and naltrexone can be considered, although their efficacy is limited.73–76 Patients with alcoholic cirrhosis should receive additional routine care such as screening and management of varices, screening for HCC, and vaccination for hepatitis A and B, among others.77
For severe AH, admission to the hospital is usually required. Patients should be assessed and closely monitored for alcohol withdrawal, encephalopathy, and bacterial infections, which are common in this patient group. Intensive nutritional support has been advocated, although its effect on patient outcomes is controversial.78,79
Corticosteroids have been the subject of numerous clinical trials since they were first introduced as a treatment for AH 40 years ago. Most have demonstrated a survival advantage when used in patients with severe disease, and current clinical practice guidelines recommend their use in patients with a Maddrey’s discriminant function ≥ 32 and those with hepatic encephalopathy.16,80,81 Pentoxifylline may also be useful in the treatment of severe AH, and is an alternative when corticosteroids are contraindicated.82 Pentoxifylline is not useful as a rescue agent in those who have not responded to corticosteroids, and the combination of these medications is not more effective than corticosteroids alone.83,84 N-acetylcysteine may offer additional incremental benefit when combined with prednisolone.85
Because of the implication of TNF-α in ALD pathogenesis and the benefit of pentoxifylline in AH, TNF-α antagonists have been studied for this condition. Early studies were promising, but larger clinical trials demonstrated an increased risk of infection and mortality with these agents.86–88 Another agent, S-adenosylmethionine (SAMe), has been shown to act as an antioxidant and downregulator of TNF-α, and therefore may be protective against ALD.89 Currently, however, clinical data are inconclusive, and further study of this agent is needed.90 Studies of other medications, such as anabolic steroids, vitamin E, silibinin, colchicine, and propylthiouracil, have likewise been disappointing.91–95
Liver transplantation is an option for patients with end-stage liver disease due to alcohol, with favorable post-transplant outcomes compared to transplantation for other indications.96 Because of concerns about recidivism and the potential for clinical improvement with alcohol cessation, current guidelines recommend a period of abstinence prior to considering transplant, in accordance with the practice patterns of the majority of transplant centers.97 This policy essentially excludes patients with severe AH, who, by definition, have not had a period of abstinence. Recently published data suggest that liver transplant may be considered for highly selected patients who have not responded to standard therapies.98 These results call into question the requirement for a strictly defined period of abstinence.72,99
New targets for therapy
Ideally, new treatments for ALD should be effective, safe, and selective. The development of such agents requires the identification of molecular targets specific for ALD. As animal models do not accurately mimic advanced ALD, and the pathophysiologic significance of serum levels of biomarkers is unclear (due to impaired liver clearance and ongoing bacterial infections), liver tissue from patients with ALD may serve as a source to identify therapeutic targets (Fig. 3). Here, we discuss the most promising targets for ALD identified in human samples.
Figure 3.
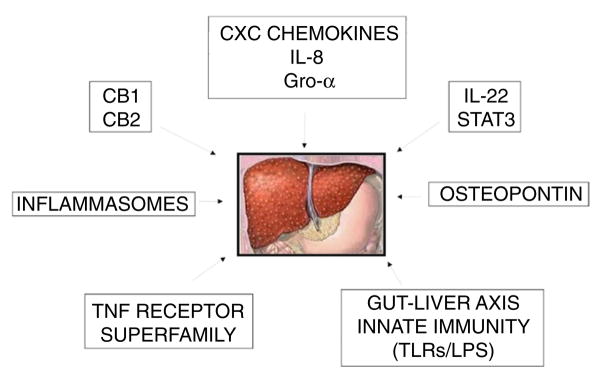
Potential therapeutic targets for alcoholic liver disease identified in translational studies. The expression and/or activation of different mediators of alcoholic liver disease in liver tissue from patients have been investigated. These findings have been correlated with disease severity and the patient’s outcome. These targets include: CXC chemokines, IL-22/STAT3, TNF receptor superfamily, osteopontin, gut microbiota and LPS, endocannabinoids and inflammasomes. CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; Gro-α growth-regulated α-protein; IL-8, interleukin 8; IL-22, interleukin 22; LPS, lipopolysaccharide; STAT3, signal transducer and activator of transcription 3; TNF, tumor necrosis factor; TLRs, toll-like receptors.
CXC chemokines
Members of the CXC family of chemokines include interleukin 8 (IL-8) and growth-regulated α-protein (Gro-α). These mediators attract polymorphonuclear leukocytes, which are the predominant inflammatory cells that infiltrate the livers of patients with ALD. In patients with AH, expression of these chemokines in the liver correlates with the severity of portal hypertension and patient survival.56,100
IL-22/STAT3 pathway
Interleukin 22 (IL-22), a member of the interleukin 10 (IL-10) family of cytokines, is important in controlling bacterial infection, homeostasis, and tissue repair. Through activation of the signal transducer and activator of transcription 3 (STAT3), it has been shown to improve ALD in rodent models.101 Furthermore, IL-22 expression is decreased, whereas IL-22 receptor 1 expression is upregulated in patients with ALD. Because of its antibacterial properties, it may be an ideal therapy in combination with corticosteroids, which predispose to bacterial infections.
TNF receptor superfamily
Tumor necrosis factor α (TNF-α) is not overexpressed in the livers of patients with AH. However, fibroblast growth factor inducible 14 (Fn14), a member of the TNF receptor superfamily (member 12A) is overexpressed in these patients.102 Moreover, its expression correlates with disease severity. This receptor is expressed primarily in hepatic progenitor cells, which accumulate in patients with severe AH.
Osteopontin
Osteopontin, an extracellular matrix protein, is upregulated in the livers of patients with ALD, and its expression correlates with disease severity.103 Animals that lack osteopontin are relatively protected from alcohol-mediated liver damage as well.104 Inhibition of osteopontin-mediated pathways may therefore be an effective therapy for ALD.
Gut microbiota and LPS
Alterations in the gut micro-biome and increased gut permeability associated with ALD can result in increased LPS in the portal circulation. Rifaximin, a nonabsorbable antibiotic that alters the gut microbiota, is efficacious in the treatment of hepatic encephalopathy, and could have a role in ALD.105 Inhibition of LPS-induced TLR4 signaling has been suggested as another target for novel therapies.106
Endocannabinoids
Endocannabinoids are involved in the pathogenesis of ALD through cannabinoid receptors 1 and 2 (CB1 and CB2).107 Animals lacking cannabinoid receptors have differential responses to alcohol-induced liver injury,108,109 suggesting the potential use of CB1 antagonists and CB2 agonists as therapeutic agents. Although CB1 antagonists are limited by their neuropsychiatric side effects, peripherally restricted agents may benefit patients with ALD.107
Inflammasome
Inflammasomes are intracellular multiprotein complexes that mediate the response to cellular danger signals activating and recruiting inflammatory cells. Inflammasome activation leads to activation of caspase-1, resulting in the release of IL-1β and IL-18.110 Serum levels of IL-1β were found to be increased in patients with ALD as well as in animal models.111,112 Recent studies demonstrated mRNA expression of several inflammasomes in the liver thus suggesting that inflammasome activation is a component of the liver pathophysiology in ALD.113
Conclusions
Alcohol consumption is a leading cause of global morbidity and mortality, with much of its negative impact as a result of ALD. Despite some advances in our understanding of the pathogenesis and clinical characteristics of ALD, many questions remain. Standardized nomenclature and histologic classifications are lacking, and there have been no significant advances in therapy in the last 40 years. Recent translational work using human liver tissue has been informative in identifying some potential therapeutic targets for this disease. However, translation of these findings into novel therapies has been lacking. Additional detailed studies of these potential targets in humans and animal models are urgently needed to improve outcomes in this patient population.
Acknowledgments
Financial support: This work was supported, in part, by the National Institutes of Health, T32 DK07634 and UL1-TR000083.
References
- 1.Lieber CS, Jones DP, Decarli LM. Effects of prolonged ethanol intake: production of fatty liver despite adequate diets. J Clin Invest. 1965;44:1009–21. doi: 10.1172/JCI105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clouston AD, Jonsson JR, Powell EE. Steatosis as a cofactor in other liver diseases: hepatitis C virus, alcohol, hemochromatosis, and others. Clin Liver Dis. 2007;11:173–89. x. doi: 10.1016/j.cld.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Gitto S, Micco L, Conti F, Andreone P, Bernardi M. Alcohol and viral hepatitis: a mini-review. Dig Liver Dis. 2009;41:67–70. doi: 10.1016/j.dld.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helman RA, Temko MH, Nye SW, Fallon HJ. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Ann Intern Med. 1971;74:311–21. doi: 10.7326/0003-4819-74-3-311. [DOI] [PubMed] [Google Scholar]
- 6.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez M, Rincon D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–56. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 8.Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr, Mezey E, White RI., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–9. [PubMed] [Google Scholar]
- 9.Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–8. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 10.Forrest EH, Evans CD, Stewart S, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–9. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–54. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 12.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 13.Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–90. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 14.Bellentani S, Saccoccio G, Costa G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. Gut. 1997;41:845–50. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23:1025–9. doi: 10.1002/hep.510230513. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 17.Stewart SH. Racial and ethnic differences in alcohol-associated aspartate aminotransferase and gamma-glutamyltransferase elevation. Arch Intern Med. 2002;162:2236–9. doi: 10.1001/archinte.162.19.2236. [DOI] [PubMed] [Google Scholar]
- 18.Raynard B, Balian A, Fallik D, et al. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–8. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 19.Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–11. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 20.Hatton J, Burton A, Nash H, Munn E, Burgoyne L, Sheron N. Drinking patterns, dependency and life-time drinking history in alcohol-related liver disease. Addiction. 2009;104:587–92. doi: 10.1111/j.1360-0443.2008.02493.x. [DOI] [PubMed] [Google Scholar]
- 21.Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut. 2010;59:1159–62. doi: 10.1136/gut.2008.162453. [DOI] [PubMed] [Google Scholar]
- 22.Bataller R, North KE, Brenner DA. Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology. 2003;37:493–503. doi: 10.1053/jhep.2003.50127. [DOI] [PubMed] [Google Scholar]
- 23.Jarvelainen HA, Orpana A, Perola M, Savolainen VT, Karhunen PJ, Lindros KO. Promoter polymorphism of the CD14 endotoxin receptor gene as a risk factor for alcoholic liver disease. Hepatology. 2001;33:1148–53. doi: 10.1053/jhep.2001.24236. [DOI] [PubMed] [Google Scholar]
- 24.Stickel F, Buch S, Lau K, et al. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 25.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42:21–3. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 26.Trepo E, Gustot T, Degre D, et al. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol. 2011;55:906–12. doi: 10.1016/j.jhep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Baraona E, Lieber CS. Effects of ethanol on lipid metabolism. J Lipid Res. 1979;20:289–315. [PubMed] [Google Scholar]
- 28.Salaspuro MP, Shaw S, Jayatilleke E, Ross WA, Lieber CS. Attenuation of the ethanol-induced hepatic redox change after chronic alcohol consumption in baboons: metabolic consequences in vivo and in vitro. Hepatology. 1981;1:33–8. doi: 10.1002/hep.1840010106. [DOI] [PubMed] [Google Scholar]
- 29.Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30:378–90. doi: 10.1055/s-0030-1267538. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134:556–67. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 31.Coll O, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692–702. doi: 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- 32.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049–63. doi: 10.1053/gast.2002.33613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–7. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 34.Yu S, Rao S, Reddy JK. Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr Mol Med. 2003;3:561–72. doi: 10.2174/1566524033479537. [DOI] [PubMed] [Google Scholar]
- 35.Wagner M, Zollner G, Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53:1023–34. doi: 10.1002/hep.24148. [DOI] [PubMed] [Google Scholar]
- 36.Viollet B, Guigas B, Leclerc J, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 2009;196:81–98. doi: 10.1111/j.1748-1716.2009.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–88. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3:178–85. doi: 10.4161/oxim.3.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farfan Labonne BE, Gutierrez M, Gomez-Quiroz LE, et al. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol Toxicol. 2009;25:599–609. doi: 10.1007/s10565-008-9115-5. [DOI] [PubMed] [Google Scholar]
- 40.Albano E, Vidali M. Immune mechanisms in alcoholic liver disease. Genes Nutr. 2010;5:141–7. doi: 10.1007/s12263-009-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mottaran E, Stewart SF, Rolla R, et al. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 42.Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis. 2004;24:273–87. doi: 10.1055/s-2004-832940. [DOI] [PubMed] [Google Scholar]
- 43.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–44. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao XJ, Dong Q, Bindas J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol. 2008;181:3049–56. doi: 10.4049/jimmunol.181.5.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrasek J, Dolganiuc A, Csak T, et al. Interferon regulatory factor 3 and type I interferons are protective in alcoholic liver injury in mice by way of crosstalk of parenchymal and myeloid cells. Hepatology. 2011;53:649–60. doi: 10.1002/hep.24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin M, Wheeler MD, Kono H, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–52. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 47.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horiguchi N, Wang L, Mukhopadhyay P, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–58. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420–9. doi: 10.1002/hep.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller AM, Wang H, Bertola A, et al. Inflammation-associated interleukin-6/signal transducer and activator of transcription 3 activation ameliorates alcoholic and nonalcoholic fatty liver diseases in interleukin-10-deficient mice. Hepatology. 2011;54:846–56. doi: 10.1002/hep.24517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Quiroz L, Bucio L, Souza V, et al. Interleukin 8 response and oxidative stress in HepG2 cells treated with ethanol, acetaldehyde or lipopolysaccharide. Hepatol Res. 2003;26:134–41. doi: 10.1016/s1386-6346(03)00010-x. [DOI] [PubMed] [Google Scholar]
- 52.Joshi-Barve S, Barve SS, Amancherla K, et al. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–30. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- 53.Joshi-Barve S, Barve SS, Butt W, Klein J, McClain CJ. Inhibition of proteasome function leads to NF-kappaB-independent IL-8 expression in human hepatocytes. Hepatology. 2003;38:1178–87. doi: 10.1053/jhep.2003.50470. [DOI] [PubMed] [Google Scholar]
- 54.Lemmers A, Moreno C, Gustot T, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646–57. doi: 10.1002/hep.22680. [DOI] [PubMed] [Google Scholar]
- 55.Maltby J, Wright S, Bird G, Sheron N. Chemokine levels in human liver homogenates: associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology. 1996;24:1156–60. doi: 10.1053/jhep.1996.v24.pm0008903391. [DOI] [PubMed] [Google Scholar]
- 56.Dominguez M, Miquel R, Colmenero J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 57.Maher JJ. Rat hepatocytes and Kupffer cells interact to produce interleukin-8 (CINC) in the setting of ethanol. Am J Physiol. 1995;269:G518–23. doi: 10.1152/ajpgi.1995.269.4.G518. [DOI] [PubMed] [Google Scholar]
- 58.Mello T, Ceni E, Surrenti C, Galli A. Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol Aspects Med. 2008;29:17–21. doi: 10.1016/j.mam.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Wang JH, Batey RG, George J. Role of ethanol in the regulation of hepatic stellate cell function. World J Gastroenterol. 2006;12:6926–32. doi: 10.3748/wjg.v12.i43.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreno M, Bataller R. Cytokines and renin-angiotensin system signaling in hepatic fibrosis. Clin Liver Dis. 2008;12:825–52. doi: 10.1016/j.cld.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Arthur MJ, Iredale JP, Mann DA. Tissue inhibitors of metalloproteinases: role in liver fibrosis and alcoholic liver disease. Alcohol Clin Exp Res. 1999;23:940–3. doi: 10.1111/j.1530-0277.1999.tb04208.x. [DOI] [PubMed] [Google Scholar]
- 62.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 63.Kluwe J, Pradere JP, Gwak GY, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–59. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 65.Jagavelu K, Routray C, Shergill U, O’Hara SP, Faubion W, Shah VH. Endothelial cell toll-like receptor 4 regulates fibrosis-associated angiogenesis in the liver. Hepatology. 2010;52:590–601. doi: 10.1002/hep.23739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–58. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–52. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 68.Muhanna N, Abu Tair L, Doron S, et al. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011;60:90–8. doi: 10.1136/gut.2010.211136. [DOI] [PubMed] [Google Scholar]
- 69.Jeong WI, Park O, Radaeva S, Gao B. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44:1441–51. doi: 10.1002/hep.21419. [DOI] [PubMed] [Google Scholar]
- 70.Chedid A, Mendenhall CL, Gartside P, French SW, Chen T, Rabin L. Prognostic factors in alcoholic liver disease. VA Cooperative Study Group. Am J Gastroenterol. 1991;86:210–16. [PubMed] [Google Scholar]
- 71.Pessione F, Ramond MJ, Peters L, et al. Five-year survival predictive factors in patients with excessive alcohol intake and cirrhosis. Effect of alcoholic hepatitis, smoking and abstinence. Liver Int. 2003;23:45–53. doi: 10.1034/j.1600-0676.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- 72.Brown RS., Jr Transplantation for alcoholic hepatitis: time to rethink the 6-month “rule”. N Engl J Med. 2011;365:1836–8. doi: 10.1056/NEJMe1110864. [DOI] [PubMed] [Google Scholar]
- 73.Fuller RK, Gordis E. Does disulfiram have a role in alcoholism treatment today? Addiction. 2004;99:21–4. doi: 10.1111/j.1360-0443.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- 74.Palmer AJ, Neeser K, Weiss C, Brandt A, Comte S, Fox M. The long-term cost-effectiveness of improving alcohol abstinence with adjuvant acamprosate. Alcohol Alcohol. 2000;35:478–92. doi: 10.1093/alcalc/35.5.478. [DOI] [PubMed] [Google Scholar]
- 75.Addolorato G, Leggio L. Safety and efficacy of baclofen in the treatment of alcohol-dependent patients. Curr Pharm Des. 2010;16:2113–17. doi: 10.2174/138161210791516440. [DOI] [PubMed] [Google Scholar]
- 76.Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 77.Kanwal F, Kramer J, Asch SM, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–17. doi: 10.1016/j.cgh.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 78.Stickel F, Hoehn B, Schuppan D, Seitz HK. Review article: nutritional therapy in alcoholic liver disease. Aliment Pharmacol Ther. 2003;18:357–73. doi: 10.1046/j.1365-2036.2003.01660.x. [DOI] [PubMed] [Google Scholar]
- 79.Cabre E, Rodriguez-Iglesias P, Caballeria J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36–42. doi: 10.1053/jhep.2000.8627. [DOI] [PubMed] [Google Scholar]
- 80.Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med. 1990;113:299–307. doi: 10.7326/0003-4819-113-4-299. [DOI] [PubMed] [Google Scholar]
- 81.Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–60. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 82.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–48. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 83.Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008;48:465–70. doi: 10.1016/j.jhep.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 84.Mathurin P, Louvet A, Dao T, et al. Addition of pentoxifylline to prednisolone for severe alcoholic hepatitis does not improve 6-month survival: results of the Corpentox Trial. Hepatology. 2011;54:391A. [Google Scholar]
- 85.Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–9. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 86.Spahr L, Rubbia-Brandt L, Frossard JL, et al. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37:448–55. doi: 10.1016/s0168-8278(02)00230-1. [DOI] [PubMed] [Google Scholar]
- 87.Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–7. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- 88.Boetticher NC, Peine CJ, Kwo P, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–60. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu SC, Martinez-Chantar ML, Mato JM. Methionine adenosyltransferase and S-adenosylmethionine in alcoholic liver disease. J Gastroenterol Hepatol. 2006;21(Suppl 3):S61–4. doi: 10.1111/j.1440-1746.2006.04575.x. [DOI] [PubMed] [Google Scholar]
- 90.Rambaldi A, Gluud C. S-adenosyl-L-methionine for alcoholic liver diseases. Cochrane Database Syst Rev. 2006;(2):CD002235. doi: 10.1002/14651858.CD002235.pub2. [DOI] [PubMed] [Google Scholar]
- 91.Rambaldi A, Iaquinto G, Gluud C. Anabolic-androgenic steroids for alcoholic liver disease: a Cochrane review. Am J Gastroenterol. 2002;97:1674–81. doi: 10.1111/j.1572-0241.2002.05826.x. [DOI] [PubMed] [Google Scholar]
- 92.Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomized placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. 2004;40:40–6. doi: 10.1016/s0168-8278(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 93.Pares A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–21. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 94.Akriviadis EA, Steindel H, Pinto PC, et al. Failure of colchicine to improve short-term survival in patients with alcoholic hepatitis. Gastroenterology. 1990;99:811–18. doi: 10.1016/0016-5085(90)90973-5. [DOI] [PubMed] [Google Scholar]
- 95.Fede G, Germani G, Gluud C, Gurusamy KS, Burroughs AK. Propylthiouracil for alcoholic liver disease. Cochrane Database Syst Rev. 2011;(6):CD002800. doi: 10.1002/14651858.CD002800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burra P, Senzolo M, Adam R, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry) Am J Transplant. 2010;10:138–48. doi: 10.1111/j.1600-6143.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 97.Murray KF, Carithers RL., Jr AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–32. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 98.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 99.Dureja P, Lucey MR. The place of liver transplantation in the treatment of severe alcoholic hepatitis. J Hepatol. 2010;52:759–64. doi: 10.1016/j.jhep.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 100.Colmenero J, Bataller R, Sancho-Bru P, et al. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687–97. doi: 10.1053/j.gastro.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 101.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Affo S, Dominguez M, Lozano JJ, et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut. 2012 May 25; doi: 10.1136/gutjnl-2011-301146. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seth D, Gorrell MD, Cordoba S, McCaughan GW, Haber PS. Intrahepatic gene expression in human alcoholic hepatitis. J Hepatol. 2006;45:306–20. doi: 10.1016/j.jhep.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 104.Morales O, Dominguez M, Juez E, et al. Osteopontin is a novel therapeutic target in patients with alcoholic hepatitis. J Hepatol. 2010;52:S23. [Google Scholar]
- 105.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–81. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 106.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–20. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. Endocannabinoids in liver disease. Hepatology. 2011;53:346–55. doi: 10.1002/hep.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeong WI, Osei-Hyiaman D, Park O, et al. Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab. 2008;7:227–35. doi: 10.1016/j.cmet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 109.Louvet A, Teixeira-Clerc F, Chobert MN, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54:1217–26. doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 110.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 111.McClain CJ, Cohen DA, Dinarello CA, Cannon JG, Shedlofsky SI, Kaplan AM. Serum interleukin-1 (IL-1) activity in alcoholic hepatitis. Life Sci. 1986;39:1479–85. doi: 10.1016/0024-3205(86)90554-0. [DOI] [PubMed] [Google Scholar]
- 112.Valles SL, Blanco AM, Azorin I, et al. Chronic ethanol consumption enhances interleukin-1-mediated signal transduction in rat liver and in cultured hepatocytes. Alcohol Clin Exp Res. 2003;27:1979–86. doi: 10.1097/01.ALC.0000099261.87880.21. [DOI] [PubMed] [Google Scholar]
- 113.Lech M, Avila-Ferrufino A, Skuginna V, Susanti HE, Anders HJ. Quantitative expression of RIG-like helicase, NOD-like receptor and inflammasome-related mRNAs in humans and mice. Int Immunol. 2010;22:717–28. doi: 10.1093/intimm/dxq058. [DOI] [PubMed] [Google Scholar]